0022-538X/79/06-0711/09$02.00/0
T-Antigen
Expression
in
Proliferating
and
Non-Proliferating
Siniian
Virus
40-Transformed
Mouse
Cells
DIMITRIS ZOUZIAS AND CLAUDIO BASILICO*
DepartmentofPathology,New York UniversitySchool ofMedicine,New York, New York 10016 Received for publication 30 October 1978
Previousstudies with simian virus 40-transformedmouse 3T3 cells whichare
temperature sensitive for theexpression of the transformed phenotype (ts SV3T3
cells) have shown that T-antigen expression and viral DNA transcription are
under cellcyclecontrol.UsingthesetsSV3T3cells,westudiedthe expression of the viral genome under proliferating and non-proliferating conditions, in the presence and absence of inhibitors ofmacromolecular synthesis and of the tumor promoterphorbolmyristate acetate. ts SV3T3 cells which were growth arrested
at39°Cbylowserumconcentrationorsaturationdensity accumulated in Gl and
didnotexpressT-antigen.When these cellswereinduced toproliferate,at either 32 or39°C,T-antigen synthesispreceded the entry of the cells into the S-phase
andwas notcoupledtoDNAreplication.Gl-arrested ts SV3T3cellswere induced
tosynthesize T-antigen by phorbol myristate acetate treatment, but T-antigen alonewas notsufficienttoinduce cellular DNAsynthesis. Isoleucine deprivation arrestedgrowth oftsSV3T3cells,but thesecells,aswellasnormal3T3, did not accumulate inGland continuedtoexpressT-antigen.The temperature-sensitive expression ofthe transformedphenotypeinthetsSV3T3cellsdoes not appear to bedueto alack oftranscriptionofspecific regions of the integrated simian virus 40genomeat390C.
Cells transformedbythe oncogenicDNA
vi-rus simian virus 40 (SV40) contain viral DNA sequences integrated into the chromosomal DNA. Viral functions expressed in these
trans-formed cells are the same as those expressed
during the early period of the lytic cycle. No
transcription of late genes orsynthesis ofviral
capsid proteins takesplace,and the viral DNA
does not replicate independently. The
expres-sion ofviralgenes intransformedcells istightly
regulatedand maycomeunderhostcell control
(37).
Studies with viral mutantshave shown that
theexpressionof viralfunction(s)isrequiredfor
theestablishmentandmaintenance of the
trans-formed state (7, 10, 16, 20, 24, 32, 37). However, the existence of revertants in which the viral genome is found toremain integrated into the chromosomal DNA and in which T-antigen
expressionpersistsindicates thataninteraction
of viral andcellular productsisnecessaryforthe
expression ofthe transformed phenotype (37).
More direct evidence ofsuch acell-virus
inter-action comes from the temperature-sensitive
SV40-transfonned mouse 3T3 cells (ts SV3T3
cells)isolated in thislaboratory (27). These cells
behaveastransfornants at the permissive tem-perature
(320C)
and lose most or all of thetransformed growthcharacteristics atthe
non-permissive temperature (39°C)dueto acellular mutation.At
390C,
tsSV3T3cellsalso resemble normal 3T3 cells inbeingabletoreachastateofGl arrest (GO) under serum starvation or at
saturationdensity. Under theseconditions, the cells become T-antigen negative, and no viral
transcriptionoccurs(4). Throughoutthispaper
we use theterm Gl arrest toindicate thestate
of cell populations which are growth arrested
with a unimodal distribution of DNA content
equivalenttothatof Gl cells. ThetermGO has
been used to indicate Gl-arrested cells which retain viability for long times and which are
presumably resting in the
early
part of Gl ormay be removed from the
cycle
(2).Thus,
GlarrestandGO may be
equivalent
insomecases,butnotnecessarilyatall times.
Acellcycle-related control appears to be
ex-erted on the expression of the integratedviral
DNA in ts SV3T3 cells at the nonpermissive
temperature. Gl-arrestedtsSV3T3cellscan be inducedtoproliferate by changing the medium
and increasing the serum concentration. Thus,
we have found thisaconvenientsystem in which
to study the regulation of viral transcription duringthecell cycle and the relationship of T-antigen production to cellular DNA synthesis.
711
on November 10, 2019 by guest
http://jvi.asm.org/
712
The results presented in this paper indicate
the following. (i) Viral transcription and T-an-tigen expression in SV40-transformed 3T3 cells
occur in the Gl phase of the cellcycleand are
notcoupledto DNAreplication. (ii)Thecellular
control of T-antigen expressionobserved in
Gl-arrested ts SV3T3 cellsisspecificfor aGOblock.
Such controlis not exerted ifcellsare arrested
by isoleucine deprivation. (iii) The tumor
pro-moterphorbol myristateacetate(PMA)induces
T-antigen synthesis. (iv) Thepresenceof
T-an-tigen alone is not sufficient to induce cellular DNAsynthesis.
MATERIALS AND METHODS
Cells. Thepropertiesof the normal 3T3 and the ts SV3T3 cells used in this work have been described
previously(4, 27). The line oftsSV3T3 cells used in
theseexperimentswas tsH6-15 (27). Cellstockswere
generally maintained inahumidified atmosphere of
10%CO2at320CinDulbecco modifiedEaglemedium containing 10% calfserum. For the experiments in-volvingrestingcellsat39°C,culturesweregrownin5 to 10% calfserum. Atthe timethey reached conflu-ence,mediumcontaining 1%calfserum wassupplied
unless otherwise stated.
T-antigen assay. Cells that had been grown on
cover slipswerefixed with acetone-ethanol (2:1) for
20minat4°C.ForT-antigendetermination,thecells
wereincubated with hamster antiserumagainst SV40
Tantigen for 45min at 37°C and then with rabbit
fluorescein-labeledantibody againsthamster
y-globu-lin. Cells were observed in a Zeiss UV microscope
equippedwithanepifluorescenceilluminator(4).
DNAsynthesisandautoradiography. Cellsthat
had been grownon coverslipswereincubated in the presenceof[3H]thymidine.Attheend of thelabeling
period, theywerefixed with 95% ethanol-acetic acid
(9:1). Coverslips werewashedextensively in 70and
95%ethanol, dried,and mountedonglass microscope
slides. The slidesweredippedin nuclear track
emul-sion(NTB-2;Kodak).After about7daysof exposure,
they weredeveloped and stained withGiemsa stain.
Flow microfluorometric analysis of cellular DNAcontent.The method describedbyKirshan(17) was used. Cells weregrown in 100-mm petridishes. After removing the medium, cellswere washed and treated in the dark withacoldsolution ofpropidium iodide (0.05 mg/ml) and sodium citrate 0.1%. After
swellingfor10minat4°C, the cellsweredetachedby
vigorous pipetting, transferredto atube onice, and analyzed on amodel 4800Acytofluorograph (Ortho
Instruments,Westwood,Mass.) within24h.
Preparation of cytoplasmic RNA. Cytoplasmic RNA waspreparedaspreviouslydescribed(4, 36).
Preparation of SV40 DNA. Plaque-purified SV40 viruswasusedtoinfect BSC-1monkey cellsat alow multiplicity of infection. Total viral DNA was
ex-tracted from infected cells bytheprocedure of Hirt (15). Supercoiled SV40 DNA (form I) was purified
fromthe HirtsupernatantbyCsCl-ethidium bromide gradient centrifugation. FormI SV40DNAwas
sub-sequently resolvedona 5 to23% sucrosegradientin
J. VIROL. lx SSC (0.15 M NaCl plus 0.015 M sodium citrate). Purifiedsupercoiled SV40 DNA was dialyzed against abuffer containing10mMTris-hydrochloride, pH 7.9, 5mMNaCl, and 0.5 mM EDTA at 4°C, followed by storageat-20°C.
Restriction enzymes and gel electrophoresis. Restriction endonucleases BglI, TaqI, and BamHI were purchased from Bethesda Research Laborato-ries. Form I SV40 DNA was digested by the endonu-cleases in a reaction mixture (total volume, 50 dl)
containing1,ugofSV40DNA, 20 mM Tris-hydrochlo-ride, pH 7.9,10mMMgCl2, 6 mM KCl, 2 mM
mercap-toethanol, and eitherBglIand TaqI or BamHI and
TaqI. The reaction was stopped by adding EDTA and sodium dodecyl sulfate to final concentrations of 20 mM and 0.2%, respectively, and the mixture was heated at 68°C for 10 min. The resulting DNA frag-ments wereresolved on cylindrical gels (0.6 by 13 cm) of 1.4% agarose,asdescribed by Sharp et al. (29). The gelswerestained with a 0.5-ug/ml ethidium bromide
solution, and the DNA bands were visualized under
UVlight. For elution of DNA fragments from agarose
gels, gel slices containing the DNA fragments were
dissolved ina small volume of 5 MNaCl04-50 mM Tris-hydrochloride, pH 7.4, at 60°C. The solution was passed through a small column of hydroxyapatite equilibrated with 0.14 M phosphate buffer. The
hy-droxyapatitecolumn was subsequently washed
exten-sively with 5 M NaCl04-50 mMTris-hydrochloride, pH 7.4, to remove the agarose. After an additional equilibration with0.14Mphosphate buffer, the DNA wasrecovered by elution with 0.4 M phosphate buffer. The eluatescontaining the DNA fragments were
di-alyzedagainst5mMTris-hydrochloride, pH 7.9-2 mM
NaClat4°C.
In vitro radioactive labeling of SV40 DNA. Radioactive labeling of SV40 DNA and its fragments wasperformed by introducing32P-labelednucleotides by the nick-translation technique as described by Maniatisetal. (19). The specific activity of the radio-active DNAwasusually between3 x107 and108cpm/ Mg.
Separation and purification of SV40 DNA
strands. The strand separation of nick-translated
SV40 fragments was performed by the method of
Sambrooketal. (28)aspreviously described (4). DNA-RNAhybridization. Hybridization experi-mentsbetweencytoplasmicRNAand theearly strand of32P-labeledSV40 DNAfragmentswereperformed aspreviously described (4).
RESULTS
T-antigen expressionand DNAsynthesis
in ts SV3T3 cells.Asreported before (4), 1or
2days after ts SV3T3 cells reach a state ofGl
arrest at39°C,T-antigenbecomesundetectable
in their nuclei and virus-specific RNA is not
transcribed. The cells remain viable for long
times. To study the time course ofT-antigen
expressionafterresumptionofgrowth,ts SV3T3
cells grown at
390C
until confluent wereincu-bated in low-serum medium (0.5 to 1%) for 3
additionaldays.T-antigen productionand DNA
on November 10, 2019 by guest
http://jvi.asm.org/
synthesis were followed after the cells were
transferred to the permissive temperature
(320C) in medium containing 20% calf serum
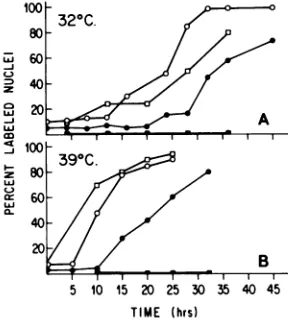
(Fig. 1). At zerotime, themajority ofthe cells
were T-antigen negative, and only a few cells weresynthesizingDNA.Flow microfluorometric analysis indicated that the cells had
accumu-lated in Gl (Fig. 2). After the medium change and shiftto320C,thecellsstartedtoproliferate
and entered DNAsynthesis with thedegree of synchrony characteristic for cells released from quiescence. Thereappearance ofT-antigen
pre-ceded DNAsynthesis, and the cells became T-antigenpositive about 10 h beforetheyentered
the S-phase. By 27 h after the shift to 320C,
most of the cells were T-antigen positive,
whereasonly 20% had entered DNAsynthesis.
In Fig. 1B the results ofsimilar experiments
performed at 390C are shown. The cells were
arrested before confluence in 0.5% serum and thenstimulated togrowbythe addition of
me-dium containing 20% serum without changing
thetemperature.Again, T-antigenreappearance
preceded DNA synthesis, the only difference
beingthat both functions occured faster thanat
the lowtemperature. The data indicate that
T-100 320C.
80
a
= 60
~40
20A0
w~~~~~~
80 9.
C-)
a:60- 40-20
B 5 10 '15 20 25 30 35 4 415
TIME (hrs)
FIG. 1. T-antigen expression and DNA synthesis intsH6-15 cellsstimulatedtoproliferateat32(A)or 390C(B).tsH6-15 cells which had been arrested in GIat390C(see text) received fresh medium contain-ing 20% calfserum in the presence or absence of cytosine arabinoside (20pg/ml).At the timeof serum anddrugaddition(zero time), half of the plates were transferredto320Candtheother halfwere kept at 390C.At the sametimehalf ofthecultures received
f3H]thymidine(1,LM;3,uCi/ml)to labelthe nuclei for DNA synthesis. T-antigen and DNA synthesis were testedby immunofluorescence and autoradiography respectively,atthe times indicated (seetext). Symbols: T-antigen-positive nuclei in the absence of (0) or presenceof(0) of cytosine arabinoside; 3H-labeled
nucleiin the absence (0)or presence (-) of cytosine arabinoside.
A B C
5?3 ~~~~~33
2 ~~~~~2-2
1020 30 40 50 6070 10 20 30 405060 70 10 20 30 405060 70
CHANNEL NUMBER
FIG. 2. Flow microfluorometric analysis of cellu-lar DNAdistribution of ts H6-15 cells at 39°C. (A) ts H6-15cells with growth arrested by low serum con-centration and saturation density. (B) ts H6-15 cells growing at 39°C. (C) ts H6-15 cells with growth rested by ileu- medium at 39°C. ts H6-15 cells ar-rested by ileu- medium at 32°C exhibited a similar pattern of cellular DNA distribution (data not shown).
antigen is synthesized while those cells are in
the Gl phase of the cellcycle. Consistent with
theseresultswasthefindingthatvirus-specific
RNAwasexpressed10 to 12h after shift-down.
This was measured by testing the ability of
RNA, pulse-labeledatvarioustimes after
shift-down,tohybridizewithSV40 DNA immobilized
onnitrocellulose filters(datanotshown).
Effect of inhibition of DNAsynthesis.To confirm the conclusion that DNA replication
was not required for T-antigen expression, the
experiment described above was performed in
the presence of cytosine arabinoside, a DNA
synthesis inhibitor (13). tsH6-15 cellswere
ar-rested in Gl at 39°C in low-serum mediumat
saturation density. Cells were then inducedto
proliferate by adding fresh, complete medium containing20
jig
ofcytosinearabinoside per ml.T-antigen-positive and DNA-synthesizing cells
were scored (Fig. 1). At both temperatures
cy-tosine arabinosideprevented thecellsfrom
en-tering the S-phase, whereas T-antigen reap-peared with almost the same kinetics asin the
controls. Similar results were obtained when hydroxyurea insteadof cytosine arabinoside was
usedtoinhibitDNAsynthesis.
Effect ofinhibitors of RNA and protein
synthesis on T-antigen reexpression. To
verify that new transcription and protein
syn-thesiswererequired for T-antigenreappearance inthe stimulatedcells,weperformedtheabove
experimentsinthepresence of an RNA
synthe-sis inhibitor (actinomycin D) or a protein
syn-thesis inhibitor (cycloheximide). As expected,
both actinomycin and cycloheximide inhibited T-antigen reexpression at both temperatures
(datanotshown).
Temperatureeffecton T-antigen
expres-sion.Theshift-down experimentsdescribed
pre-viously were donewith a simultaneous change
of the growth medium, with the fresh medium
30,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.505.251.448.58.159.2] [image:3.505.74.218.351.513.2]containing 10 or20%serum. We wanted to
de-termine the effects of shifting Gl-arrested ts
SV3T3 cells from 39 to 32°C without changing
the low-serum medium(Fig. 3). Both T-antigen
reappearanceand the initiation of DNA
synthe-siswere delayed and increasedataslowerrate
thanwas the case with thecultures which had
received complete fresh medium (Fig. 1). Again,
T-antigenreappearancepreceded DNA
synthe-sis, although the interval between thetwo
phe-nomena became very long. Nevertheless, we
have shown thatasimple change oftemperature
from 39 to 32°C releases these cells from Gl
arrest.
The results of these experiments are
some-what paradoxical. Although it is clear that a
simple change in temperature is sufficient to
makethesecellslose the normalphenotypeand,
albeitslowly,reenteraproliferativestate
accom-paniedby the expressionofviral functions,the
primary change mustoccur when the cells are
devoid of viral gene products. In addition, the
extremely long time interval between
appear-anceofT-antigen andtheentry intoDNA
syn-thesissuggests thatthepresenceofT-antigenis
notsufficienttoinduce thecellstoenterthe
S-phase. Some other temperature- and
time-de-pendent changes apparently must occur. The
hypothesis that T-antigen is not sufficient to
induce DNA synthesis inthese cells in the
ab-sence of other temperature-dependent
func-tion(s) issupported bythe results of the
experi-mentdescribed below.
Effect of tumor promoter PMA on
Gi-arrestedtsSV3T3 cells. PMA isa
represent-ative of various phorbol esters which enhance
100
LAJ
LUI
LUI
-j
LUJ
LU.
0-80
60
40
20
20 40 60 80 100 TIME (hrs)
FIG. 3. Temperature effect on T-antigen
expres-sion. tsH6-15 cells werearrested in Gl at39°Cby celldensityand lowserumconcentration(1%). After 3days at39°Cin low-serum medium the cellswere
transferred to 32°C without medium change (zero time). [3H]thymidine (1 pM; 3,uCi/ml) was added, and the nucleiwerescoredfor T-antigen positiveness
(0)or3H-labeling (0)atdifferenttimes(see text).
tumorigenicity ofmany chemicalcarcinogensin
vivo (30). In the presence ofPMA, normalcells
in vitro reversibly acquire some of the
charac-teristics of transformed cells (30, 39). Sincethe
data shown abovesuggestedto usthat T-antigen
was not necessarily coupled to cellular DNA
synthesis, we wanted to determine whether T-antigen-negative ts SV3T3 cells could be
in-duced toproduceT-antigenwithoutsubsequent
cellular DNA replication. ts SV3T3 cells were
arrested in Gl at 39°C as described previously
and held at low serumconcentration (1%) for3
days. At this point,PMA (in 50% ethanol
solu-tion) wasaddedtotheplatesatafinal
concen-tration of 0.1 ,ug/ml without medium change.
The additionof PMAinduced thereappearance
of T-antigen in the majority of the cells, al-though very few cellssynthesized DNA (Table
1). Thesynthesis of T-antigen occurredin most
of the cells by 13 h with the same kinetics as
those of serum-stimulated cells at 39°C, but little cellularDNAsynthesiswasdetectedeven
after 31 h (cf. Fig. 1). Atlater times, the cells returned to quiescence. Thus, the addition of PMA enabled the ts SV3T3 cells to overcome
theGO blockand tosynthesize T-antigen.
How-ever, the cells did not enter the S-phase,
sug-gestingthatT-antigenper se may not be able to
trigger the DNA replication machinery of the
cells.
Effect ofisoleucine deprivation. In
expo-nentially growingcellpopulations,cells progress
through the mitotic cycle in an asynchronous
manner. Different metabolic blocks, nutrient
deprivation, ortemperature-sensitivemutations
are ableto stop cellproliferation andcause the
TABLE 1. Effect oftumorpromoterPMA in Gl-arrestedtsH6-15cellsa
% ofT-anti- DNA
synthesis'
Time(h) gen-positive % of
DNA-syn-cells thesizing cells cpm/culture
0 5 1 1,400
6 ND 1 1,000
9 36 ND ND
13 75 5 1,800
24 82 6 3,500
27 ND 4 4,300
31 78 6 4,200
atsH6-15cellsweregrown in 10%serum at
390C
untilthey becameconfluent; thennewmedium with 1%serum wasadded. After3days (zero time) PMA (0.1ug/ml)wasaddedwithoutchangingthe medium.
bCells were pulse-labeled with [3H]thymidine (3
,tCi/ml) for 1 h; thefrequency of labeled nucleiwas
determinedbyautoradiography,andtheradioactivity incorporated into DNAwasmeasuredin trichloroace-ticacid-precipitablematerial.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.505.271.463.477.582.2]cells to accumulateatspecific pointsofthe cell cycle. Such blocks can be utilized to studythe
eventsoccuring duringcellcycle progression (3).
Low serum concentrationorisoleucine depri-vation have been used to viablyarrest normal cells in Gl (8, 18, 25). Transformed cells,
how-ever, generally either do not respond to these
blocks or die during DNAsynthesis (9, 18, 23,
25, 26, 35, 38). Since ts SV3T3 cells at 39°C
behaveasnormalcells,it was assumed thatthey would respond to isoleucine deprivation. We wished to compare the effects of such a block with thoseresulting fromdensityorserum
star-vation.
ts SV3T3 cells were G1 arrested at 39°C in
serum-depleted medium. After 3 days (zero time) the cells were washed and incubated in medium with or without isoleucine (ileu+ and
ileu- media, respectively) and supplemented
with extensively dialyzed calf serum at a final concentration of10%,andtheywerethen
trans-ferred to 320C. Figure 4 shows that isoleucine deprivation didnotpreventT-antigen
reappear-ance. DNA synthesis was inhibited, although
notcompletely,since a small percentage ofcels
synthesized DNA in ileu- medium. Similar
re-sults were obtained withcellskeptat390Cafter serumaddition.
Thisunexpected resultpromptedthe investi-gation of the cell cyclearrestpoint oftsSV3T3
100
i
-J
z 2
80 2
-LJ
40-LAJ
10 2
3000 5
TIME (hrs)
FIG. 4. Effect of isoleucine deprivationon
T-anti-genexpressionand DNA synthesisintsH6-15cells
arrested inGIat39°Cand thenstimulatedto prolif-erateat320C. Gi-arrestedtsH6-15 cellswerewashed, and new medium with or without isoleucine and supplemented with 10% dialyzed calf serum was added(zerotime).Atzerotime[3H]thymidine(1 zM;
3,Ci/ml)wasalso added.T-antigen-positive and
3H-labeled nuclei were tested by immunofluorescence
andautoradiography, respectively,atdifferent times.
Symbols: T-antigen-positive nuclei in the presence
(0)orabsence(E)ofisoleucine;3H-labelednuclei in the presence(0)orabsence(U)of isoleucine.
cells at 32 and390Cin ileu- medium. tsSV3T3
cells growing at 32 or390Cin complete medium were washed twice with ileu- medium and in-cubated in ileu- medium supplemented with 10% dialyzed calf serum. Under these conditions the cells remainedT-antigen positive, and DNA syn-thesis dropped to zero. However, measurements of the distribution of DNA content of the ts SV3T3 cellsinileu- medium showed that cells were distributedin the Gl and S-phases of the cell cycle (Fig. 2). In other words, isoleucine deprivation stopped the proliferation of the ts SV3T3cells but did not cause them to accumu-late inG1.
It isclear that ts SV3T3cellsrespond differ-ently than other cell types to isoleucine depri-vation. This apparently results, however, not from the fact that these cells are SV40
trans-o
x
c-LIi
Cm
-i
if,
0
C-)
x co
D
1O 20 30 40 50 60
TIME (hrs.)
10203040 5060 70
CHANNEL NUMBER
FIG. 5. DNA content and DNA synthesis of3T3 ME cells keptin ileu- medium. 3T3 ME cells were
growninDulbeccomodified Eaglemedium
contain-ing10%calfserum.Beforeconfluencethe cellswere
washed,and ileu- mediumcontaining10% dialyzed
calfserum wasadded. Flowmicrofluorometric anal-ysisofcellular DNA distributionwasperformnedas
described in the text. (A) 3T3 cells in complete
me-dium.(B) 3T3 ME cells in ileu- mediumfor38h. (C)
3T3 ME cells in ileu- mediumfor50h. (D)Rateof
DNAsynthesisin 3T3MEcellswhiletheywerekept
in ileu- medium. The rate of DNA synthesis was measured bypulse-labeling thecells with
[3H]thy-midine(0.5stM;3,uCi/ml)attheindicated timesfor Ih and thencountingthetrichloroacetic
acid-precip-itablematerial.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.505.258.448.274.517.2] [image:5.505.74.234.398.545.2]716
formed, but from an inherent property of the
3T3 mouse cell line. This was shown by an
experiment in which 3T3 ME cells growing at
37'C in mediumcontaining10% calf serumwere
washed and incubated in ileu- medium. DNA
synthesis, as measured by the rate of
incorpo-ration oftritiatedthymidineintotrichloroacetic
acid-precipitable material, dropped rapidly, and
flowcytofluorometric analysis indicated thatthe
cellswerearrestedinthe GlandS-phases (Fig.
5). A similar effect ofisoleucine deprivation on
3T3cellshas beenreportedrecentlyby Yen and Pardee (40). The above data confirm the fact
that only a GO-like block suppressed T-antigen expression, whereas isoleucine deprivation, al-thoughstoppingcellproliferation, didnotinhibit it.
Viraltranscription in ts SV3T3. Ina
pre-vious paper (4) we reported that virus-specific
RNA is detected in ts SV3T3 cells only while
theyaregrowingat 32 or39°C.Whenthesecells
are arrested in G1 at 39°C, they become
T-antigennegative,andviraltranscription ceases. Wealsohadindications thatthetranscription of theearly strand of theSV40genome was more
extensive incells growing at 32°C than in cells
growingat39°C.
We attempted to determine whether there
was a part of the early region of the SV40
genomewhichwaspreferentially transcribed at
the permissive temperature, 32°C. It has been
shownthat theearly region of the SV40genome
encodes two polypeptides, large T and small t,
responsible for viral replication and initiation and/or maintenance ofcellulartransformation, respectively (14, 31). A possible
temperature-dependent control of thetranscription ofsmall
tgenescouldhaveexplained the'behaviorofthe
tsSV3T3cells.
To determine whether the integrated viral
genome wastranscribeddifferentlyat39°C than
at 32'C,wecleaved SV40 formIDNAwith the
restriction enzymes BamHI, BglI, and TaqI (Fig. 6). Thefragmentswereresolved inagarose
gels,eluted,and nick-translated in thepresence
of 32P-labeled nucleoside triphosphates, as
de-scribed above.Fragments B and C (Fig. 6)
com-prise the early portion of the SV40 genome.
Fragment B encompasses the sequences where
all of the SV40 gene A mutants have been
mapped.Consequently,itstranscriptionsecures theintegrityofthelargeT.Fragment Ccontains
thesequence where the viable deletionmutant
d1890wasmapped,and itstranscriptionappears
tobe responsible for small tsynthesis (14, 31).
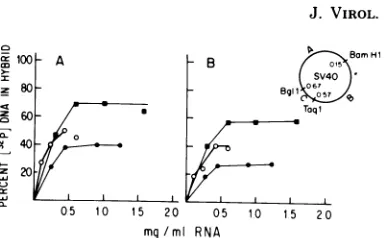
Wetested thehybridizabilityof theearlystrands
of fragments B and C with cytoplasmic RNA
extracted from ts H6-15 cells under different
growth conditions (Fig. 6). The extent of
tran-cioro0 A B BrH
a - B I{ 1
60 Taqi
m40
X 20
X05 10 15 20 05 1o 15 20 mg/ml RNA
FIG. 6. Hybridization of cytoplasmic RNA ex-tractedfrom tsH6-15 cellswith theearly strands of fragmentsB (0.15 to 0.57 map unit) and C (0.57 to 0.67 mapunit)of the early regionof SV40 genome (insert). Hybridization was carriedout at68°Cfor40 h as described in the text. The reaction mixture (0.2 ml) contained2 x 10-5to 5 x10-5,tgof[2P]DNAper ml (specificactivity,3x107to108cpm/p.g).(A) Fragment B withcytoplasmic RNA fromtsH6-15 cells growing at32°C (U),growing at 39°C (0), and arrested at 39°C and then stimulatedtogrow(0). (B) Fragment C with thesameRNApreparations.
scriptionof these two fragmentswasgreater at
32°C than at 39°C. ts H6-15 cells growing at
39°C contained viral RNA sequences derived
from bothfragmentsBandC. Neitherfragment
wastranscribedat39°Ctothesamedegreeasit
was at32°C. From theabove analysiswe were
unable to detect preferential transcription at
39°Cbetween thetwoselectedfragmentsof the
SV40 genome. It has been shown recently in
lytically infected cells that the mRNA's forlarge
T and small t bothencompass the wholeearly
region of the SV40 genome (5, 14). Inview of these new data, such preferential transcription
should notbeexpected.
Todetermine whethervirus-specific RNAwas
possibly degraded fasterat39°C, tsH6-15cells
weregrown at39°C until they became arrested
in Gl. Then these cellswerestimulated with a
highserumconcentration to enter DNA
synthe-sis. When all cellswere T-antigenpositive,
cy-toplasmic RNA was extracted and tested for
hybridizationwith theearlystrandsoffragments
B and C (Fig. 6). The amountofvirus-specific
RNA and the extent ofhybridization increased
compared with cells exponentially growing at
39°C, although they were still lower than the
values detected at32°C. It seems likely that in
ts H6-15 thenonpermissive temperature lowers
the rate of the transcription of the integrated
SV40 as well as the stability of the viral
tran-scripts. Whether this differencemay be related to the temperature-sensitive expression of the
transformedphenotypeinthetsSV3T3 cells is
unknownatthis time.
DISCUSSION
The SV40 gene A product (T-antigen) is a
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.505.270.462.57.176.2]DNAbinding protein which is expressed in cells lytically infectedortransformed withSV40and the synthesis of which is selfregulated (1, 12,
34). T-antigen appears to be required for the
initiation of viral DNA replication and the
es-tablishment of cell transformation (10, 20, 32,
33). A functionalT-antigen is alsoprobably
re-quiredtomaintainsome,ifnotall, of the
prop-erties oftransformed cells (7, 11, 20, 24, 32). It
hasbeensuggestedthatT-antigenpromotes
un-regulated cellgrowth by actingas aninitiator of
DNAreplication (10, 21, 22).
Abiologicalsystemin which theexpression of
T-antigencanbe controlled should be usefulto
study the role of this protein in viral transfor-mation. Ina previous paper weshowed that a
cellular controlwasexertedonT-antigen
expres-sion in cells whichexpressthetransformed
phe-notype inatemperature-dependent manner(ts
SV3T3) (4). Gl (GO)-arrestedtsSV3T3cells do
notexpressT-antigen and donotcontain
virus-specific RNA, whereas growing cells expressed
T-antigenandvirus-specificRNA.
Inthispaper wehave extended the
character-ization of thisphenomenoninanattempt togain
informationonthe roleplayed byT-antigenin promoting cellular DNA synthesis and on the regulation of itsexpression in transformed cells. Our results show that when Gl-arrested ts
SV3T3 cellsareinducedtoproliferate, T-antigen
expressionfollowsafteravariableperiodoftime,
but alwaysprecedes the entry of the cells into
the S-phase. Accordingly,theuseof DNA
syn-thesis inhibitors does notpreclude the
appear-ance of T-antigen. T-antigen reappearance is
clearly not related to the exposure of the ts
SV3T3 cells tothe temperature permissive for the expression of the transformed phenotype, butonlytotheresumption ofcell cycle
progres-sion. Inaddition, the results obtained with ileu-medium confirm the hypothesis thatonlycells viably arrested in Gl fail toexpressT-antigen, whereas othertypesofgrowtharrestdonotlead
tosucharesult.
Itis,unfortunately, difficulttodefmethe
pre-cise point in Gl at which T-antigen becomes reexpressedorwhere viraltranscription restarts.
This is due not onlyto the fact that acertain
amountofT-antigenmustaccumulatebefore it
canbe detected, but, more importantly, to the
abnormally longGllagexhibitedbyGl-arrested
cells after stimulation (2).Therefore,wedonot
know at which point in G1 the expression of
viral functions restart, nor can we relate this
activitytoanyspecificGlevent.
The kinetics of resumption of growth and
entry into DNA synthesis exhibited by the ts
SV3T3 cells are not different from those
ex-hibited by normal3T3cellsupon readdition of
serumafterserumstarvation. Thus,the appear-ance ofT-antigen does not seem toaccelerate the recruitment of cells intothe S-phase. In this
respect, it isinterestingto compare the behavior
ofour ts SV3T3 cells upongrowth stimulation
or shift to the temperature permissive for the transforned phenotype with the behavior of
cellstransformed byAmutantsofSV40 under
similar conditions (6, 11, 23). Inbothts SV3T3 cells and tsA SV40 transformants, the
trans-formed phenotype is temperature sensitive. However, thets SV3T3 cellsowe their charac-teristicsto a cellularmutation,whereas the be-havior of SV40-transformed cells is presumably determined byaviral mutationaffecting
T-an-tigen function. Atthe nonpermissive
tempera-ture, exponentially growing ts SV3T3 cells
ex-press anapparentlyfunctional T-antigen. When
these cells become confluentor aredepleted of
serum, this expression ceasesdue to acellular
control atthe level oftranscription. Under the
same conditions, tsA SV40 transformants
con-tinuously produce T-antigen which is
presum-ablyinactive because of the temperature-sensi-tive mutation. It is unknown whetherT-antigen isexpressed when these cells reach Glarrest at
39°C, and,infact,it hasonlybeen shown inone
casethat these cellscanreachaGlarrest atthe nonpermissivetemperature(23).
Theabilitytoreacha stateofviable Glarrest
under conditions ofserum starvation, density,
etc.,isanimportantcriteriondistinguishing
nor-malcellsfromSV40-transformedcells (9, 18, 23, 25, 26, 35,38).The tsSV3T3 cellsclearlyhave thesepropertiesat39°C.Inashift-down
exper-iment with tsA SV40transformants, T-antigen should becomefunctionallyactive and then
di-rectlyorindirectlyexertitseffectonthe
induc-tionof cellular DNAsynthesis. Inthecaseofts
SV3T3cells,however, newT-antigenshould be
synthesized before such an induction effect is
manifested. Indeed,as indicated above,
T-anti-gen expression precedes DNA synthesis. After
shift to the permissive temperature, ts SV3T3 cellsresumed DNAsynthesis afteralagperiod
of20 to 25h inserum-containing medium and
60 to80hunder conditions ofserumstarvation.
Thesevaluesarecomparabletothevaluesfound
byMartin and Stein (23) and Brockman (6) in studies withtsA SV40-transformed cells.
How-ever, Butel and Soule(11) observedavery short
lag period of4to 8 hbefore thesecellsentered DNA synthesis. The temperatureeffect in the
resumption ofDNAsynthesisintsA
transform-ants was serumindependent.Thisfindingraises
the question of whether the tsA transformed
cells used by these authors were indeed Gl arrestedat39°C. Inourcase, readdition of
nor-mal medium to ileu- medium-arrested cells,
on November 10, 2019 by guest
http://jvi.asm.org/
718 ZOUZIAS AND BASILICO
which doesnotbringabout aGlarrest,resulted
in a very rapid resumption of DNA synthesis
(datanotshown).
Thetemperatureshiftexperiments performed
with tsA SV40-transformed hamster or mouse
cellssuggest thatthe continuous expression of gene A is required for the maintenance of the transformed phenotype and that the gene A productmaydirectly induce cellularDNA
syn-thesis in SV40-transformed cells (11,21,22).Our results are not intotal agreementwith this
in-terpretation. Asmentioned before, the kinetics
ofentryintoS-phaseofourcellsafter
stimula-tionatboth32and390Caresimilartothose of
3T3 cells. Thus, T-antigen, which appears in
these cells inmid-Gl doesnotseem tobe capable
ofinitiating cellDNAsynthesis directly. Sucha
mechanism of action would belikelytoresult in
an accelerated recruitment of cells into the
S-phase. Inaddition, thetemperatureshift
exper-iments without addition ofserum also suggest
that T-antigen does not play a direct role in
initiation of DNA synthesis.
In most of the experiments described, the startingpoint(Gl arrest)wasacharacteristicof thenontransformedphenotype.WhentsSV3T3
cellsareinducedtoproliferate byincreasingthe
serumconcentration,T-antigen expressionis
co-ordinated with theexpression ofother cellular
geneswhich pull the cells out ofGO and push
them toward theS-phase.It isnotclearwhether,
after shift to 320C, ts SV3T3 cellsacquire the transformedphenotype beforeorafterT-antigen
expression. When thecells are shiftedto 32°C
without mediumchange,both theappearanceof T-antigen and the resumption of growth are
quite delayed. T-antigen cannot be obviously
implicated in thisprocess,asitwas notpresent at the beginning. Furthermore, the extremely
long lag separatingT-antigenappearance from
DNAsynthesis in theseexperimentsstrengthens
theconclusion thatT-antigenisnotsufficientto
induce cellular DNA synthesiswithout the
ac-tivation ofsome other cellular function. If
T-antigen could induce cellular DNA synthesis
directly,thelag between its appearance and the
entry into S-phase would be expected to be
approximately 10 h rather than the 40 h
ob-served in this type ofexperiment. The results
with PMA also show that,under conditions of
serum starvation and temperature
nonpermis-sive for the expressionof the transformed
phe-notype, induction of T-antigen is notfollowed
byDNAsynthesis.Thus,webelievethatSV40 T-antigenintransformed cellsismorelikely to
act as an inducer of cell proliferation, rather
than as adirect initiator of DNAsynthesis.
Further work in this area will include the
search for virus-specific proteinsand their char-acterization in ts SV3T3 cells under different growth conditions. Preliminary experiments
with[35S]methionine-labeledcells indicatedthat
polypeptides corresponding to small, medium, and large T-antigens are immunoprecipitated
fromextractsoftsSV3T3cells growingat 39 or
32°C. Thus, these cells express all known
T-antigensatthenonpermissivetemperaturewhen in exponential growth. It will be interesting to
estimate the relativeamountsof these viral
pro-teinsatthetwotemperatures. Itis possiblethat
the cellular control of the transformed
pheno-typeoftsSV3T3 cells is manifested by regula-ting thesynthesisofthesepolypeptides.
ACKNOWLEDGMENTS
Thisinvestigationwassupported by Public Health Service grants CA 11893 and CA 16239 from the National Cancer Institute.
We thank PatSantanellofor herexcellenttechnical
assist-anceand EricEilenfor thehelpful discussions.
LITERATURE CITED
1. Alwine, J. C., S. I. Reed, and G. R. Stark. 1977. Characterizationof theautoregulation of simian virus
40geneA. J.Virol. 24:22-27.
2. Baserga, R. 1976.Multiplication and division in mam-maliancells.Dekker,NewYork.
3. Basilico, C. 1977. Temperature sensitive mutations in animal cells.Adv.Cancer Res. 24:223-266.
4. Basilico,C.,and D.Zouzias.1976.Regulationof viral transcription and tumor antigen expression in cells transformedby simian virus40.Proc.Natl. Acad.Sci. U.S.A. 73:1931-1935.
5. Berk, A. J., and P. A.Sharp.1978.Spliced early mRNAs of simian virus40. Proc. Natl. Acad. Sci. U.S.A.75:
1274-1278.
6. Brockman,W. W. 1978.Transformation ofBALB/c-3T3 cellsby tsAmutantsof simian virus 40:temperature sensitivity of the transformedphenotype and retrans-formationbywild-typevirus. J.Virol. 25:860-870.
7. Brugge, J.S.,and J. S. Butel.1975.Role of simian virus
40geneA function in maintenance of transformation. J. Virol. 15:619-635.
8. Burk,R. R. 1970.One stepgrowth cycleofBHK21/13 hamster fibroblasts.Exp.Cell. Res. 63:309-316. 9. Burstin,S.J.,and C.Basilico.1975.Transformationby
polyomavirus altersexpressionofacellmutation af-fectingcycletraverse.Proc.Natl. Acad.Sci. U.S.A. 72: 2540-2544.
10.Butel, J. S.,J. S.Brugge, andC.A. Noonan. 1974.
Transformation ofprimateandrodentcellsby temper-ature-sensitivemutants ofSV40. ColdSpringHarbor Symp. Quant.Biol.39:25-36.
11.Butel, J. S., and H. R. Soule. 1978. Role of the simian virus 40gene A product inregulation of DNA synthesis in transformed cells. J. Virol.26:584-594.
12. Carroll,R.B., L. Hager, and R.Dulbecco.1974.Simian virus40T-antigen binds toDNA. Proc. Natl. Acad. Sci.
U.S.A.71:3754-3757.
13. Cozzarelli, N. R. 1977. The mechanism of action of
inhibitors of DNAsynthesis. Annu. Rev. Biochem. 46:
641-668.
14. Crawford, L. V., C. N. Cole, A. E. Smith, E. Paucha,
P.Tegtmeyer, K. Rundell, and P. Berg. 1978. Or-ganizationandexpression of early genes of simian virus
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
40.Proc.Natl. Acad. Sci.U.S.A. 75:117-121. 15.Hirt,B. 1967. Selective extraction ofpolyomavirusfrom
infectedmousecellcultures. J. Mol. Biol. 26:365-369. 16.Kimura, G., and R.Dulbecco. 1973. A temperature-sensitive mutant of simian virus40affecting transform-ingability. Virology 52:529-534.
17. Kirshan,A. 1975.Rapidflowcytofluorometric analysis ofmammaliancellcycle by propidiumiodidestaining. J.Cell Biol. 66:188-193.
18. Ley, K.D.,and R. A.Tobey.1970.Regulationof DNA synthesis in Chinese hamster cells. II. Induction of DNAsynthesis and cell divisionbyisoleucine and glu-tamineinGI arrested cells in suspension culture. J. Cell Biol. 47:453-459.
19. Maniatis,T.,A.Jeffrey, and D.G. Kleid. 1975. Nu-cleotide sequence of the rightwardoperator ofphage A. Proc.Natl. Acad.Sci. U.S.A. 72:1184-1189. 20. Martin,R.G., andJ. Y.Chou. 1975. Simianvirus40
functionsrequired for theestablishment and mainte-nance ofmalignanttransformation. J. Virol. 15:599-612.
21.Martin,R.G.,J. Y.Chou,J.Avila,and R. Saral. 1974. The semiautonomousreplicon:amolecularmodelfor theoncogenicityofSV40. ColdSpring Harbor Symp. Quant. Biol. 39:17-24.
22. Martin, R. G., and A. Oppenheim. 1977. Initiation points for DNA replication in non-transformed and simian 40-transformed Chinese hamsterlungcells. Cell 11:859-869.
23. Martin,R.G.,and S. Stein. 1976.Restingstatein normal and simian virus 40-transformed Chinese hamsterlung cells.Proc.Natl. Acad.Sci.U.S.A. 73:1655-1659. 24. Osborn, M.,and K. Weber. 1975. Simian virus40gene
A functionand maintenance of transformation. J. Virol. 15:636-644.
25. Pardee, A. B. 1974. A restriction point for controlof normal animal cell proliferation.Proc. Natl. Acad.Sci. U.S.A.71:1286-1290.
26. Pardee,A.B., andJ. J.Lynne.1975.Selectivekillingof transformed babyhamsterkidney (BHK) cells. Proc. Natl. Acad.Sci. U.S.A.72:4994-4998.
27.Renger, H.C., and C. Basilico.1972.Mutationcausing temperaturesensitiveexpressionofcelltransformation byatumorvirus. Proc.Natl.Acad.Sci.U.S.A. 69:109-114.
28. Sambrook,J.,P. A.Sharp, andW.Keller.1972. Tran-scription ofsimianvirus 40. I.Separation of the strands of SV40 DNA and hybridization of the separated
strands to RNA extracted fromlytically infected and transformed cells.J.Mol.Biol. 70:57-71.
29.Sharp, P. A., B. Sugden, and J. Sambrook. 1973. Detectionof two restriction endonuclease activities in Haemophilus parainfluenzaeusing analytical agarose-ethidium bromide electrophoresis. Biochemistry 12: 3055-3063.
30.Slage,T. J., A.Sivak,and R. K.Boutwell(ed.). 1978. Carcinogenesis, vol. 2. Mechanism of tumor promotion and carcinogenesis. RavenPress,New York. 31.Sleigh,M.J., W. C. Topp, R. Hanich, and J. F.
Sam-brook. 1978.Mutants ofSV40 with an altered small-t protein are reduced in their ability to transform cells. Cell14:7948.
32. Tegtmeyer,P. 1975.Function of simian virus 40 gene A in transforming infection. J. Virol. 15:613-618. 33. Tegtmeyer, P., and K. Rundell. 1977. The role of the
papovirusgene A in oncogenic transformation, p. 957-970. InH. H. Hiatt, J. D. Watson, and J. A. Winsten (ed.), Origins of human cancer. Cold Spring Harbor Laboratory,ColdSpring Harbor, N.Y.
34. Tjian,R.1978.Thebindingsite onSV40 DNA for a T-antigen related protein.Cell13:165-179.
35. Toniolo, D., andC. Basilico. 1975.SV40-transformed cellswith temperaturedependent serum requirements. Cell4:255-262.
36.Toniolo, D., H. K. Meiss, and C. Basilico. 1973. A temperature-sensitive mutation affecting 28S ribosomal RNAproduction inmammaliancells. Proc. Natl. Acad. Sci.U.S.A. 70:1273-1277.
37. Tooze, T. (ed.). 1973. The molecularbiologyof tumor viruses.Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
38.Vogel A., andR.Pollack.1975. Isolation and charac-terizationof revertant cell lines. VII. DNAsynthesis and mitotic rate of serum sensitive revertants in non-permissivegrowth conditions.J.Cell.Physiol. 85:151-162.
39. Weinstein, I. B., M. Wingler, and C. Pietropaolo. 1977. The action of tumorpromotingagents in cell culture,p. 751-772. InH. H.Hiatt,J. D.Watson,and J. A. Winsten (ed.), Origins of human cancer. Cold SpringHarborLaboratory, ColdSpringHarbor,N.Y. 40. Yen,A.,andA.Pardee.1978.Arrestedstatesproduced by isoleucinedeprivationandtheirrelationshiptothe low serumproduced arrestedstatein Swiss3T3cells. Exp.CellRes.114:389-395.
on November 10, 2019 by guest
http://jvi.asm.org/