EXPRESSION OF CANCER STEM CELL MARKER
CD44 IN ORAL SQUAMOUS CELL CARCINOMA OF
TONGUE
Dissertation submitted to
THE TAMILNADU Dr.M.G.R. MEDICAL UNIVERSITY In
partial fulfilment for the Degree of
MASTER OF DENTAL SURGERY
BRANCH VI
ORAL PATHOLOGY AND MICROBIOLOGY
ACKNOWLEDGEMENT
I bow in gratitude to the Almighty for all His shower of blessings on me.
Words are just not expressive enough to extend my sincere gratitude to my parents who helped me throughout all my endeavours.
My heartfelt gratitude to my teacher Dr. K. Ranganathan, MDS., MS (Ohio), Ph.D., Professor and Head of Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital for his active guidance, help, encouragement and ample time spent throughout my course. Without his conscientious support, I would not have made headway in this project. Thank you so much sir.
I am extremely thankful and pay my sinceregratitude to Professor, Dr. Uma Devi K. Rao, Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital whose expertise, consistent guidance and moral support helped me to succeed in my study. I take this opportunity to acknowledge her for her care which helped me to become responsible doctor.
I am thoroughly grateful to Professor, Dr. T. Rooban, Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital for his immense interest in my topic of research, for providing me with material and links that I could not have possibly discovered on my own to complete this study. Thank you so much Sir.
I extend my personal gratitude to Readers Dr. N. Lavanya and Dr. C. Lavanya, and Senior lecturers Dr. Sudharsan, Dr. Kavitha and Dr. Joseph, Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital for giving the right advice at the right time and for being a source of motivation in completing the study.
I am grateful to our Geneticist and Lab Manager Mrs. Kavitha Wilson, for her advices to carry out my study successfully. Much of my experimental work would have not been completed without the assistance of our Lab Technician, Mr. Rajan, Department of Oral and Maxillofacial Pathology. Thank you so much sir.
At this juncture, I would like to thank Dr. Vidhyarani Shyamsundar, Preventive Oncology Section, Cancer Institute, Adayar for lending her hands in the technical aspects of the study.
CONTENTS
S. No Titles Page No
1. INTRODUCTION 1
2. AIM AND OBJECTIVES 3
3. MATERIALS AND METHODS 4
4. REVIEW OF LITERATURE 12
5. RESULTS 49
6. DISCUSSION 55
7. SUMMARY AND CONCLUSION 61
8. BIBLIOGRAPHY 64
9. ANNEXURES 75
I. Institutional Ethics Committee form II. IHC Training Form
III. Dissertation protocol
IV. Primary and secondary antibody
Introduction
1
Oral cancer is the eleventh most common cancer in the world1. Globally, the Age-Standardised Incidence Rate per 100,000 population for oral cancer in men and women is 5.5 and 2.5, respectively. India accounts for one-fifth of all oral cancer cases and one-fourth of all oral cancer deaths. Oral cancers include cancers of the mucosal lip, tongue, gum, floor of the mouth, palate and mouth1. In spite of significant advances in treatment, 5-year survival rate after diagnosis of OSCC remains low due to advanced stage at diagnosis and tumor recurrence2. The predominant cause of death in patients with cancer is loco-regional invasion, distant metastasis, and recurrence3. Even after therapies that aim to completely regress tumours, it is possible that Cancer Stem Cells (CSCs) are left behind allowing tumour recurrence. Therapies that are specifically directed against CSCs result in much more durable responses and eliminate metastatic tumours4.
Introduction
2
The most common CSC surface marker and a potential therapeutic target for many cancers is CD448,9. It is a transmembrane glycoprotein involved in cell-cell and cell-cell-matrix adhesion and in cell-cell signaling10. It is broadly distributed on hematopoietic cells, fibroblasts, and numerous tumor cells of melanoma, leukemias and cancers of breast, ovary, liver, pancreas, lung, colon, brain, vulva and prostate8,11.
CD44 interacts with Hyaluronic Acid (HA) to influence cytoskeleton function, cell survival, growth and migration through various processes such as RhoA signalling, Ankyrin co-localization and Ezrin recruitment12,13. Profiling of CD44 with respect to tumour type and subtype may help in determining tumor growth, survival and metastasis14.
Aim and Objectives
3
AIM:
To evaluate the expression of CD44 in Oral Squamous Cell Carcinoma.
OBJECTIVES:
1. To investigate CD44 expression in Formalin Fixed Paraffin Embedded tissues of Squamous Cell Carcinoma of Tongue using mouse monoclonal CD44 primary antibody and SS Polymer HRP/DAB detection kit by Immunohistochemistry.
2. To investigate CD44 expression in Formalin Fixed Paraffin Embedded tissues of Squamous Cell Carcinoma of Buccal mucosa using mouse monoclonal CD44 primary antibody and SS Polymer HRP/DAB detection kit by Immunohistochemistry.
3. To investigate CD44 expression in Formalin Fixed Paraffin Embedded tissues of normal mucosa using mouse monoclonal CD44 primary antibody and SS Polymer HRP/DAB detection kit by Immunohistochemistry.
4. To compare the CD44 expression in normal mucosa, Squamous Cell Carcinoma of Tongue and Squamous Cell Carcinoma of Buccal mucosa.
Materials & Methods
4
STUDY DESIGN:
This comparative study was done to evaluate the CD44 expression in normal mucosa, Oral Squamous Cell Carcinoma of Tongue and Buccal mucosa.
STUDY GROUPS:
Group 1 - Tissues from normal buccal mucosa (n=6)
Group 2 - Tissues from Oral Squamous Cell Carcinoma of Tongue (n=16)
Group 3 - Tissues from Oral Squamous Cell Carcinoma of Buccal mucosa (n=16) Immunohistochemistry Control : Human tonsil tissue 15
(previously known positive case for CD44)
STUDY SETTING:
This study was done at Ragas Dental College and Hospital and Cancer
Institute, Adayar and approved by the Institutional Ethics Committee of Ragas
Materials & Methods
5
ARMAMENTARIUM USED:
• Microtome • Autoclave • Hot air oven • Slide warmer • Coplin jars • Measuring jar • Weighing machine
• APES (3 amino propyl triethoxysilane) coated slides • Slide box
• Micro-pipettes • Toothed forceps • Electronic timer • Beakers
• Rectangular steel tray with glass rods • Sterile gauze
• Cover slips • Light microscope
REAGENTS USED:
1) Xylene
2) Absolute alcohol (Isopropyl alcohol) 3) Harris Hematoxylin
Materials & Methods
6 5) Eosin
6) APES
7) 1 N sodium hydroxide 8) 1 N Hydrochloric acid
9) Tris EDTA (Ethylene Diamine Tetra Acetate) buffer 10) 3% Hydrogen peroxide
11) Tris buffered Saline
12) Distilled water
ANTIBODIES USED:
• Primary antibody –Anti-CD44 mouse monoclonal antibody,
Clone: DF1485, Concentrated (1ml) CAT NO- MU310-UC, BioGenex
(ANNEXURE IV).
• Secondary antibody - SS Polymer-HRP/DAB IHC Detection system, BioGenex (ANNEXURE IV).
PROCEDURE:
1) A detailed case history including patient’s age, gender, past medical and dental
history, history of drug intake, deleterious habits and trauma was taken from records for control and study group.
Materials & Methods
7
3) From the Formalin Fixed Paraffin Embedded tissues, 5 micron thick sections were cut and used for routine Hematoxylin and Eosin (H&E) staining and Immunohistochemical (IHC) staining.
4) Positive control for CD44 was a section of human tonsil tissue15 (previously known positive case for CD44)
H & E STAINING:
The slides were deparaffinized in xylene and hydrated through grades of alcohol
to water. The sections on the slides were flooded with Harris Hematoxylin for 5 minutes. The slides were washed in running tap water for 5 minutes. The slides were differentiated in 1% acid alcohol for 5 minutes. The slides were washed well in running tap water for 5 minutes. The tissue sections on the slides were then stained in eosin for 30 seconds. The slides were washed in running tap water for 1 minute. The slides were then dehydrated through alcohol, cleared, mounted and viewed under light microscope and tumors were graded into varying histologic gradings of malignancy as well, moderately and poorly differentiated.
IMMUNOHISTOCHEMICAL STAINING OF CD44:
Materials & Methods
8
xylene to remove paraffin wax. They were put in descending grades of alcohol and then rehydrated with water. Circles were drawn using a diamond marker around the tissues, so that the antibodies added later are restricted to the circle. The slides were transferred to TRIS EDTA buffer of pH 9 and steamed in pressure cooker for antigen retrieval at 15 lbs pressure for 15 minutes. Slides were then treated with 3 % hydrogen peroxide for 7 minutes to quench endogenous peroxidase activity of cells that would result in non-specific staining. Then, the slides were dipped in Tris buffered saline with Tween 20 for 6 minutes. The slides were wiped carefully without touching the tissue section. The sections were incubated at room temperature with mouse monoclonal primary CD44 antibody (BioGenex). Primary antibody was detected using SS Polymer-HRP/DAB IHC Detection system (BioGenex). After thorough washing with Tris buffered saline at pH 7.4, sections were treated with super enhancer for 20 min at room temperature followed by incubation with SS Polymer-HRP reagent for 30 min at room temperature. After three washes with TBS, substrate DAB was applied to the sections for 2 min in the dark. Slides were then washed in distilled water to remove excess chromogen and counterstained with hematoxylin, dehydrated with ethanol and xylene and mounted permanently with DPX. The slides were then observed under the Light Microscope (LM).
POSITIVE AND NEGATIVE CONTROL:
Materials & Methods
9
STEPS INVOLVED:
1. APES coated slides with 2 paraffin embedded tissue placed in warming table. 2. Placed in xylene twice (5 minutes each)
3. Placed in 100% isopropanol (5 minutes) 4. Placed in 90% isopropanol (5 minutes) 5. Placed in 70% isopropanol (5 minutes) 6. Washed in distilled water (2 minutes each)
7. Keep in Tris EDTA buffer at pH 9 under steam pressure for 15 min for antigen retrieval
8. Cooling of solution done for 10 minutes 9. Slides were transferred to distilled water. 10. Placed in 3% hydrogen peroxide (7 minutes) 11. Washed in Tris buffer saline (2-3 minutes)
12. Primary antibody added and incubated (30 minutes) 13. Washed in Tris buffer saline (2-3 minutes)
14. Poly excel target binder reagent added and incubated (12 minutes) 15. Washed in Tris buffer (2-3 minutes)
16. SS Polymer-HRP added and incubated (12 minutes) 17. Washed slides in Tris buffer (2-3 minutes)
Materials & Methods
10
20. Stained with Harris Hematoxylin (20 seconds) 21. Washed in tap water
22. Placed in 70% alcohol (1 minute) 23. Placed in 100% alcohol (1 minute) 24. Placed in xylene (1 dip)
25. Slides to be mounted using DPX
26. Slides to be observed under the LM and graded
CRITERIA FOR EVALUATION OF STAINING:
Evaluation of H & E sections:
Tumors were graded as well, moderate and poorly differentiated tumors.
Evaluation for IHC16:
CD44 expression was evaluated as brown membranous staining in tumor nests & stromal cells (fibroblasts). The staining intensity was graded semi-quantitatively as follows:
Materials & Methods
11
To calculate the percentage of CD44 positive cells, atleast four high-power fields were chosen randomly, and 1000 cells were counted for each slide. The percentage positivity was graded semi-quantitatively as follows:
o Grade I – 0-10 % of positive cells o Grade II – 11-24 % of positive cells o Grade III – 25-49 % of positive cells o Grade IV – 50-74 % of positive cells o Grade V – 75-100 % of positive cells16
Each case was evaluated by two blinded observers independently with respect to positive control.
Statistical analysis:
Review of Literature
12
ORAL CANCER:
Oral cancer is the eleventh most common cancer in the world. Globally, the Age-Standardised Incidence Rate per 100,000 population for oral cancer in men and women is 5.5 and 2.5, respectively. India accounts for one-fifth of all oral cancer cases and one-fourth of all oral cancer deaths. The observed trends in incidence and mortality among men and women are closely correlated with the patterns in tobacco and alcohol use.
Tobacco use, in any form is the major risk factor for oral cancer. With dietary deficiencies, these factors cause more than ninety percent of oral cancers which include cancers of the mucosal lip, tongue, gum, floor of the mouth, palate and mouth, corresponding to the ICD, 10th revision. The buccal mucosa is the most common site for oral cancer in South and Southeast Asia; in all other regions, tongue is the most common site.
Review of Literature
13
Personalised diagnostics using biomarker can guide treatment and consequently improve the chance of curing the disease19.
CAUSES FOR POOR PROGNOSIS IN OSCC20:
RECURRENCE, SECOND PRIMARY TUMOR (SPT) & SECOND FIELD
TUMOR:
Long-term exposure to tobacco and/ or alcohol causes “field cancerisation” of
the upper digestive tract, thereby predisposing patients towards tumor recurrence, second field tumor formation and development of SPT at a separate anatomic site from the index tumor. It is said to be synchronous if it is diagnosed within 6 months after the index tumor or metachronous if it is diagnosed more than 6 months.
LYMPH NODE METASTASIS:
Review of Literature
14
EXTRACAPSULAR SPREAD:
Metastases may penetrate the lymph node capsule and infiltrate the perinodal tissue which has been referred to as Extracapsular Spread. It is a significant predictor of regional recurrence and the development of distant metastases.
DISTANT METASTASIS:
In patients with head and neck cancer, distant metastasis is defined as metastases below the clavicle. It may be the result of lymphogenic or haematogenous spread. High incidence of distant metastases has been reported in hypopharyngeal SCC, followed by SCC of the tongue. The average survival once distant metastases have been diagnosed ranges between 4 and 7 months. Tumor differentiation, immune status and genetic susceptibility of the host also plays a role in determining prognosis20.
MARKER OVERVIEW:
A cancer biomarker is a biological molecule found in blood, other body fluids,
Review of Literature
15
MARKER BIOLOGICAL
PROCESSES
CANCER
CONTEXT
I.SUSTAINED PROLIFERATIVE SIGNALING19
Ki-67 Cell cycle, cell proliferation
Best prognostic factor of the survival rate and recurrence
CDKN2A Cell cycle, cell cycle arrest Tumor suppressor gene
HPV16 High-risk HPV type HPV(+) tumors demonstrated favorable outcomes compared to TP53 mutants
DLC1 Negative regulation of cell proliferation
Tumor suppressor
CYR61 Regulation of cell growth and adhesion
Function as an oncogene or a tumor suppressor, depending on the cancer origin
TP53 Cell cycle, cell cycle arrest Tumor-suppressor
CCND1 Cell cycle, cell division Biomarker of cancer phenotype and disease
Review of Literature
16
MARKER BIOLOGICAL
PROCESSES
CANCER CONTEXT
RB1 Cell cycle, cell cycle arrest Tumor-suppressor protein. Defects in this gene are a cause of retinoblastoma and osteogenic sarcoma
CA9 Response to hypoxia Intracellular pH maintenance by which cancer cells adapt to the toxic conditions of the extracellular environtment EGFR Positive regulation of cell
proliferation
Overexpression is associated with poor prognosis
II. EVADING GROWTH SUPPRESSORS
MYC Positive regulation of cell proliferation
Deregulation of cell growth, proliferation, metabolism and genome stability
ALDH1A1 Ethanol oxidation Regulates growth and differentiation of CSCs PROM1 Retina layer formation Maintains stem cell
properties by suppressing differentiation
Review of Literature
17
MARKER BIOLOGICAL
PROCESSES
CANCER
CONTEXT
MAP1LC3A Autophagy Positive expression had a shorter survival
FAS Apoptosis Promotes tumor growth
III. ANGIOGENESIS
HMOX1 Angiogenesis Highly expressed in cancer than in healthy tissues
PDPN Lymphangiogenesis Identifies lymphatic endothelial
differentiation in vascular endothelial neoplasms
CTTN Cell motility and focal adhesion assembly
Overexpressed in breast cancer and OSCC
PTK2 Angiogenesis Tumor progression and metastasis
CTNND1 Cell adhesion Downregulation
Review of Literature
18
MARKER BIOLOGICAL
PROCESSES
CANCER CONTEXT
MMP1 Proteolysis Angiogenesis, response to hypoxia and proteolysis progression of cancer
VIM Movement of cell EMT
CDH1 Cell adhesion Loss of function cause cancer proliferation and metastasis
VCAN Cell adhesion Associated with poor outcome
AMFR Movement of cell Tumor motility-stimulating protein
IV. REPROGRAMMING OF ENERGY METABOLISM
HIF-1α Angiogenesis and response to hypoxia
Anaerobic metabolism
V. TUMOR-PROMOTING INFLAMMATION
IL4R Regulation of cell proliferation and Immune system
Review of Literature
19
MARKER BIOLOGICAL
PROCESSES
CANCER
CONTEXT
CXCL8 Movement of cell, chemotaxis
Neovascularisation
CD163 Inflammatory response Anti-inflammatory myeloid marker SERPINB3 Regulates cell
proliferation
EMT
CRP Inflammatory response High baseline CRP patients had a greater risk of early death
STEM CELL MARKER IDENTIFIED IN OSCC:
STEM CELL
MARKER
FUNCTIONAL RELEVANCE IN OSCC
CD44 Cell surface glycoprotein which causes proliferation, differentiation, migration, invasion, tumour sphere formation and resistance to chemotherapy.
Review of Literature
20
STEM CELL
MARKER
FUNCTIONAL RELEVANCE IN OSCC
CD133 Transmembrane glycoprotein displaying increased clonogenicity, EMT phenotype, tumour sphere formation, self-renewal and proliferation.
c-Met Tyrosine kinase receptor for HGF is associated with metastasis, tumour invasion and decreased survival. Side
populations
Subpopulations of Hoechst 33342 dye-resistant cells demonstrate tumourigenic, chemoresistance and self-renewal properties in vivo22.
Musashi-1(Msi-1)
RNA binding post transcriptional gene regulator whose over expression correlates with advanced stage and poor differentiation.
CD97 EGF - 7 transmembrane surface protein which is highly expressed in OSCC.
Cripto-1 Key regulator of embryonic development which plays a vital role in malignant transformation.
Bone
Morphogenetic Proteins (BMPs)
Review of Literature
21
STEM CELL
MARKER
FUNCTIONAL RELEVANCE IN OSCC
CXCR4 Chemokine receptor promote migration and invasion by regulating MMP-9 &13 via ERK signaling pathway in OSCC.
CD166 Over expression demonstrate tumour formation ability in vivo and are positively correlated with poor patient outcome
SLC2A13 A solute carrier protein family member which facilitates glucose transport whose overexpression is observed in sphere forming cells of primary cultures of OSCC samples. Nestin Up-regulated in metastasizing samples of OSCC CD29/β1
integrin
An integrin unit associated with very late antigen receptors. When combined with CD44high cells, tumors exhibit molecular characteristics of EMT. p63 A member of the p53 family of transcription
Review of Literature
22
STEM CELL
MARKER
FUNCTIONAL RELEVANCE IN OSCC
GRP78 Key role in both stem cell and cancer by mediating tumour proliferation, metastasis and conferring resistance to treatment. ALDH1+ cells in OSCC showed increased GRP78 anchored at the plasma membrane, exerting stemness properties.
HMGA2 A transcriptional factor initiating mesenchymal tumour formation in OSCC which initiates sphere and colony formation.
p75 A low affinity neurotrophic receptor that binds to NGF and protects stem cells from apoptosis.
Podoplanin Podoplanin positive tumours demonstrated better patient survival.
Review of Literature
23
STEM CELLS – REVIEW:
Stem cells are undifferentiated, self-renewing cells with the potential to
produce specialized differentiated cells24. Stem cells are divided into three categories:
Embryonic Stem Cells (ESCs) Germinal Stem Cells
Somatic Stem Cells (SSCs)
ESCs: These cells originate from the inner cell mass of the blastocyst. They are
totipotent and have indefinite replicative potential.
Germinal stem cells: These are derived from primary germinal layers of embryo.
They differentiate into progenitor cells of specific organs.
SSCs: These cells are less totipotent than ESCs and exists in haematopoietic,
neural, gastrointestinal and mesenchymal tissues25.
Stem cell potency is defined as the potential to give rise to a range of new cell phenotypes.
1. Totipotent: Cells which can differentiate into all cells of the three embryonic germ layers and extraembryonic tissue. E.g., A fertilized egg.
2. Pluripotent: Cells which can differentiate into all cell types of the three germ layers, but they lose the ability to differentiate into cells of the extraembryonic tissue. E.g., ESCs
Review of Literature
24
4. Unipotent: It is capable of differentiation into one cell type only. E.g., Muscle stem cells24
EMBRYONIC STEM CELL MARKERS26:
STEM CELL
MARKERS
CHARACTERISTICS CLASSIFICATION
SSEA-1 Murine embryos, mouse ESCs, mouse and human germ cells, EC cells
Carbohydrate associated molecules SSEA-3, SSEA-4
Primate ESCs, human embryonic germ cells, human ESCs, EC cells
CD90 (Thy-1)
Human ES cells, mouse ES cells, hematopoietic stem
cells, EC cells Surface marker CD31, CD59, CD9,
CD 29
Review of Literature
25
STEM CELL
MARKERS
CHARACTERISTICS CLASSIFICATION
CD326 Human ES cells, mouse ES cells, EC cells
Surface marker CD133 Human ES cells, mouse ES
cells, embryonal carcinoma (EC) cells, Hematopoietic stem cells
TRA-1-81 Human ESCs, teratocarcinoma, embryonic germ cells, EC cells
Surface antigen TRA-1-60 Human ESCs, teratocarcinoma,
embryonic germ cells, EC cells
Cripto (TDGF-1) Mouse ES cells, human ESCs, cardiomyocyte, EC cells
Review of Literature
26
BEST STEM CELL MARKER IN VARIOUS TUMORS22,27:
STEM CELL MARKERS COMMON
CANCERS
CD44+, ALDH, CD133, c-Met, Side populations OSCC
CD44+, ESA+, CD24–/low lineage, ALDH-1high Breast cancer
CD44+, CD133+, EpCAM+, CD24+ Pancreatic cancer
CD44+, α2β1high, CD133+ Prostate cancer
CD44+, CD133+, ALDH-1, CD166+, CD29, CD24+, CD26, Msi-1, Lgr-5, Wnt activity/β-catenin
Colon cancer CD133+, BCRP1+, A2B5+, SSEA-1+ Brain
cancer CD34+, CD38–, HLA–DR–, CD71–, CD90–, CD117–,
CD123+
Leukemia
CD133+, CD49f+, CD90+ Liver
Review of Literature
27
CD44:
Synonyms: Pgp-1, Ly-24, Hermes, lymphocyte homing receptor, H-CAM, and HUTCH-110.
One of the most common CSC surface marker and a promising therapeutic target for many cancers8. It is a transmembrane glycoprotein involved in cell-cell and cell-matrix adhesion and in cell signaling10. It was first described by Dalchau et al9. It is broadly distributed on hematopoietic cells, fibroblasts, and numerous tumor cells. It was first identified as gp85 which was then shown to be a HA receptor in placenta cells11. CD44 is encoded by the highly conserved CD44 gene on chromosome 11 in humans. The full-length CD44 gene consists of 20 exons and 19 introns. Ten of these exons are expressed in all isoforms (constant exons). The ten central exons (variable exons) undergo extensive alternative splicing via excision or inclusion in various combinations in the membrane proximal stem region to generate splicing variants (CD44v isoforms), which accounts for CD44 heterogeneity.
VARIANTS OF CD44:
1. CD44s - Smallest or standard isoform lacks all variant exons in the membrane proximal domain and is expressed on most vertebrate cells.
Review of Literature
28
CD44 molecular weight ranges from 85-230 kD for three reasons:
1. Variety of mRNAs are generated by alternative splicing of ten variant exons in its pre-mRNA.
2. Undergo various post-translational modifications
3. They get shortened in vivo, owing to partial cleavage by matrix metalloproteases, and this effect is most pronounced in human cancer of all types10.
CD44 also contributes to T cell activated extravasation of lymphocytes into sites of inflammation and plays substantial role in angiogenesis28.
MOLECULAR STRUCTURE OF CD4429:
Review of Literature
29
Hyaluronan binding domain:
This domain forms the primary ligand binding receptors for HA. Other CD44
ligand includes collagen, osteopontin, integrin, fibronectin, laminin, and MMPs.
Membrane proximal domain:
This is the most diverse region of the CD44 molecule in which introduction of new exons modulates HA-binding affinity by inducing conformational changes or allowing CD44 isoforms to function as a co-receptor by generating new binding sites for many growth factors, receptors, and non receptor protein-tyrosine kinases. For example, the sequence encoded by exon v6 contains a binding site for HGF and VEGF. The binding of HGF with CD44v6 induces CD44v6/HGF/cMet complex formation, leading to c-Met or HGF-induced Ras signaling activation. The inclusion of variant exons has been found to be dependent on mitogenic or oncogenic signals that regulate alternative splicing. Therefore, cancer cells express a variety of CD44 variants, particularly in an advanced stage.
Transmembrane (TM) domain:
It provides a platform for CD44v oligomer formation or coupling to cofactors, adaptor proteins, and receptor or non receptor protein-tyrosine kinases.
Cytoplasmic Domain:
Review of Literature
30
CD44 INTERACTIONS IN CANCER:
CD44 AND HA:
CD44-HA binding is tightly coupled with Leukemia-Associated Rho Guanine nucleotide exchange factor (LARG) which induces LARG-mediated RhoA activation. When activated by RhoA, Phospholipase C (PLC) hydrolyze inositol diphosphate into inositol trisphosphate (IP3), resulting in Ca2+ release from intracellular stores. This in turn activates Ca2+/calmodulin-dependent kinase-II. It then phosphorylates the cytoskeletal protein, filamin, leading to cytoskeleton reorganization and tumor cell migration.
CD44-HA binding activates one of the RhoGTPase i.e., ROK. It phosphorylates myosin phosphatase and myosin light chain, thereby activating myosin adenosine triphosphatase (ATPase) and generating actomyosin-mediated membrane motility and cell migration.
Review of Literature
31
CD44 AND ANKYRIN:
CD44-HA binding also promotes recruitment of the cytoskeletal protein, ankyrin, and inositol-1,4,5-triphosphate (IP3) receptor into cholesterol-containing lipid rafts. The Ankyrin Repeat Domain of ankyrin is responsible for binding IP3 receptor to CD44 at lipid rafts which subsequently triggers intracellular Ca2+ mobilization leading to HA-induced membrane–cytoskeleton interactions and tumor cell-specific behaviors (e.g., cell survival, growth and migration)12,30.
CD44 AND THE ERM (EZRIN, RADIXIN, AND MOESIN):
ERM proteins regulate the structural assembly and stabilisation of plasma membrane domain. They are concentrated at the surface projections such as microvilli and membrane ruffles where they link the microfilaments to the membrane. They also serve as linkers between molecules such as CD44 and ICAM-2 and the cytoskeleton. ERM proteins are recruited to the cytoplasmic tail of CD44 and so linking to the cytoskeleton, under the influence of growth factors, results in proliferation and invasion of tumors13.
CD44 REGULATION OF DRUG TRANSPORTER EXPRESSION:
Review of Literature
32
hyaluronan interactions, viz., hyaluronan oligomers, rapidly induces internalization of the transporters and CD44 into the cell29.
CD44 IN INFLAMMATION:
Binding between CD44 and E-selectin ligand support neutrophil slow rolling.
Interaction of CD44 with P-selectin causes initial tethering and fast rolling. These interactions play a key role in the pathogenesis of Leucocyte Adhesion Deficiency syndrome in which binding of CD44 with E-selectin is inhibited. Bi-molecular complex formation between CD44 and VLA-4 integrin is required for firm adhesion of neutrophils. Neutrophil transit across epithelial monolayers in vitro was found to be attenuated when neutrophil CD44 was activated. In contrast, visualization of neutrophil recruitment in vivo within the inflamed cremaster
Review of Literature
33
Activated cells present in tonsillar lymphocyte preparations possess CD44 which is capable of binding hyaluronan. It is this that accounts for lymphocytes to adhere under flow with tonsillar stromal cells, stromal cell extracellular matrix, purified hyaluronan, and reticular fibers in the T cell areas of secondary lymphoid
organs33. In the context of autoimmune disease, CD44 also enables rolling
subsequent to T cell stimulation, in the peripheral circulation during chronic inflammatory diseases which supports their role in the enhanced homing of activated lymphocytes to target sites of autoimmunity33.
CO-FACTORS INTERACTIONS WITH CD44:
HYALURONAN:
Hyaluronan is a major ligand for CD44 and can bind CD44v isoforms. Through binding of CD44, HA can activate cytoskeleton and MMPs signaling involved in tumor progression. It exists in high molecular weight or low molecular weight due to cleavage into varying sizes. High molecular weight HA is involved in tumorigenesis, anti-angiogenic and anti-inflammatory responses. However, low molecular weight HA promote cell motility, CD44 cleavage and angiogenesis. Therefore, the size of HA ligand determines biological function.
OSTEOPONTIN (OPN):
Review of Literature
34
N-terminal domain of CD44. OPN binding to CD44 variants/beta1-containing integrin promotes cell spreading, motility and chemotaxis. OPN increases expression of both CD44s and variant isoforms in human melanoma.
MATRIX METALLOPROTEASES (MMPS):
Extracellular matrix proteins that are involved in extracellular matrix degradation which are important during development, wound healing, bone resorption, and angiogenesis. MMP-9 and CD44 binding results in MMP9 activity localization on the cell surface. Ability of CD44 to localize proteolytically active MMP-9 to the tumor cell surface is important for tumor invasion34.
CD44 - THERAPEUTIC TARGET IN CANCER:
CD44 ANTIBODIES AND VACCINES:
Review of Literature
35
HYALURONAN OLIGOMERS:
Hyaluronan oligomers act in vivo by disrupting endogenous hyaluronan-CD44 interactions that are necessary for tumor progression. Hence, inhibits local tumor growth, primary tumor spreading and residual tumor growth following surgical removal of the tumor mass38. It also suppresses resistance to anticancer drugs, including, doxorubicin, taxol, vincristine and methotrexate29.
HYALURONAN -COATED NANOPARTICLE SYSTEMS:
Review of Literature
36
vessels to tumor tissue rather than their normal counterpart. Also, the lack of lymphatic vessels causes lymph circumfluence to suffocate. Under these circumstances, nanoparticles, accumulate in tumor tissues. Magnetic nanomaterials are good candidates for disease treatment because tissues rarely absorb magnetic waves, which penetrate deeper than visible light and infrared rays without side effects. It contains iron oxide, which can be locally heated by a magnetic field. The walls of microcapsules are integrated with 18 nm-diameter iron oxide nanotubes. Under an alternating magnetic field, magnetic nanotubes can heat their surroundings and destroy the walls of the microcapsules, thereby releasing the encapsulated material into the surrounding solution38.
E.g., HA– Iron Oxide Magnetite nanoparticles are efficient in labelling CD44 containing tumor cells in targeted drug delivery systems39.
HYALURONIDASES:
Hyaluronidases improve access of drugs to cancer cells through effects on cell adhesion and matrix barriers. Its oligosaccharide products inhibit hyaluronan-CD44 signaling, resulting in decreased cell survival and chemoresistance35.
Review of Literature
37
CD44 IN OSCCs AND OPMD:
An Immunohistochemical study by Flavia Paiva Prudente de Moraes et al
concluded that CD44 might be associated with patient outcome in OSCC in which evaluation of the expression of CD44, CD24, CD133, ALDH1, CD29 and Ki-67 in 52 OSCC specimens [Oropharynx not otherwise specified (n=4), Tonsils (n=10), Soft palate (n=2), Mobile tongue (n=20), Gingiva (n=5), Floor of the mouth (n=4), Hard palate (n=4), Retromolar trigone (n=2), Cheek mucosa (n=1)] and 21 metastatic lymph nodes and correlation with clinicopathologic features was done. The study shown CD44 positivity in 30 OSCC specimens (57.7 %), 7 metastatic lymph nodes (33.3%) and in the basal and parabasal layers of normal mucosa. In the study, a statistically significant association between CD44 expression and well-differentiated tumors (P=.045) was seen. Also, the median 5-year survival rate of patients who shown negative and positive expression for CD44 was 74% and 38%, respectively40.
Among the CD44 variants, CD44v6 proved to be a poor predictor of tumor
Review of Literature
38
subsites (n = 32) presented a high expression of this marker only in 22 cases (53.7%). However, CD44v6, p63 and podoplanin expression did not show significant relationship with clinico–pathological variables. But, a significant
relation was found between MMP-9 and tumour stage (p=0.002) and also with nodal metastasis (p=0.018)16.
CD44 was found to be useful in predicting the disease course in which
immunohistochemical expression of HIF-1α, CD44, p16, Ki67, and podoplanin in 35 OSCC specimens was investigated and correlation with clinical findings was done by
Johannes Dunkel et al. It was found that high CD44 expression and low HIF-1α
expression had a strong positive correlation with the 5-year disease-free survival (P < .001). Whereas, p16, Ki67 and podoplanin did not correlate with the course of the disease41.
CD44 was found to be useful in assessing the prognosis of OSCC in relation to different sub-sites of the oral cavity in an immunohistochemical study by
Michal Krump and Jiri Ehrmann which analyzed 29 OSCC samples {floor of
Review of Literature
39
statistically significant CD44 negativity in tongue tumour group (0%) compared with that of the floor of the mouth (85%; p=0.003) and other sites (100%; p=0.003). But, no statistically significant difference was found in histologic grades of differentiation42.
With regard to premalignant conditions, CD44v6 serves as a useful marker for detecting high-risk leukoplakias. This finding was demonstrated in a study by
Pournima Y Godge and Leena S Poonja which analyzed the
Review of Literature
40
CD44 appears to be the most useful among other CSCs markers in OSCC in a
study by Cl. Margaritescu et al which evaluated immunohistochemical expression of three CSC markers, namely, CD44, CD133 and CD117. Out of 30 specimens, CD44 positivity was seen in 80–100% of well-differentiated, 60–85% of moderate differentiated and 40–62% of poor differentiated cases. In normal mucosa, the strongest reaction was seen in basal and parabasal layers and became progressively weaker in the upper granular layers. Both in normal and tumor tissues, there was no expression for CD133 and CD11744.
Review of Literature
41
Taking into account the staining patterns, irregular staining of CD44 was found to predict more advanced disease and shortened survival of the patients in an immunohistochemical study by Kosunen A et al which evaluated CD44 and MMP-9 expression in OSCC. The study included 138 and 124 specimens for CD44 and MMP-9 analyses, respectively and correlated with clinicopathological factors and survival in OSCC. The results revealed association between irregular staining pattern of CD44 and poor tumour differentiation (p = 0.003), clinical stage III–IV (p = 0.049) and the presence of T3-4 tumour stage (p = 0.03). It was also found that MMP-9 intensity was strong in 21 (17%), moderate in 78 (63%) and weak in 25 (20%) cases and MMP-9 stromal staining intensity was strong in 31 (25%), moderate in 61 (49%) and weak in 32 (26%) cases. A significant association was found between irregular staining of CD44 in tumour cells and strong staining intensity of MMP-9 in stromal tissue (p < 0.001) whereas CD44 expression and MMP-9 intensity were not related to each other in tumour cells46.
CD44 IN OSCC OF TONGUE:
CD44 targeting was found to increase therapeutic success, thereby decreasing
morbidity and mortality of OSCC in an immunohistochemical study by Nasrollah
Saghravanian et al which investigated CD44 and p63 expression in two groups
Review of Literature
42
in high grade tumors (p < 0.001), advanced stage tumors (p = 0.009) and overall survival (p < 0.05) which was statistically significant. And, the death risk for cases with CD44 expression more than 30% was 2.08 fold higher than those with immunoreactivity less than 30%. Whereas, increased expression of p63 correlated with higher grades of disease and a significant difference was found between p63 expression and different grades of OSCC (p= 0.004)47.
CD44 was not found to be useful in predicting tumor prognosis in an
immunohistochemical study by Reza Kaboodkhani et al which evaluated the expression of CD44 in 51 OSCC specimens of tongue. The study did not show significant correlation with survival (p=0.77), age (p=0.4), clinical lymphadenopathy (p = 0.155), lymph node metastasis (p=0.87), sex (p=0.947), smoking (p= 0.287), and tumor size (p=0.813)48.
Irrespective of HPV status, CD44 was found to be a prognostic marker in
Review of Literature
43
(p=0.174 and p=0.345), E-cadherin (p=0.355 and p=0.395), COX-2 (p=0.944 and p=0.599), Ki-67 (p=0.628 and p=0.145) and p27 (p=0.690 and p=0.471). And, also expression and intensity of CD44, EGFR, COX-2, Ki-67, VEGF, E-cadherin and p27 was not correlated to HPV status. In the multivariate analyses, CD44, p16 and EGFR were included together with age, TNM stage, grade, tumor location and sex. CD44 was found to be statistically significant prognostic marker (p=0.026), while expression of p16 and EGFR failed to show a statistically significant correlation49.
CD44 was found to be a useful in predicting the invasive behaviour of OSCC
Review of Literature
44
CD44 expression was found to associate only with histological grading of
malignancy in OSCC of tongue in an immunohistochemical study by Maria
Carmen Fontoura Nogueira da Cruz et al which associated the expression of
CD44v6 and E-cadherin in 30 OSCC specimens [Tongue (n=15) and lower lip (n=15)] with the anatomic location, nodal metastasis and histological grading of malignancy. The results revealed OSCCs with low score of malignancy had a higher immunoexpression of E-cadherin (p = 0.005) and CD44v6 (p = 0.003). However, no significant difference in the expression pattern and number of positive cells for CD44v6 or E-cadherin in OSCCs of tongue and lower lip (p>0.05) and also with and without nodal metastasis (p>0.05) was found15.
Loss of CD44 expression was found to be an early event in lingual carcinogenesis and a marker of major alterations in non-tumour adjacent epithelium in an immunohistochemical study by Miguel Angel Gonzalez-Molesa
Review of Literature
45
Among the isoforms, CD44v9 proved to be a useful indicator in assessing
metastatic potential of OSCCs of Tongue in which the immunoexpression of CD44 and their correlation to lymph node metastasis and survival was done by S.
Sato et al. In 120 specimens, CD44v9 expression did not correlate with the
histological grading of the tumors (p=0.109), whereas positive correlation was seen between reduced expression of CD44v9 and without metastatic lesions. Also, shorter survival time (p=0.0125) showed CD44v9 reduction. Hence, reduced expression of CD44v9 serves in assessing tumor prognosis52.
OTHER METHODS OF STUDYING CD4453,54:
Other methods in demonstrating CD44 expression were as follows:
Fluorescence Activated Cell Sorting (FACS), Immunofluorescence, Polymerase Chain Reaction (PCR), Western Blot, Immunocytochemistry.
Review of Literature
46
expressed in higher levels in the cell lines of stage IV SCC which was resistant to the conventional treatment regimens (11A). Real time quantitative RT-PCR shown high levels of CD44v 4 and CD44v 6 in primary tumor cell lines rather than their corresponding recurrences. The levels of expression increased with the tumor stage. And, also CD44 v4 and CD44 v6 was high in CD44 enriched cells sorted by FACS by 4 and 2 fold correspondingly. CD44v 1 and CD44v 2 was expressed equally in CD44high and CD44low cells. Immunofluorescence staining with CD44s were shown to delineate the plasma membrane and cell-cell boundaries. CD44v 1, CD44v 2, CD44v 4, and CD44v 6 were seen in cells with mesenchymal morphology. Specifically, CD44v1 and CD44v 2 in higher magnification participated in philopodia. CD44 staining in dividing cells labeled the centers of organization of microtubules. Immunocytochemistry did not pick up any variant forms for this cell line, indicating that both FACS and real time qRT-PCR have higher sensitivity53.
CD44 does not confer a survival advantage in head and neck cancers, however its variants offers a survival advantage or stem cell properties. In a study
by M. Athanassiou-Papaefthymiou et al, In Silico Transcriptomics database
Review of Literature
47
six probes, respectively, whereas decreased CD44 was found for five out of six probes in disease and only one cell line i.e., SCC of tongue. In Immunocytochemistry, all cell lines stained 50% for CD44s, except for HN12 where only 10% cells were stained. All cell lines also stained 50% for CD44 variants namely, v3, v5, v6, and v9. CD44v10 had occasional staining in all cell lines except cell line 20. HN4 cell line did not stain for CD44v 7, while its metastasis-derived HN12 stained sparsely.
Review of Literature
48
expressed another band at 180–190 kDa.. Variants i.e., v5, v7/8, and v10 were expressed by all cell lines but there were no differences in their expression between them. Flow Cytometry analysis shown that all cell lines expressed total CD44 greater than 50% increase in mean channel fluorescence (MCF). The most frequently undetected variant exon was v7, followed by v10 and v4. OSC-20 cell line expressed the fewest variant exons. And, also HN4 lacked v7 while its metastasis-derived partner, HN12, lacked v10. Expression of v4 was infrequent in HN30 (50% increased MCF) but in HN31 it was highly expressed (50% increased MCF). However, HN22 could not be analysed54.
CD44 IN VARIOUS CANCERS:
CD44 also plays a key role in the prognosis of tumors such as Prostate cancer,
Results
49
SAMPLE CHARACTERISTICS:
The study population comprised of 38 cases taken from the archival blocks.
They were categorized into three groups. Group 1 (n=6) comprising of normal mucosa samples, Group 2 (n=16) comprising of Oral Squamous Cell Carcinoma of Tongue and Group 3 (n=16) comprising of Oral Squamous Cell Carcinoma of buccal mucosa. All the samples were analyzed for immunohistochemical expression of CD44.
Distribution of age in the study groups (Table 1 & Graph 1):
The age of patients were divided into 3 groups: 20 – 40 years, 41 – 60 years and those above 61 years. Group 1 consisted of 6 (100%) cases. All were in the age group of 20 – 40 years. Group 2 consisted of 2(12.5%) cases in 20 – 40 years, 11(68.8%) cases in 41 – 60 years and 3(18.8%) cases above 61 years. Group 3 consisted of 3(18.8%) cases in 20 – 40 years, 7(43.8%) cases in 41 – 60 years and 6(37.5%) cases above 61 years. A significant difference was found with respect to age in the study groups (p=0.001*)
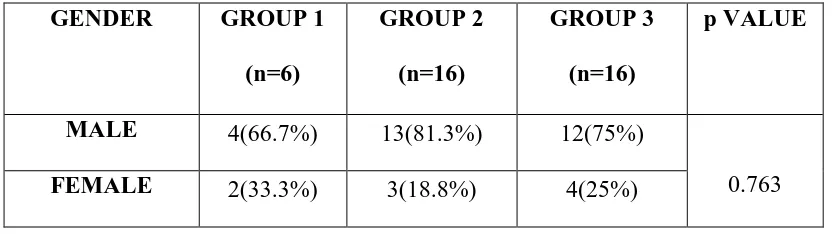
Distribution of gender in the study groups (Table 2 & Graph 2):
Results
50
Distribution of habits in the study groups (Table 3 & Graph 3):
Based on the prevalence of habits in the study groups, they were categorized in to six groups. They were those with
1. No habits
2. Habit of tobacco (chewing only)
3. Habit of tobacco usage (smoking and chewing)
4. Habit of chewing tobacco in combination with alcohol usage 5. Habit of smoking in combination with alcohol consumption
6. Habit of tobacco (smoking and chewing) and alcohol consumption
Results
51
All the cases enrolled in the study showed CD44 staining. In group 1, group 2 and group 3, CD44 staining was present in all of the 6(100%) cases, 16(100%) cases and 16(100%) cases, respectively.
The following parameters were used to evaluate CD44 in the study groups: Staining intensity and
Percentage of positive cells stained
Comparison of staining intensity of CD44 between the study groups (Table 4 &
Graph 4):
On comparing the staining intensity between the study groups, out of 6(100%) cases in group 1, 5 (83.3%) cases exhibited strong staining, whereas only 1(16.7%) case demonstrated weak staining. In group 2, 4(25%), 6(37.5%) and 6(37.5%) cases showed weak, moderate and strong staining, respectively. In group 3, 4(25%), 5(31.3%) and 7(43.8%) cases showed weak, moderate and strong staining. However, no significant difference was found with respect to CD44 staining intensity between the study groups (p=0.363*).
Comparison of percentage of CD44 positive cells between the study groups
(Table 5 & Graph 5):
Results
52
% and 0 to 10 % of positive cells, respectively. However, the results were statistically insignificant (p=0.177*).
Comparison of staining intensity of CD44 between histologic grades of
differentiation in OSCC (Table 6 & Graph 6):
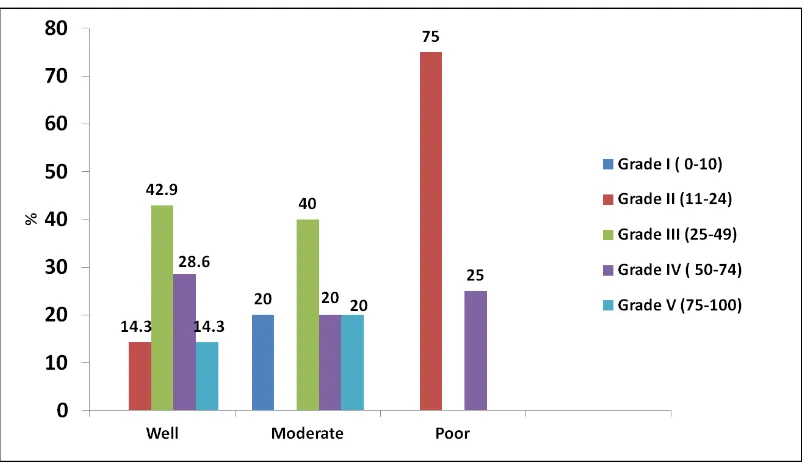
Out of 32 cases of OSCC, 13, 10 and 9 were well, moderate and poorly differentiated tumors, respectively. Among the well differentiated tumors, strong and moderate intensity was present in 8(61.5%) and 5(38.5%) cases, respectively. Among the moderately differentiated tumors, strong, moderate and weak intensity was present in 4(40%), 4(40%) and 2(20%) cases. Within the poorly differentiated tumors, strong, moderate and weak intensity was found in 1(11.1%), 2(22.2%) and 6(66.7%) cases. When the sub-site of tumors were not taken into account, staining intensity of CD44 correlated with histologic grades of differentiation (p=0.009*)
Comparison of percentage of CD44 positive cells between histologic grades of
differentiation in OSCC (Table 7 & Graph 7):
Results
53
Comparison of CD44 staining intensity between histologic grades of group II
(Table 8 & Graph 8):
In group 2, i.e., Oral S quamous Cell Carcinoma of Tongue, out of 16(100%) cases, 7, 5, 4 cases were well, moderate and poorly differentiated tumors, respectively. Among well differentiated, 4(57.1%) and 3(42.9%) cases exhibited strong and moderate intensity. In moderately differentiated tumors, 2(40%), 2(40%) and 1(20%) cases exhibited strong, moderate and weak intensity. In poorly differentiated tumors, 1(25%) and 3(75%) cases exhibited moderate and weak intensity. But, no significant difference was found with respect to CD44 staining intensity in the group 2 (p=0.083*).
Comparison of CD44 staining intensity between histologic grades of group III
(Table 9 & Graph 9):
Results
54
Comparison of percentage of CD44 positive cells between histologic grades of
group II (Table 10 & Graph 10):
In group 2, i.e., out of 16(100%) cases of Oral Squamous Cell Carcinoma of Tongue, 7, 5, and 4 were well, moderate and poorly differentiated tumors, respectively. In well differentiated type, 1(14.3%), 2(28.6%), 3(42.9%) and 1(14.3%) cases demonstrated 75 to 100 %, 50 to 74 %, 25 to 49 % and 11 to 24 % of positive cells, respectively. In moderately differentiated tumors, 1(20%), 1(20%), 2(40%) and 1(20%) cases exhibited 75 to 100 %, 50 to 74 %, 25 to 49 % and 0 to 10 % of positive cells, respectively. In poorly differentiated tumors, 1(25%) and 3(75%) cases exhibited 50 to 74 % and 11 to 24 % of positive cells, respectively. However, no significant difference was found (p=0.246*).
Comparison of percentage of CD44 positive cells between histologic grades of
group III (Table 11 & Graph 11):
Out of 16(100%) cases of Oral Squamous Cell Carcinoma of buccal mucosa, 6, 5, and 5 were well, moderate and poorly differentiated tumors, respectively. Among the well differentiated type, 3(50%), 2(33.3%), and 1(16.7%) cases demonstrated 75 to 100 %, 50 to 74 % and 25 to 49 % of positive cells, respectively. In moderately differentiated tumors, 1(20%), 2(40%) and 2(40%) cases exhibited 75 to 100 %, 50 to 74 % and 25 to 49 % of positive cells, respectively. In poorly differentiated tumors, 1(20%), 2(40%) and 2(40%) cases exhibited 75 to 100 %, 25 to 49 % and 0 to 10% of positive cells, respectively. However, the results were insignificant (p=0.238*).
Tables and Graphs
TABLE 1: DISTRIBUTION OF AGE AMONG THE STUDY GROUPS (N=38)
AGE GROUPS
IN YEARS
GROUP 1
(n=6)
GROUP 2
(n=16)
GROUP 3
(n=16)
p VALUE
20-40 6(100%) 2(12.5%) 3(18.8%)
0.001*
41-60 0(0%) 11(68.8%) 7(43.8%)
61 AND
ABOVE
0(0%) 3(18.8%) 6(37.5%)
*p Value < 0.05 is significant
GRAPH 1:DISTRIBUTION OF AGE AMONG THE STUDY GROUPS
GROUP I – Normal mucosa
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:73.595.119.507.411.625.2]Tables and Graphs
TABLE 2: DISTRIBUTION OF GENDER AMONG THE STUDY GROUPS (N=38)
GENDER GROUP 1
(n=6)
GROUP 2
(n=16)
GROUP 3
(n=16)
p VALUE
MALE 4(66.7%) 13(81.3%) 12(75%)
0.763
FEMALE 2(33.3%) 3(18.8%) 4(25%)
GRAPH 2:DISTRIBUTION OF GENDER AMONG THE STUDY GROUPS
GROUP I – Normal mucosa
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:74.595.110.517.391.615.2]Tables and Graphs
TABLE 3: DISTRIBUTION OF HABITS AMONG THE STUDY GROUPS (N=38)
HABITS GROUP 1
(n=6) GROUP 2 (n=16) GROUP 3 (n=16) p VALUE
NO HABITS 6(100%) 1(6.3%) 0(0%)
0.000*
TOBACCO CHEWING 0(0%) 4(25%) 5(31.3%)
TOBACCO
(SMOKING+CHEWING)
0(0%) 3(18.8%) 2(12.5%)
TOBACCO
CHEWING+ALCOHOL
0(0%) 0(0%) 4(25%)
SMOKING+ALCOHOL 0(0%) 1(6.3%) 2(12.5%)
SMOKING+TOBACCO
CHEWING +ALCOHOL
0(0%) 7(43.8%) 3(18.8%)
*p Value < 0.05 is significant
GRAPH 3:DISTRIBUTION OF HABITS AMONG THE STUDY GROUPS
GROUP I – Normal mucosa
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:75.595.109.514.492.718.2]Tables and Graphs
TABLE 4: COMPARISON OF STAINING INTENSITY OF CD44 BETWEEN THE STUDY GROUPS (N=38)
STAINING
INTENSITY
GROUP 1
(n=6)
GROUP 2
(n=16)
GROUP 3
(n=16)
p VALUE
WEAK 1(16.7%) 4(25%) 4(25%)
0.363
MODERATE 0(0%) 6(37.5%) 5(31.3%)
STRONG 5(83.3%) 6(37.5%) 7(43.8%)
GRAPH 4:DISTRIBUTION OF STAINING INTENSITY OF CD44
BETWEEN THE STUDY GROUPS
GROUP I – Normal mucosa
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:76.595.118.504.396.652.2]Tables and Graphs
TABLE 5: COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS BETWEEN THE STUDY GROUPS (N=38)
PERCENTAGE OF
CD44 POSITIVE
CELLS
GROUP 1
(n=6)
GROUP 2
(n=16)
GROUP 3
(n=16)
p VALUE
0-10 0(0%) 1(6.3%) 2(12.5%)
0.177
11-24 0(0%) 4(25%) 0(0%)
25-49 1(16.7%) 5(31.3%) 5(31.3%)
50-74 1(16.7%) 4(25%) 4(25%)
75-100 4(66.7%) 2(12.5%) 5(31.3%)
GRAPH 5:COMPARISON OF PERCENTAGE OF CD44 POSITIVE
CELLS BETWEEN THE STUDY GROUPS
GROUP I – Normal mucosa
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:77.595.120.512.424.670.2]Tables and Graphs
TABLE 6: COMPARISON OF CD44 STAINING INTENSITY BETWEEN HISTOLOGIC GRADES OF DIFFERENTIATION (N=32)
STAINING
INTENSITY
OF CD44
HISTOLOGIC GRADES OF
DIFFERENTIATION
p VALUE
WELL
(n=13)
MODERATE
(n=10)
POOR
(n=9)
WEAK 0(0%) 2(20%) 6(66.7%)
0.009*
MODERATE 5(38.5%) 4(40%) 2(22.2%)
STRONG 8(61.5%) 4(40%) 1(11.1%)
*p Value < 0.05 is significant
GRAPH 6:COMPARISON OF CD44 STAINING INTENSITY BETWEEN
HISTOLOGIC GRADES OF DIFFERENTIATION (N=32)
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:78.595.124.499.442.696.2]Tables and Graphs
TABLE 7: COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS BETWEEN HISTOLOGIC GRADES OF DIFFERENTIATION (N=32)
PERCENTAGE
OF CD44
POSITIVE
CELLS
HISTOLOGIC GRADES OF
DIFFERENTIATION p VALUE WELL (n=13) MODERATE (n=10) POOR (n=9)
0-10 0(0%) 1(10%) 2(22.2%)
0.277
11-24 1(7.7%) 0(0%) 3(33.3%)
25-49 4(30.8%) 4(40%) 2(22.2%)
50-74 4(30.8%) 3(30%) 1(11.1%)
75-100 4(30.8%) 2(20%) 1(11.1%)
GRAPH 7:COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS
BETWEEN HISTOLOGIC GRADES OF DIFFERENTIATION
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:79.595.119.512.453.693.2]Tables and Graphs
TABLE 8: COMPARISON OF CD44 STAINING INTENSITY BETWEEN HISTOLOGIC GRADES OF GROUP II (N=16)
STAINING
INTENSITY
OF CD44
HISTOLOGIC GRADES OF
DIFFERENTIATION
p VALUE
WELL
(n=7)
MODERATE
(n=5)
POOR
(n=4)
WEAK 0(0%) 1(20%) 3(75%)
0.083
MODERATE 3(42.9%) 2(40%) 1(25%)
STRONG 4(57.1%) 2(40%) 0(0%)
GRAPH 8:COMPARISON OF CD44 STAINING INTENSITY BETWEEN
HISTOLOGIC GRADES OF GROUP II
[image:80.595.107.511.435.705.2]Tables and Graphs
TABLE 9: COMPARISON OF CD44 STAINING INTENSITY BETWEEN HISTOLOGIC GRADES OF GROUP III (N=16)
STAINING
INTENSITY
OF CD44
HISTOLOGIC GRADES OF
DIFFERENTIATION
p VALUE
WELL
(n=6)
MODERATE
(n=5)
POOR
(n=5)
WEAK 0(0%) 1(20%) 3(60%)
0.222
MODERATE 2(33.3%) 2(40%) 1(20%)
STRONG 4(66.7%) 2(40%) 1(20%)
GRAPH 9:COMPARISON OF CD44 STAINING INTENSITY BETWEEN
HISTOLOGIC GRADES OF GROUP III
[image:81.595.110.516.431.706.2]Tables and Graphs
TABLE 10: COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS BETWEEN HISTOLOGIC GRADES OF GROUP II (N=16)
PERCENTAGE
OF CD44
POSITIVE
CELLS
HISTOLOGIC GRADES OF
DIFFERENTIATION p VALUE WELL (n=7) MODERATE (n=5) POOR (n=4)
0-10 0(0%) 1(20%) 0(0%)
0.246
11-24 1(14.3%) 0(0%) 3(75%)
25-49 3(42.9%) 2(40%) 0(0%)
50-74 2(28.6%) 1(20%) 1(25%)
75-100 1(14.3%) 1(20%) 0(0%)
GRAPH 10:COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS
BETWEEN HISTOLOGIC GRADES OF GROUP II
GROUP II – Oral Squamous Cell Carcinoma of Tongue
[image:82.595.108.512.475.707.2]Tables and Graphs
TABLE 11: COMPARISON OF PERCENTAGE OF CD44 POSITIVE CELLS BETWEEN HISTOLOGIC GRADES OF GROUP III (N=16)
PERCENTAGE
OF CD44
POSITIVE
CELLS
HISTOLOGIC GRADES OF
DIFFERENTIATION
p VALUE
WELL
(n=6)
MODERATE
(n=5)
POOR
(n=5)
0-10 0(0%) 0(0%) 2(40%)
0.238
11-24 0(0%) 0(0%) 0(0%)
25-49 1(16.7%) 2(40%) 2(40%)
50-74 2(33.3%) 2(40%) 0(0%)
75-100 3(50%) 1(20%) 1(20%)
GRAPH 11:COMPARISON OF PERCENTAGE OF CD44 POSITIVE
CELLS BETWEEN HISTOLOGIC GRADES OF GROUP III
[image:83.595.112.513.486.705.2]Photographs
ARMAMENTARIUM
ANTIBODY KIT
Photographs
CD44 EXPRESSION IN POSITIVE CONTROL
10 X 40 X
CD44 EXPRESSION IN NORMAL MUCOSA
10 X 40 X
Photographs
CD44 EXPRESSION IN OSCC – WELL-DIFFERENTIATED
10 X 40 X
CD44 EXPRESSION IN OSCC – MODERATELY-DIFFERENTIATED
10 X 40 X
Photographs
CD44 EXPRESSION IN OSCC – POORLY-DIFFERENTIATED