JOURNAL OFVIROLOGY,Aug. 1973, p. 310-319 Copyright © 1973 American Society for Microbiology
Vol.12,No. 2 PrintedinU.S.A.
Host-Mediated Repair
of Discontinuities
in
DNA
From T4 Bacteriophage
K. CARLSON,I Z. K. LORKIEWICZ, AND A. W. KOZINSKI
Department of Human Genetics, Universityof Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104
Received forpublication21February 1973
Discontinuities of T4 DNA whicharecaused byexcisionofUV-damagedareas,
by decay of 32p atoms, or which are present in DNA from
rII-lig.m-
phage produced inahostnonpermissive for ambermutantsareall repaired by bacterialenzymes after infection in the presence of chloramphenicol. Escherichia coli DNA polymerase I participates in the host-mediated repair, but an approxi-mately 20-fold variation in the levels of host polynucleotide ligase does
not affect either the kinetics ortheextent of repairobserved. Upon removal of chloramphenicol, host-repaired DNA from UV-irradiated phage undergoes a secondarycycle of breakage, which ultimately results in solubilization ofmostof thephage DNA. If the cellsareco-infected withnonirradiated helper phage, the
secondary breaks arerepaired and the continuity of the polynucleotide chain is restored. The close coincidence in theextentofprimary and secondarybreakage
suggests that phage-coded enzymes recognize and excise areas improperly
repaired by the host. In contrast to host-mediated repair, repair mediated by rescuingphage probably restored functionality tothe damaged DNA.
Although bacteriophage T4, after infectionof
bacterial cells, codes for a number ofenzymes
involved in its DNA metabolism (review in
reference 19), it is quite
possible
that some ofthe enzymes involved in this process may be injected into the cell together with the phage DNA upon infection or be of bacterial origin. This possibility was
explored by
Kozinski and Lorkiewicz in 1967 in astudy
ofintracellular
breakage
andrepair ofUV-damaged
DNA(15). ("Break" and"breakage"
refer to single-stranded discontinuities in DNA with no im-plications tothe natureandsize ofthis discon-tinuity."Repair"
refers to the restoration ofintegrity of broken
DNA.)
The authors foundthat when the synthesis of new proteins was
inhibited by the addition of chloramphenicol (CM) shortly before infection, DNA from UV-irradiated phage acquiredsingle-standed inter-ruptions shortly after infection. (DNA of the radiation-sensitive mutant V,
[11]
didnotac-quire such breaks.) The broken strands were subsequently repairedto full integrity, still in the presence of CM. The results were
inter-preted to indicate that bacterial enzymes
per-formed both breakageandrepair.
IPresent address: McArdle Laboratory for Cancer
Re-search University of Wisconsin Medical Center Madison,
Wis.53706.
This work
provided
the first indication that DNAfrom a virulentphage
can berepairedby
host enzymes. More recentexperimentsin this laboratory have shown that a phage enzyme
injected with the DNA is
responsible
for the intracellularbreakage
ofUV-damaged
DNA(R.
Shames,
Z. K.Lorkiewicz,
and A. W.Kozinski,
J. Virol., in press). This enzyme is the
product
of the v gene in
T4,
is found in the mature phage, and hasendonucleolytic
activity specific forUV-damages
whenassayed
in vitro(C.
J.Castillo,
Ph.D.thesis,
University
ofPennsyl-vania, in preparation).
Theobjective ofthe present communication
is to characterize the repair event
following
breakage. Evidence will be
presented
showing
thatseveral different types of
damage
inphage
DNA can berepaired,
and that the enzymesresponsiblefortherestoration of
integrity
areofbacterial origin. Escherichia coliDNA
polymer-ase Iparticipates inthisfunction,
buta20-fold variation in the levels ofbacterial polynucleo-tide ligase does not affect either kinetics or extent ofrepair. The host-repaired DNA fromUV-damaged phageisgeneticallyincompetent, and only a second round of phage-directed excision repair restores functionality to the
damagedDNA. 310
on November 10, 2019 by guest
http://jvi.asm.org/
REPAIROF PHAGE T4 DNA
MATERIALS AND METHODS
Strains. The bacterial strains E. coli W3110
su-thy- and its derivatives P3478polA- anda
spontane-ousrevertantof thismutant(6)wereobtained from J.
Caims. E. coli strains N953 lac- galE-
galKam-trpam-araam- T6Rsu- and itsderivatives N1323lop8
andN1325 lop8 lig2 correspondtothe strainsN1071,
N1072 lop8, and N1252 lop8 lig2 of Gellert and
Bullock (9) which have been cured of their lambda
prophage. These strains were obtained through H.
Krisch. E. coli B23 isthenormal host for T4used in
thislaboratory.
ThebacteriophagesusedwereT4BO,r, anosmotic
shock-resistant mutant, and T4D r59 H39X, an
rII-ligam- doublemutant(2).
Phage preparation. Methods forpreparing
radio-active phage have been described (15, 16). (i) UV
irradiation of bacteriophage was performed with a
germicidal lamp (GE) without a filter, with adose
corresponding to 6 to 9 lethal hits. The phage was
suspendedin 0.15 MNaCl-0.01MTris-hydrochloride,
pH7.4, duringirradiation.
(ii) To obtain phages with DNA nicks due to 32p
decay, 32P-labeledphageofaspecific activityof 10to
20 mCi of phosphorus per mg were stored at a
concentration of 1010 particles per ml in 3 x D
medium (8)at+4C until about6to8single-stranded
scissions due to 32P decay had accumulated, as
determined by alkaline sucrose gradient
sedimenta-tion.
(iii) rII-ligam- B denotes phage which were
pre-pared byasingle growth cycleinE.coli B23 (2). This
strain isnonpermissive for the amber ligase mutation,
butpartial suppression of the amber phenotype by the
rII mutation results in productive infection. About
10%oftheresultingphagesareviable, and the phages
contain DNA with numerous single-stranded inter-ruptions (2).
Determination ofintracellularstrand integrity
ofphageDNA.Allexperimentswereperformedat37
C in TCG medium 16)at acell concentration of 3 x
108/ml.The medium wassupplementedwith
thymi-dine (TdR) at 5 ,ug/ml in experiments with
thy-strains and L-tryptophan at20/g/ml inexperiments
with trp- strains. CMwasaddedtothe culturesata
final concentration of 150jg/mlimmediately before
the cells were infected with 32P-labeled phage at a
multiplicity of 3 to 5 particles per cell. At desired
intervals after infection the samples were lysed
(together with 3H-labeled matureT4phage as
refer-ence) with lysozyme, Triton X-100, andNaOH to a
final concentration of 0.2 M (20). The lysates were
sedimented through alkaline sucrosegradients (5 to
20% in1M NaCl-0.2 MNaOH; 26,700rpmfor 3h ina
SpincoSW50rotor).Thedistance sedimentedbythe
intracellular DNA molecules relativetothe distance sedimentedbythe marker DNA(D2DJ)(4)serves as a measureof strandintegrity.
Enzymological techniques. DNA extracted from
phagewithphenolwasdialyzed against1mMEDTA,
pH 7.4, andincubated with T4polynucleotide ligase
bythe method of Weiss and Richardson(25). Incuba-tion with T4 DNApolymerase wasbythe method of
Aposhian (1). After conclusion of the enzymatic
reactions the mixtures were againdialyzed against1
mM EDTA and analyzed onalkaline sucrose
gradi-ents.
RESULTS
Intraceliular repair of different types of
single-stranded discontinuities in phage DNA. It was demonstrated previously that,
shortly after infection in the presence of CM, UV-damaged T4 DNA acquires a number of
single-stranded nicks (15) correspondingto the
number of lethal hits (17). These nicks are
repaired upon prolonged incubation (15). To test the generality of such repair, we prepared
phages with different types ofinterruptions in
their DNA.
(i) Breaks in DNA due to 32P-Decay.
Lit-win etal.(17) showed that there is aone-to-one
relationship between the numberofdecayed32P atoms and the number of resulting single-stranded interruptions, which indicates that there is a chain break adjacent to the decayed
32p.
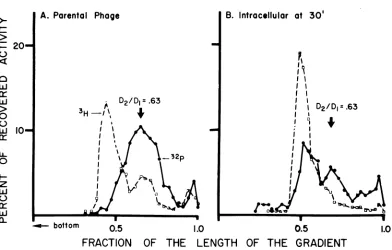
Decayed phage were prepared by allowing 32P-labeled phage (specific activity 20 mCi of phosphorus per mg to age in 3 x D medium. Such phage acquired a number of single-stranded breaks (Fig. 1A). Bacteria were
in-fected (MOI 1) with the decayed phage in the presence ofCM. Thirtyminuteslaterthe
integ-rity of thephage DNAwasanalyzed (Fig. 1B).
Most ofthe radioactivity now coincided fairly well with the integral reference. This indicates that discontinuities due to 32p decay can be
repaired.
(ii) Discontinuities in DNA from rll-ligam- B
phage.
In a bacterial hostnon-permissivefor ambermutants, partial suppres-sion ofthe amberligasemutationby rII muta-tionsallows the productionof maturephages(2, 7,13).Such phages, however, contain
discontin-uousDNA (2). Thenatureofthese
discontinui-ties is not known. Toobtain informationabout this, wetested therepair ofsuchdiscontinuous DNA invitroby subjecting the DNA to
repair-ing enzymes. DNAextractedfromrII-ligam-.B phage (Fig. 2A, insert) was incubated withT4
polynucleotide ligaseor a combination ofboth ligase andT4DNApolymerase. It was expected that asimple break not involving loss of nucleo-tides should be repaired by ligase alone, whereas a combination of the two enzymes wouldberequired if the break consisted of gaps in thepolynucleotide chain. Sampleswerethen
subjected to alkalinesucrose gradient analysis.
Ligase alone (Fig. 2A) could not effect any repair of the DNA. A combination ofthe two enzymes, however, achieved significant extent
311
VOL. 12, 1973
on November 10, 2019 by guest
http://jvi.asm.org/
CARLSON, LORKIEWICZ, ANDKOZINSKI
A. Parental Phage
^
D2/Di
=.633H-
\
+
B. Intracellular at 30'
78
I I
I I
I I I I
IDD
.1.0
FIG. 1. Repairof breaks inflicted by 32Pdecay in T4 DNA. 3P-labeledphage (specific activity 20 mCi of
phosphoruspermg)wasstoredin3x Dmedium allowing limiteddecaytooccur.The decayedphagewasused
toinfect E. coli B in thepresenceofCM (150
gg/ml).
Thirty minutesafter infectionpartof the infected cellswere lysed and analyzed on alkaline sucrose gradients. A, Analysis of the parental phage used for the
experiment; B, intracellularDNA from thesamephage. Note significantrepair. 3Hrepresentsreference DNA
from mature T4 phage.
ofrepair(Fig. 2B).Thisresultindicatesthatthe discontinuities in rII-ligam- .B DNA are gaps, not nicks, requiring both repair synthesis and
sealing.
The extent of in vivo repair of this same
phageDNAwastesteduponinfectionofE.coli
B (MOI 3) inthe presenceofCM.Intracellular DNA was analyzed on alkaline sucrose
gradi-ents (Fig. 2C andD). The intensityofrepairis
quitesimilartowhatwasobservedin vitrowith
acombination ofligaseandpolymerase (cf.Fig.
2B andC). Note that extensive repairoccurred
already at 5 min. There is no further repairif incubation is prolonged beyond 5 min. In both
cases about 35% of the DNAmass isintegral. A
sizablefraction of the DNA isunrepairedinvivo
aswellasin vitro. This fractionmaycorrespond
to a fragmented moiety of DNA, i.e., where double-stranded scissions have occurred in
ad-dition to the single-stranded gaps. Invariably,
when DNA fromrII-lig- phageis extracted with
phenolandsedimentedthrough neutralsucrose gradients, a sizable fraction sediments more
slowly then integral DNA. It is uncertain
whether fragments are found in the phage, or
whetherthegappedDNAisunusually
suscepti-ble to fragmentation during extraction. The
extent ofgapping varies considerably from ex-periment to experiment. In other experiments wherethestartingmaterialwaslessbroken, full
restoration ofintegritywasobservedupon
infec-tion in thepresence ofCM.
Role of bacterial enzymes in repair of
discontinuous phage DNA. Although the
oc-currenceofrepairafter infection in the absence of new protein synthesis suggested that pre-existing (bacterial) enzymes were responsible (15), the host origin of the repairenzymes was
not proven. The repair may be effected by a phage protein injectedfrom theinfecting
parti-cle or by a protein the synthesis of which is unusually resistant to CM. The notion that
repairingenzymes areindeed of bacterialorigin
is supported by the experiments described as
follows.
Role of hostpolynucleotideligaseand DNA
polymerase I in repair. Polynucleotide ligase
sealsasingle-strandednickinbihelicalDNA in
vitro(26), joininga 3'OH end toa5'P end.This
enzyme, therefore, is an obvious candidate for
performingthe last step inrepair.
E.coli mutantsdeficientinDNApolymerase
I (6) are fully viable, but display increased
sensitivity to UV (6).The increased sensitivity
H )0.
CR
0
w
lII
cr
w
0w
LL
0
z
w
0
w
a-0.5 1.0 0.5
FRACTION
OF THE
LENGTH OF THE
GRADIENT
312 J VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.498.65.457.65.315.2]* 100
* *
SSlw
X
(
LLJ
O~~~~~~~~~~~~~~~~~~~~~~L
o IIm*-3H** ~~~~~~~~~~~~~~~~~~~*~~* * ~
32p~ ~ ~ ~ *
0 0~~~~~~~~~~~~~~~~
I-~
bott0
LLII'0
LL
z~~~~~~~~~~~~~~
0 2. R oLL
**~~~C
F-
LLJI
'.- 1an invio hgswr banda ecie nMaeil n ehd n nlzdi nak ln urs
I
I.-~~~~~~~~~~D
o, C. n 0trcellularfh i
E.cl ntepeecf0M h netdclswr sntacpled ar n60' mi fe nfcinadnlzdi
W
0~~~~~~~~~~~~~~~~~~~~
copaison2
RpairofdscontinduCtreeasinDafromprI-leiextentBofareparofdthediscntinuonprusDAivehotivitroadi
fanviivo..h
Phages
we,resentainmed aspesctribveediMateiaiyybls:anMtodsendanalyze
3H; ansoklinesasucrS2pereprsens
compreden lckfrpi
maturnA n DNAD nrcluaphghage.nfctono
from aeo samE.olBnhe reenc o CM Te
ifete
cells* weesmlda100d6iftrifcinannlzdi
repesntrfernc DA ro maur
4(phage
313
on November 10, 2019 by guest
http://jvi.asm.org/
CARLSON, LORKIEWICZ, AND KOZINSKI
is probably due to diminished repair after excsion ofUV-damaged DNA (12). Both a 5' to 3' and a 3' to 5' exonuclease are firmly as-sociated with polI; the combination of
exonu-clease and polymerase activities inthe purified
enzyme will excise and repair UV-damaged
DNA in vitro (14). These findings all suggest a
role of polI in repair synthesis and not in replication per se. The involvementofpolIand
ligase in the repair of damagedphageDNA was tested in experiments analogous to those de-scribed above.
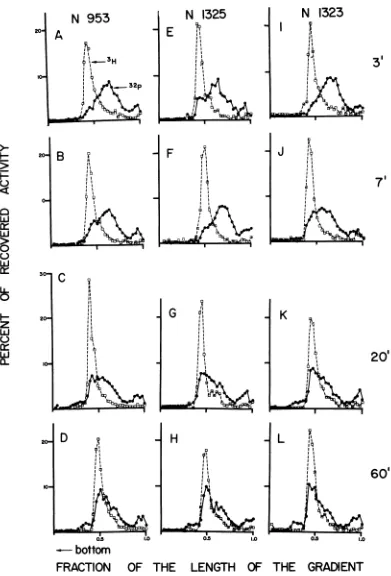
Ligasemutants. Two types ofligasemutants
inE. coli characterized by Gellert (9) andtheir
parental strain were scrutinized: N1323, which
overproduces ligase, N953 producing normal amounts, and N1325 which isdeficient in
poly-nucleotideligase.Theligaselevels differatleast 20-fold between the
overproducer
andthedefi-cient strain (9). Intracellular repair of DNA from UV-irradiated phage is illustrated inFig.
3. Neitherdeficiency (Fig. 3E, F,G, andH)nor overproduction (Fig. 31, J, K,
1)
affected eitherthe extent or the kinetics of repair of
UV-damagedDNA. The same was trueforrepairof
breaks due to 32P decay and for breaks in
rll-ligag
-.B DNA(data notshown).
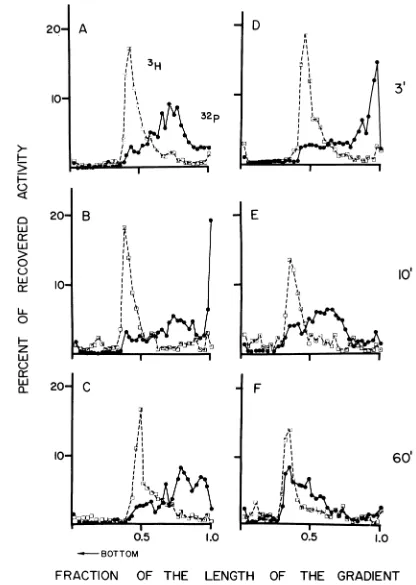
DNA polymerase I mutant. Similar
experi-ments wereperformedwithbacteria deficient in
polI (Fig. 4).
Deficiency
inpolI
impaired
the repair process. While similar extent of initialbreakage of
UV-damaged
DNA was found inboth the
poll-deficient
strainandina spontane-ous polI+ revertant thereof (Fig. 4A andD),
subsequent restoration of strand
integrity
was less efficient inpolA- cells(Fig.
4B,
C, E,
andF).Even 60 min ofincubationinthe presence of
CM didnot restore the
integrity
inpolA-
cells(cf. Fig. 4C andF).
The fact that
deficiency
inpolI
reduced the extent of observedrepair
furtherstrengthens
the argument that bacterial enzymesperform
this repairofphageDNA andstrongly
suggests thatpolIispartially
responsible
fortherepair.
While there are no doubts thatligase
is capable of repairingsingle-stranded
breaks inDNA (22,25), we feel that its
physiological
role is still uncertain. It isinteresting
toobserve thatligase deficiency does not
impair
therepair
of discontinuities inphage
DNA.This observation leaves room forspeculations
about the role of ligase inthecell.Host-repaired areas may be
recognized by
phage-codedrepairingenzymes. Kozinski and Lorkiewicz (15) showed
that,
despite
anearly
perfect restoration ofintegrity
ofUV-damaged
DNAupon removal ofCM,therewas noaccom-panying restoration of
phage
viability,
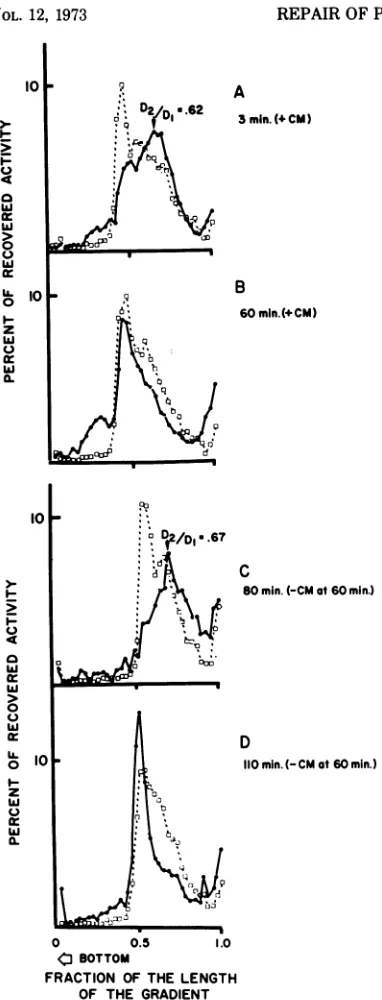
as ifrepair wasincompetent. UV excisions in E. coli DNA produce quite large gaps (3, 24). If the same is true for UVexcisions in T4 DNA (23), the filling of such gaps would lead toinsertion of cytosine instead of hydroxymethylcytosine (HMC) (since phage enzymes required to syn-thesize HMC and glucosylate it are not pro-duced in the presence of CM). Phage-coded excision (and repair) enzymes may be able to recognize such inadequately repaired areas of the DNA. If this were the case, expression of phage functions after removal of CM from the culture may result in a second round of excision and possibly repair. This was tested in the following experiment. A culture of B23 was divided into two parts. One part was infected with 32P-labeled, UV-irradiated T4 phage (MOI 0.1), and the second part was mixedly infected with the same UV-irradiated phage (MOI 0.1) and nonirradiated helper phage (MOI 3). CM was added to both parts at the moment of infection. Sixty minutes later the infected bac-teriaweresedimented and resuspended in fresh
medium without CM, and incubation was con-tinued.Figure5 showsalkaline sucrose gradient analysis of samples from the mixedly infected culture at different times after infection. Prompt nicking occurs at 3 min after infection (Fig. 5A), and repair in the polynucleotide chain has occurredby60 min (Fig.5B).Twenty minutes after the removal of CM, the DNA repaired by the host has undergone a second
cycle of nicking (compare Fig. 5C and A and note that the average DJ/Dl of secondarily
nicked DNA is virtually identical). The pat-ternsofdistributiondepictedinFig.5A, B, and
C are, for all practical purposes, identical also for bacteria which were singly infected with
UV-irradiated phage without any unirradiated
helper phage (notdocumentedhere). Figure5D
represents the fate of DNA fromUV-irradiated
phage 100 min after infection together with helper phage and shows that there is secondary repair. This is a typical pattern only for the
suspensions co-infected with rescuingcold, via-ble phage. In the case of infection with UV-irradiated phage alone, there is no secondary repair, but gradualsolubilization of DNA (up to 80% of the parental label, not documented
here). In a typical experiment the secondary nicking is first detectable at 10 min after removal of CM, reaching its maximum at ap-proximately 20 min after removal of CM. The close coincidence in the extents ofprimary and secondary nicking indicates that the phage enzymes may recognize excise and repair those areas that were previously subjected to host-mediatedrepair. This experiment also indicates
314 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
3'
cr_~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~i
>
0
w
0
CD~~
20 G ~'K
a.<
20'
<<X60
'
0.5 1.0 OS 1.0 0.5 1.0
bottorn
FRACTION
OF THE
LENGTH
OF
THE
GRADIENT
FIG. 3. RepairofWV-damagedDNA inligasemutantsofE.coli. E. coli N953(wild-typeligaselevels), Nl325 (deficientinligase),orN1323overproducing ligase) wasinfectedwithUV-irradiated phagein thepresenceof
CM.Atintervalssampleswerewithdrawn andanalyzedasdescribed inpreceding legends.Note thestriking
similarityin thepatternof occurringrepairas afunction oftime and theseeminglackofeffect of ligaselevelson
thekinetics oftherepair. 3H represents referenceDNA frommature T4phage.
315
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.498.56.447.42.618.2]P 3478
0.5
1.0
W
3110 Thy- Pol
Rb
E
F
'- BOTTOM
FRACTION
OF THE
LENGTH
OF
THE
GRADIENT
FIG. 4. Repair of UV-damagedDNA inaDNApolymerasemutantofE. coli. E. coli P3478polA-orapolA+
revertantofthis strainwereinfectedwith UV-irradiatedphageasdescribed inlegendtoFig. 3,andtheinfected
cellsweresampledatintervals. Both strains allow theinfliction ofnicks at 3min;however,adrasticdifference
is observedintheextentof subsequent repairinpol+andpol-mutants.A, B,andC, polA-;D, E,andF,polA+
revertant.
316
0
LUJ
cr-LLJ
0
LL
U-x
LLJ
0
z
LUi
LUI
0l.
31
l0'
60'
1.0
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.498.43.459.59.644.2]REPAIROF PHAGE T4 DNA
A
3 min.(+ CM)
B
60min.(+CM)
C
80min.(-CMat60min.)
D
110min.(-CMat 60min.)
0 0.5 1.0
3BOTTOM
[image:8.498.57.248.50.550.2]FRACTION OF THE LENGTH OF THE GRADIENT
FIG. 5. Primary and secondary breakdown and
repair of UV-damaged phageDNA. E. coli B23was
infectedinthepresenceofCM withanMOIof0.1of
32P-labeled, UV-inactivated (6lethalhits) T4phage
andanMOIof3.0of cold,nonirradiated T4phage. A,
Pattern obtained at 3 min after infection in the
presenceofCM; notethe peaking ofthe breakdown
product at DJ/D, = 0.62. This is the product of
primary nicking.B, Fateof injfectedDNA at60min
after infection; note the extensive repair (primary
repair). The reference 3H DNA (broken line) is
that host repair of UV damages in phage DNA doesnotaffect thecoding for excision enzyme(s)
recognizing host-repaired areas, but that
pro-duction ofenzyme(s) restoring strand integrity
areprevented.
DISCUSSION
Experiments documented here support our
earlier conclusion that there is host-mediated
repair ofdiscontinuous phage DNA under
con-ditions when the synthesis of phage-induced
proteins is prevented. Physiological (rHIligam-.B) and physical (32P decay)
discon-tinuitiesin phage DNA, aswell as
discontinui-ties inflicteduponthe DNAafterithasentered the cell (UV damage), are promptly repaired
upon infection of E. coli B in the presence of
CM. The three types of damage are quite
different: rH-ligam- B phage DNA has single-stranded gaps, 32p decay causes a single-stranded nickwhere the newly exposedend may
have an S terminus, which would be a rather
unyielding candidate for repair, and UV-irradiated phage have damaged DNA which is not discontinuous to begin with but becomes
broken after infection ofthe cells.
It is possible that the repair itself it quite
similarinall threecases. Ithas beenshown that
excision ofUV-irradiated DNA both invivo in
E. coli (3, 24) andinvitro using purified E. coli polymerase I (14) or a mixture of T4
endonu-cleaseI and T4"excision enzyme" (23) results inthereleaseof oligonucleotides in size exceed-ing a thymine-thymine dimer by 10 to 30
residues. The terminus at the nick arising due to 32P decay provides a veryunusual substrate
for repairing enzymes. It appears simpler to
postulate that in thiscaserepair is preceded by a preparatory, "editing" enzyme which recog-nizes that the DNA termini are somehow
un-usual, and excises adjacent nucleotidesin
"an-ticipation" of repair ofthe damage. Possibly,
this excision ofnucleotides may proceed by a
similar mechanism as the UVexcision, maybe utilizing the same enzyme(s). The
discontinu-ousDNAofrII-ligam-
.B
phagewasshown here to contain single-stranded gaps of undefinedpartially degraded, therefore after repairthe
distribu-tionof 32ptendstobemorehomogeneousthan thatof
thereference. C, Secondary nicking occurring at20
min after the removalofCM; note that theD2/D, of
thefragmented moiety = 0.67; this isverysimilarto
the product of primary nicking shown in A. D,
Secondary repair which occurs at 40min after the
removal ofCM; note therepair in the continuity of
polynucleotidechain andcomparewith therepairin
B. I0
1--4% 0
U
U.
0.
I--CL
1Lu
I--z
0.
317
VOL.12, 1973
on November 10, 2019 by guest
http://jvi.asm.org/
CARLSON, LORKIEWICZ,AND KOZINSKI
length, sincethe discontinuities are repairable
only byacombination of DNApolymerase and ligase. In all three cases, then, repair may involve fillingof a single-stranded gap.
Among several candidates for the role of
bacterialrepairing enzyme(s),DNApolymerase I and polynucleotide ligase attract special
at-tention, being well characterized enzymes
which havebeen shown to repair DNA
discon-tinuities in vitro (18, 22, 25). Our finding that
deficiencyinpolIinterferes withtherepair of T4
DNAis well in line with thediminishedrepair
activities in this strain reported from another
laboratory(12). Deficiency inpolI hasalso been
reported to interfere with replication and
re-combination of T4 DNA (21). In some repeti-tions of our polA experiments (notdocumented here), some residual repair was observed,
though this repair was significantly less than
whatwasfoundinthepol+revertant. The origin of the residual repair activity in the
polI-mutant is not known.recA- bacteria lack
abil-ity to repairdiscontinuities inphage DNA (15)
andarealso UVsensitive (5). Thissuggeststhat
therecA protein maybe involvedinthe residual
repair observed inpolA- cells. It isof interest
here to note that it is not possible to obtain a
doublemutantdeficientinbothrecA andpolA,
suggesting that the two enzymes perform
similar complementary functions (10).
More surprising was the
discovery
thatnei-ther deficiency nor overproduction of ligase affected therate or extentofrepair. The
ligase-deficient
mutant employed inthisstudy shows increased UV sensitivity, suggesting impaired repair activity, although there is no effect on DNAreplication or ontheviability
ofthe cells (9). It is possible that the small amounts ofligase found in the ligase-deficient mutant are
sufficientto"seal" thegaps in
phage
DNAoncethey
are filled. Anotherpossibility
is that the"sealing"
maybeperformedby
ahighly
special-ized, asyet undescribed ligatingenzyme, orby thegap-filling enzyme itself.
It wasshown
by
Kozinskiand Lorkiewicz(15)
that functionality can not be restored by the host-mediated repair of phage DNA.Experi-mentsdescribedinthis paperprovidean
expla-nation for this observation. Upon removal of
CM there is a
secondary
breakdown ofUV-irradiated, host-repaired phage DNA. The ex-tent of this secondary breakdown coincides
closelywith that of theprimary
breakdown,
and proceeds with an identicalpattern
both insingly and in
mixedly
(UV-damaged
andnon-UV-damaged
phage)
infected bacteria. Aftercompletion ofthe
secondary
round ofnicking,
secondary repair takes
place,
butonly
if thecells have been co-infected with non-UV-damaged "rescuer". In the absence of unir-radiated helper phage the irunir-radiated DNA is gradually solubilized. Thus, host-mediated re-pair not only did not improve the DNA, but
actually provedvery detrimental.
This suggests that secondary nicking and repair are caused bynewlysynthesized
phage-codedenzymes which atfirst areable to
recog-nize areas improperly repaired by the host
enzyme(s) and thenrestorethedamagedarea to its integrity. Theformer, but not the latter, may
be coded from UV-irradiated DNA. There are reasons to believe that viability is also restored,
since it was demonstrated that the parental
labelofsuchrescued phageisbeingtransferred to progeny phage as segments of parental
strandswhich underwentsemiconservative
rep-lication (E. Shahn, Ph.D. thesis, 1965,
Univer-sityofPennsylvania, Philadelphia).
Stringent
proof that thehost-repaired
areasare recognized, excised, and repaired
by
phageenzymescould beobtained
if,
forinstance, 3H-TdR was incorporated during the host-medi-ated repair in CM. Upon removal of CM oneshould then observe solubilization of this 3H
label coincidentally with the appearance of
secondary breaks. However, after infection in
thepresence ofCM mostofthe 3H-TdR
uptake
will go to bacterial DNA. This bacterial DNA
synthesis, and the phage-mediated breakdown oflabeled hostDNA afterremovalof
CM,
would overshadow any label entering andbeing
re-leased from therepaired
areas of the phage DNA.ACKNOWLEDGMENTS
We thankD. T.Denhadtforperforming theinvitrorepair ofrIl-liga,&- BphageDNA,and J. Cairns and H. M. Kirsch forgenerousgifts of strains. P. B. Kozinski and M. Mitchell provided excellent assistance with the experiments. This investigationwassupportedbyNationalScience Foundation grantGB-29637,andbyPublic Health Service grantCA-10055 andinpartbygrant 1-F02-CA-51268-01,the lattertwofrom the National Cancer Institute.
LITERATURE CITED
1. Aposhian,H. V. 1966. DNApolymerasefromT2-infected Escherichiacoli,p.277-283.InG. L. Cantoni and D. R. Davies (ed.), Procedures in nucleic acid research. Harper andRow,New York.
2. Berger, H., and A. W. Kozinski.1969.SuppressionofT4D ligase mutations by rIlA and rlIB mutations. Proc. Nat. Acad.Sci., U.S.A.64:897-904.
3. Boyce, R. P., andP.Howard-Flanders. 1964.Release of ultravioletlight-inducedthyminedimers fromDNAin Escherichia coli K-12. Proc. Nat.Acad. Sci. U.S.A. 51:293-300.
4. Burgi, E.,and A.D.Hershey.1963.Sedimentationrateas
a measure ofmolecular weightof DNA. Biophys.J. 3:309-321.
5.Clark, A.J., and A. D. Margulies. 1965.Isolation and
318 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
REPAIR OF PHAGET4DNA
characterization of recombination-deficientmutantsin Escherichia coli K12. Proc. Nat. Acad. Sci. U.S.A. 53:451-459.
6. DeLucia, P., and J. Cairns. 1969. Isolation ofan
Esch-erichia coli strain with a mutation affecting DNA
polymerase.Nature(London) 224:1164-1166. 7. Ebizuzaki, K., and L. Campbell. 1969. On the role of
ligase in genetic recombination inbacteriophage T4. Virology38:701-703.
8. Fraser, D., and E. A. Jerrel. 1953. The amino acid composition of T3 bacteriophage. J. Biol. Chem. 205:291-295.
9. Gellert, M., and M. L. Bullock. 1970. DNA ligase
mutants of Escherichia coli. Proc. Nat. Acad. Sci. U.S.A.67:1580-1587.
10. Gross, J. D., J. Grunstein, and E. M. Witkin. 1971. Inviability of recA- derivatives oftheDNApolymerase
mutant of DeLucia and Cairns. J. Mol. Biol. 58:631-634.
11. Harm, W. 1963. Mutants ofphage T4 with increased sensitivitytoultraviolet. Virology 19:66-71. 12. Kanner, L., and P. C. Hanawalt.1970.Repair deficiency
in abacterial mutant defective in DNApolymerase. Biochem. Biophys. Res. Commun. 39:149-155. 13. Karam, J. D. 1969. DNA replication by phage T4rII
mutantswithoutpolynucleotide ligase (gene 30). Bio-chem.Biophys. Res. Commun. 37:416-422.
14. Kelly, R. B., M. R. Atkinson, S. A. Huberman, andA. Kornberg.1969.Excisionofthyminedimersand other mismatchedsequencesbyDNApolymeraseof E. coli. Nature(London) 224:495-501.
15. Kozinski, A. W., and Z. K. Lorkiewicz. 1967. Early intracellular events in the replication of T4 phage DNA. IV. Host-mediatedsingle-stranded breaks and repairinultraviolet-damagedDNA.Proc. Nat.Acad. Sci. U.S.A.58:2109-2116.
16. Kozinski, A. W., and W. Szybalski. 1959. Dispersive
transfer of the parental DNA moleculetotheprogeny
ofphageOX174.Virology 9:260-274.
17. Litwin, S., E. Shahn, and A. W. Kozinski.1969. Interpre-tation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from
ran-dombreaks. J. Virol.4:24-30.
18. Masamune, Y., and C. C. Richardson. 1971. Strand displacement duringdeoxyribonucleic acid synthesisat
single strand breaks. J.Biol. Chem.246:2692-2761. 19. Mathews, C. K. 1971. Bacteriophage biochemistry.Van
Nostrand Reinhold Company, New York.
20. Miller, R.C., andA.W.Kozinski.1970.Early intracellu-lar events in the replication of bacteriophage T4 deoxyribonucleic acid. Further studiesonthe T4
pro-tein-deoxyribonucleic acid complex. J. Virol. 5:490-501.
21. Mosig,G.,D. W.Bowden, and S. Bock.1972.E.coli DNA polymeraseIand other hostfunctions participatein T4 DNA replication and recombination. Nature N.Biol. 240:12-16.
22. Olivera, B. M., and I. R. Lehman. 1967. Linkage of polynucleotides through phosphodiester bonds by an
enzyme from Escherichiacoli. Proc. Nat. Acad. Sci. 57:1426-1433.
23. Sekiguchi, M., S. Yasuda, S. Okubo, H. Nakayama, K. Shimada, and Y.Takagi. 1970. Mechanismsofrepair of DNA in bactriophage. I. Excision of pyrimidine dimers from ultraviolet DNA by an extract of T4-infected cells. J. Mol. Biol.47:231-242.
24. Setlow, P. B., and W. L. Carrier. 1964.The
disappear-anceofthymine dimersfromDNA:anerror-correcting mechanism. Proc. Nat.Acad. Sci. U.S.A.51:226-231. 25. Weiss, B., and C. C. Richardson. 1967. Enzymatic breakage and joiningofdeoxyribonucleic acid. I. Re-pair of single-stranded breaksin DNA byan enzyme
systemfromEscherichia coliinfected withT4 bacterio-phage. Proc.Nat.Acad. Sci.U.S.A.57:1021-1028.
VOL. 12, 1973