Dissertation on
STUDY OF OCULAR MOTOR NERVE
PALSIES IN DIABETES MELLITUS
Submitted in partial fulfilment of requirements of
M. S. OPHTHALMOLOGY
BRANCH III
Of
REGIONAL INSTITUTE OF OPHTHALMOLOGY
MADRAS MEDICAL COLLEGE
CHENNAI – 600 003
THE TAMILNADU DR.M.G.R. MEDICAL UNIVERSITY
CHENNAI-600 003
CERTIFICATE
This is to certify that this dissertation titled “ Study of ocular motor
nerve palsies in diabetes mellitus” is a bonafide record of the research work
done by Dr.V.SARANYA., Post graduate in Regional Institute of
Ophthalmology, Madras Medical College and Research Institute, Government
General Hospital,Chennai-03, in partial fulfilment of the regulations laid down
by The Tamil Nadu Dr.M.G.R. Medical University for the award of
M.S.Ophthalmology Branch III, under my guidance and supervision during the
academic years 2016-2019.
Prof. Dr.M.V.S. PRAKASHMS.DO.,
Department of Orbit and Oculoplasty services,
Regional Institute of Ophthalmology Madras Medical College & Research Institute, Govt. General Hospital, Chennai – 600 008
Prof. .M.ANAND BABU M.S. D.O.,
Director and Superintendent
,
Regional Institute of Ophthalmology Madras Medical College & Research Institute,
Govt. General Hospital, Chennai – 600 008
Prof. DR.R.JAYANTHI M.D.,FRCP(Glas) DEAN
Madras Medical College,
ACKNOWLEDGEMENT
I would like to thank Prof. DR.R.JAYANTHI., M.D.,FRCP (Glas),
Dean, Madras Medical College and Research Institute for giving me
permission to conduct the study in this Institution.
With due respect and gratitude, I thank Prof.Dr.M.ANAND BABU,
M.S., D.O., Director and superintendent, Regional Institute of Ophthalmology
and Govt. Ophthalmic Hospital, Chennai for permitting me to conduct this
study.
Prof.Dr.M.V.S.PRAKASH, M.S.,D.O., Unit Chief, Orbit and
Oculoplasty services, and my guide for assigning me this topic for study and
guiding me throughout my Post graduate course. I wish to express my sincere
thanks for the valuable help, encouragement and guidance at various stages of
the study.
My sincere thanks to my Assisstant Professors
Dr. T.G. Uma Maheswari, MS, Dr.P.Geetha, MS.DO, Dr.R.Sujatha,MS.,
for their timely help and guidance in conducting this study.
I wish to express my sincere thanks to my family, friends and all my
colleagues who helped me in bringing out this study. Last but not the least, my
heartful gratitude and sincere thanks to all my patients without whom this
DECLARATION BY THE CANDIDATE
I hereby declare that this dissertation entitled, “STUDY OF OCULAR
MOTOR NERVE PALSIES IN DIABETES MELLITUS” is a bonafide and
genuine research work conducted by me under the guidance of
Prof. Dr.M.V.S.PRAKASH, M.S., D.O., Head of Department of Orbit and
Oculoplasty services, Regional institute of ophthalmology & Government
Ophthalmic hospital. Chennai-600008.
Dr. V.SARANYA
Place: Chennai
Date:
CERTIFICATE OF THE GUIDE
This is to certify that this dissertation work titled “STUDY OF
OCULAR MOTOR NERVE PALSIES IN DIABETES MELLITUS” of the
candidate DR.V.SARANYA with registration number 221613012 for the
award of MS in the branch of OPHTHALMOLOGY.
I personally verified the urkund.com website for the purpose of
plagiarism Check. I found that the uploaded thesis file contains from
introduction to conclusion pages and result shows 0% percentage of plagiarism
in the dissertation.
CONTENTS
S.No. Title Page No.
PART 1
1 INTRODUCTION AND REVIEW OF
LITERATUREND REVIEW OF
LITERATURE
2 DEFINITION AND CLASSIFICATION
3 BIOCHEMICAL MECHANISM OF
DIABETIC TISSUE DAMAGE
4 OCULAR MANIFESTATIONS OF
DIABETES MELLITUS
5 OCULOMOTOR NERVE
6 TROCHLEAR NERVE
7 ABDUCENS NERVE
8 FEATURES OF OCULAR MOTOR NERVE
PALSY IN DM
9 TREATMENT OF OCULAR MOTOR
NERVE PALY
PART 2
10 AIM OF THE STUDY
11 MATERIALS AND METHODS
12 RESULTS AND ANALYSIS
13 DISCUSSION
14 CONCLUSION
PART 3
15 BIBLIOGRAPHY
16 PROFORMA
17 KEY TO MASTER CHART
18 MASTER CHART
ABBREVIATIONS
DM - Diabetes mellitus SOF - Superior orbital fissure
AGEs - Advanced glycation end products VEGF - Vascular endothelium growth factor NPDR - Non proliferative diabetic retinopathy PDR - Proliferative diabetic retinopathy NAD - No abnormal deviations
INTRODUCTION
A perfect alignment between the motor system of two eyes is responsible
for viewing an object as single. The extraocular muscles of both eyes work in
co-ordination. When any one or more of these falter, it may manifest as double
vision, drooping of eyelids, deviation of eyes or sometimes with pain. Patients
may present to the ophthalmologist for one of these complaints, may be
referred by another physician or be seen accidentaly while they come for a
routine checkup. This may be one of the first manifestation of a multisystem
REVIEW OF LITERATURE
The best early evidence of description of diabetes in worlds literature is
recorded in EbersPapyres dated 1550 BC. Arateus of Cappadocia coined the
term `diabetes’ meaning “siphon”, to explain the liquefaction of flesh and bone
into urine
In 400B.C. Susrata , an indian surgeon had described the diabetic
syndrome as characterised by a “honeyed urine”.
Diabetic retinopathy was first described in 1869 by Eduard von
Jauger,and specific lesions like –“Microaneurysms and new vessels”-were
described by Stephan Mackenzie and Edward Nettleship in 1880.
In 1964 ,Marchal de calvi associated neuropathy with diabetes and
symptoms were clearly reported by Frederide pavy in1885.Albuminuria was
DEFINITION AND
DEFINITION
Diabetes mellitus is defined as a group of metabolic diseases
characterized by hyperglycaemia resulting from defects in insulin secretion,
insulin action or both. The chronic hyperglycaemia is associated with
long-term damage, dysfunction and failure of various organs, especially the eyes,
kidney, nerves, heart and blood vessels.
CLASSIFICATION OF DIABETES MELLITUS
Type 1 (β-cell destruction usually leading to absolute insulin deficiency )
Autoimmune
Idiopathic
Type 2
Ranges from predominantly insulin- resistant with relative insulin
deficiency to a predominantly insulin - secretory defect with or without insulin
Other specific types
Genetic defects β – cell function
Genetic defects of insulin action
Diseases of exocrine pancreas
Endocrinopathies
Drug induced or chemical – induced
Infections
Uncommon form of immune mediated diabetes
Other genetic syndromes sometimes associated with diabetes
BIOCHEMICAL MECHANISMS OF
BIOCHEMICAL MECHANISMS OF DIABETES TISSUE DAMAGE
• Chronic tissue damage in diabetes is generally related to the severity and
duration of hyperglycaemia. Other determinants of specific complication
include genetic predisposition and hypertension. Tissue damage may
continue to evolve even after hyperglycaemia has been improved
(hyperglycaemic memory).
• Diabetes particularly affects tissue in which glucose uptake increases
during hyperglycaemia, leading to raised intracellular glucose
concentration. High glucose levels may cause cumulative and
progressive tissue damage through irreversible alteration of structural
protein and other long-lived molecules, or (e.g.in the retina) through the
summation of micro vascular occlusion.
• At cellular level, hyperglycaemia may damage tissues by enhanced
glucose flux through the polyol pathway; formation of advanced
glycation end-product (AGEs); activation of protein kinase (PKC); and
stimulation of the hexosamine pathway. All these mechanism may stem
ultimately from overproduction of superoxide by mitochondria, which
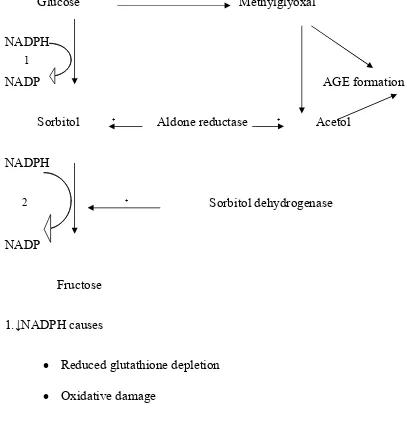
• The polyol pathway, whose rate-limiting enzyme is aldose reductase,
reduce glucose and several other sugars (e.g. glucose to sorbitol). The
pathway operates in tissues that express aldose reductase (e.g. lens,
retina, endothelium), particularly during hyperglycaemia. Cellular
damage may result from enhanced production of glycating sugars (e.g.
methyglyoxal) and thus AGE formation, and/or depletion of reduced
glutathione (a scavenger of reactive oxygen species ,ROSs),resulting in
oxidative damage.
Normal glucose
↓
Normal intracellular glucose
↙ ↘
Glucokinase Aldose reductase
(low Km high affinity) (high Km low affinity)
↓ ↓
Glycolytic > polyol
Pathway pathway
Fig 1 a : The Polyol pathway
The pathway is normally inactive ,but becomes active when intracellular
Glucose Methylglyoxal
NADPH
NADP AGE formation
Sorbitol ₊ Aldone reductase ₊ Acetol
NADPH
₊ Sorbitol dehydrogenase
NADP
Fructose
1.↓NADPH causes
• Reduced glutathione depletion
• Oxidative damage
2.↑NADH/NAD⁺ causes
• Increase Triose Phosphates(AGE formation)
[image:20.595.103.510.118.543.2]• PKC activation
Fig.1 b: Polyol Pathway
1
Consequences of increased glucose flux through polyol pathway
includes the generation of powerful glycating sugars (methyglyoxal, acetol and
triose phosphates), enhanced oxidative damage protein kinase C (PKC)
activation.
• AGEs are the irreversible products of proteins that react with glycating
sugars such as 3- deoxy glucosone, methyglyoxal and the relatively
weak glucose. Covalent cross-linking and other changes damage
structural proteins (e.g.collagen) and extracellular matrix components,
while circulating AGE-modified proteins bind to specific receptors for
advanced glycation endproducts (RAGEs) on macrophages and
endothelial cells. Macrophages release proinflammatory cytokines,
while endothelial cells express procoagulant and adhesion proteins that
favour thrombosis and ultimately atheroma formation; endothelial
expression of vascular endothelium-derived growth factor (VEGF) also
Glucose Early glycation
₊ products
Protein
↕ Advanced glycation
Schiff base end products
↕
Amadori product
↓ Electrophilic pyrolle 3 deoxy glucosone intermediates
Fig.2:
Formation of reversible, early, non-enzymatic glycation products and of
irreversible advanced glycation and products (AGEs). Through a complex
series of chemical reaction, amadori products can form families of imidazole
based and pyrrole – based glucose derived cross-link.
[image:22.595.100.507.96.559.2]PKC isoforms (especially β and δ) are activated by diacyl glycerol (DAG),
synthesized de-novo from increased intracellular glucose. This may decrease
tissue blood flow (by inhibiting production of the potent vasodilator, nitric
oxide) and enhance vascular permeability via increased VEGF expression,
induction of plasminogen activator inhabitor – 1 (PAI-1) expression may
favour thrombosis.
Fig.3
Glucose
flux
Diacylglycerol
Protein
kinase
C
activity
Hyperglycemia
Permeability vasoactive harmones basement membrane
VEGF Blood flow changes synthesis
[image:23.595.114.549.277.731.2]Activation of protein kinase – C by de-novo synthesis of diacylglycerol,
following increased glucose utilization.
• High intracellular glucose levels result in increased production of
glucosamine,which by leading to glycation of transcription factors
(forming their O-Glc Nacylated derivatives) may enhance transcription
of specific genes including PAI – 1 and transforming growth factor β1
Fig. 4 The Glucosamine pathway
Glucosamine-6phosphate,generated from fructose-6 phosphate and
glutamine(gln) ,is converted to UDP-N acetyl glucosamine, which can glycate
transcription factors and thus enhance transcription of genes including PAI-1
Extra cellular
Cystosol
Nucleus
UDP ‐ Glc NAc
Transcription PAI‐1 TGF‐ β₁
Glucosamine 6 phosphate AZA Glu
Fructose 6 phosphate
Gln
Glucose 6 phosphate Glucose
Glycation of transcription factors – o – Glc NAc
and TGF β₁ Glutamine:fructose-6 phosphate amidotransferase(GFAT),the rate -
limiting enzyme is inhibited by azaserine(AZA).
Excess mitochondrial production of the ROS, superoxide, may cause all
the above abnormalities. Superoxide production via the tricarboxylic acid(
TCA) cycle is increased by hyperglycaemia; consequences include
stimulation of aldose reductase activity, enhanced formation of methylglyoxal
and thus AGE, increased DAG synthesis and PKC activation, and hexosamine
OCULAR MANIFESTATIONS OF DIABETES MELLITUS
S. No STRUCTURE MANIFESTATIONS
EXTRAOCULAR
1 LIDS Ptosis, xanthelasma, chronic blepharitis,
Recurrent stye & chalazion 2 EXTRAOCULAR
MUSCLES
Mononeuropathy
3 LACRIMAL APPARATUS
Decreased tear secretion, increased tear glucose levels
4 ORBIT Orbitorhinomucormycosis
OCULAR
1 CORNEA Corneal hypoesthesia, Recurrent erosions,
corneal abrasions, punctate keratopathy, neurotrophic keratitis
2 IRIS AND PUPIL Rubeosis iridis, small pupil, neovascular
glaucoma 3 ANGLE
STRUCTURES
Open angle glaucoma, secondary glaucoma
4 LENS Fluctuating myopia, snowflake cataract,
senile cataract
5 VITREOUS Posterior vitreous detachment, asteroid
bodies
6 RETINA Diabetic retinopathy, lipaemia retinalis,
decreased contrast sensitivity and colour vision
7 OPTIC NERVE Anterior ischemic optic neuropathy, optic
atrophy
These are the associated findings in diabetes. We can look for them ,so that
EARLY TREATMENT DIABETIC RETINOPATHY STUDY (ETDRS)
CLASSIFICATION OF DIABETIC RETINOPATHY
NON PROLIFERATIVE DIABETIC RETINOPATHY(NPDR)
1.MILD NPDR Any or all of microaneurysms,
retinalhaemorrhages, exudates, cotton wool spots, upto the level of moderate NPDR
2.MODERATE NPDR Severe retinal haemorrhages: about 20
medium-large per quadrant in 1-3 quadrants or mild IRMA
Significant venous beading can be present in no more than one quadrant
Cotton wool spots commonly present
3.SEVERE NPDR The4-2-1 rule;one or more of:
• Severe haemorrhages in all 4
quadrants
• Significant venous beading in 2 or more quadrants
• Moderate IRMA in 1 or more
quadrants
PROLIFERATIVE DIABETIC RETINOPATHY (PDR)
1.EARLY PDR New vessels on the disc (NVD) or new
vessels elsewhere(NVE) but extent insufficient to meet the high risk criteria
2.HIGH RISK PDR NVD about one 1/3 disc area
OCULOMOTOR NERVE
ANATOMY
The third cranial nerve is entirely motor in function. It supplies all the
extraocular muscles of the eyeball except the lateral rectus and superior
oblique. It also supplies the intraocular muscles namely the sphincter pupillae
and the ciliary muscles.
FUNCTIONAL COMPONENTS
1.SOMATIC EFFERENT- concerned with movements of the eyeball.
2.GENERAL VISCERAL EFFERENT(parasympathetic)- for accommodation
and constriction of the pupil.
3.GENERAL SOMATIC AFFERENT- for carrying proprioceptive impulses
from the muscles supplied by the third nerve.
THE OCULOMOTOR NUCLEAR COMPLEX
LOCATION
It is situated in the midbrain at the level of the superior colliculus in the
ventromedial part of the central grey matter that surrounds the cerebral
It is a longitudinal column, 10mm long extending above from the floor of the
third ventricle and below it is related to the nucleus of the fourth nerve. There
are two motor nuclei.
1.Main motor nucleus of the large multipolar neurons.
2.Accessory Edinger Westpal nucleus of small multipolar neurons.
The main motor nucleus has the following subnuclei:
1.DORSOLATERAL NUCLEUS-supplies ipsilateral inferior rectus
2.INTERMEDIATE NUCLEUS-supplies ipsilateral inferior oblique
3.VENTROMEDIAL NUCLEUS-supplies ipsilateral medial rectus
4.PARAMEDIAL NUCLEUS-supplies contralateral superior rectus
5.CAUDAL CENTRAL NUCLEUS-supplies bilateral levator palpebrae
superioris.
The Edinger Westphal nucleus lies posterior to the main occulomotor
nuclear mass.It consists of a median and two lateral parts.It gives rise to
CONNECTIONS OF THE NUCLEUS
1.Cerebral cortex
• Motor cortex of both sides through the corticonuclear tracts
• Visual cortex through the superior colliculus
• Frontal eye field
2.Nuclei of 4,6 and8 cranial nerves through the medial longitudinal fasciculus.
3.Pretectal nucleus of both sides
4. Vertical and torsional gaze centres.
5. Cerebellum through the vestibular nuclei.
COURSE AND DISTRIBUTION
It can be divided into four parts
1. The fascicular part
2. The basilar part
3. The intracavernous part
THE FASCICULAR PART
It consist of efferent fibres that pass from the third nerve nucleus
through the red nucleus and the medical and small lateral root, which unite to
from a flattered nerve, which then gets twisted bringing the inferior fibres
superiorly and vice versa. Thus the nerve becomes a rounded cord. The nerve
then passes between the posterior cerebral and superior cerebellar arteries.
Then it runs forward in the interpeduncular cistern (running lateral and parallel
to the posterior communicating artery) to reach the cavernous sinus.
THE INTRACAVERNOUS PART
The nerve enters the cavernous sinus by piercing the posterior part of its
roof on the lateral side of the posterior clinoid process. It then descends on the
lateral wallsinus, where it lies above the trochlear nerve. In the anterior part of
the cavernous sinus, the nerve divides into superior and inferior divisions
which enter the orbit through the middle part of the superior orbital fissure
within the annulus of Zinn.
THE INTRAORBITAL PART
In the orbit the smaller superior divisions ascends on the lateral side of
the optic nerve and supplies the superior rectus and levator palpebrae
i. Nerve to medial rectus passes inferior to the optic nerve.
ii. Nerve to inferior olique (longest of the three branches) passes in
between the inferior rectus and lateral rectus and supplies the
oblique from its posterior border. It gives off the motor root to
the ciliary ganglion.
iii. Nerve to inferior rectus passes and enters the muscle on its upper
aspect.
THE FEATURES OF THIRD NERVE PALSY
It may be complete or incomplete and it may be congenital or acquired.
1. Ptosis-due to paralysis of LPS.
2. Deviation –eyeball is turned down, out and slightly intorted due to
unopposed action of the lateral rectus and the superior oblique.
3. Ocular movements-restriction of the following movements.
• Adduction –due to paralysis of medial rectus
• Elevation –due to paralysis of superior rectus and inferior oblique
• Depression- due to paralysis of inferior rectus
• Extortion-due to paralysis of inferior rectus and inferior oblique
4. Pupil-is fixed and dilated due to paralysis of sphincter pupillae.
5. Accommodation – completely lost due to paralysis of ciliary muscle.
6. Crossed diplopia – appears on manually raising the eyelid, which occurs
due to paralytic divergent squint.
7. Head posture – if the papillary area is uncovered the head takes a
posture consistent with the directions of actions of paralysed muscle i.e
head is turned to the opposite side, titled towards the same side and chin
PUPIL SPARING ISOLATED III NERVE PARESIS
The pupillomotor fibres of the III nerve travel in the outer layers of the
nerve and are therefore closer to the nutrient blood supply enveloping the
nerve. The outer fibres are supplied by the pial plexus whereas the inner fibres
are supplied by the vasa nervosum.
So this explains why the diabetics (where the vasa nervonum are
affected) have pupillary sparing in 80% and similarly in any ischaemic vascular
etilogy. In contradiction when compressive lesions involve the III nerve the
superficial fibres are affected resulting in pupillary involvement in 90%.
Most patients with ischaemic III nerve paresis demonstrate improvement
in motility measurements within one month or may have complete recovery by
3 months (maximum : 6 months).
LOCATION OF PUPILLOMOTOR FIBRES WITHIN THE TRUNK OF
Cranial imaging like MR scanning – MRI, MRA, Four vessel angiography and
Lumbar puncture are recommended if:
i. The pupil is involved i.e. dilates or becomes dilated in the initial
5 – 7 days after onset.
ii. No significant improvement in 3 months.
iii. The patient develops signs of aberrant regeneration of III nerve.
iv. Other neurologic findings develop.
ABERRANT REGENERATION OF III NERVE
This is seen after trauma and tumour compression of the III nerve, but
never after an ischaemic III nerve paresis. If the patient is followed with a
presumed diagnosis of ischaemic III nerve palsy and then develops signs of
aberrant regeneration, then MR scanning and cerebral angiography are
TROCHLEAR NERVE
The trochlear nerve is entirely motor in function and supplies only the superior
oblique muscle of the eyeball.
PECULIARITIES
• The only cranial nerve to arise from the dorsal aspect of the brain.
• The only cranial nerve to cross completely to the other side i.e.the
trochlear nerve arises from the contralateral nucleus.
• The longest and thinnest of all cranial nerves.
FUNCTIONAL COMPONENTS
1. SOMATIC EFFERENT- concerned with the primary,secondary and
tertiary actions of superior oblique.
2. GENERAL SOMATIC AFFERENT-carries proprioceptive impulses
from the superior oblique. The impulses are relayed to the mesencephalic
NUCLEUS
Situated in the ventromedial part of the central gray matter of the
midbrain at the level of inferior colliculus. It is continuous with the III
nerve nuclear complex. It belongs to the somatic efferent column of nuclei.
CONNECTIONS OF THE NUCLEUS
1. Cerebral cortex
i. Motor cortex-of both sides through the corticonuclear tracts.
ii. Visual cortex-through the superior colliculus
iii. Frontal eye fields.
2. Nuclei of 3, 6 and 8 cranial nerves through the medial longitudinal
bundle.
3. Superior colliculi through the descending predorsal bundle.
4. Vertical and torsional gaze centres.
5. Cerebellum through the vestibular nuclei.
COURSE AND DISTRIBUTION
It is divided into
i. The fascicular part
ii. The precavernous part
iii. The intracavernous part
TROCHLEAR NERVE
THE FASCICULAR PART
It consists of efferent fibres which after leaving the nucleus ,pass
posteriorly around the aqueduct in the central grey matter and decussate
completely in the anterior medullary velum.
THE PRECAVERNOUS PART
The trochlear nerve trunk emerges from the superior medullary velum
just below the inferior colliculus on the dorsal aspect of midbrain. It then winds
round the superior cerebellar peduncle and the cerebral peduncle just above the
pons.
It runs beneath the free edge of the tentorium, and like the III nerve
passes between the posterior cerebral and superior cerebellar arteries to appear
ventrally lateral to cerebral peduncle. It then pierces the dura on the posterior
corner of the roof of the cavernous sinus to enter into it.
THE INTRACAVERNOUS PART
In the cavernous sinus, the nerve runs forwards in its lateral wall lying
below the III nerve and above the first division of the fifth cranial nerve. In the
anterior part of the cavernous sinus, it rises, crosses over the III nerve and
(where it passes superolateral to the annulus of Zinn and medial to the frontal
nerve).
THE INTRAORBITAL PART
After entering through the lateral part of the superior orbital fissure, the
nerve passes medially above the origin of the LPS and ends by supplying the
superior oblique on its orbital surface.
The number of fibres in the intraorbital part of the trochlear nerve are
greater than its intracranial part. These extra fibres carrying the proprioceptive
impulses from the superior oblique leave the trochlear nerve to join the
ophthalmic division of fifth nerve in the cavernous sinus.
FEATURES OF IV NERVE PALSY
1. Hyperdeviation due to weakness of superior oblique. This becomes
more obvious when the head is titled towards ipsilateral shoulder (Park
Bielchowsky head tilt test).
2. Ocular movements – depression is limited in adduction. Intorsion is also
limited .
4. Abnormal head posture – To avoid diplopia head adopts a posture such
that the action of superior oblique is less needed i.e face is slightly
turned to opposite side, chin is depressed and head is titled towards the
opposite side.
PARK-BIELCHOWSKY’S THREE STEP TEST
The medial and lateral rectus muscles do not have a vertical
action. Therefore hypertropia of paretic etilogy is due to weakness of one or
more of the following vertically acting muscles. If the hypertropia is due to
weakness of only one of these eight muscles, answering the following three
questions identifies the paretic muscle.
1. First step - which is the higher eye?
a) If the patient has a right hypertropia then the weak muscle is
either a depressor of the right eye (right inferior rectus / right
superior oblique) or an elevator of the left eye (left superior
rectus / left inferior oblique).
b) If the patient has left hypertropia then the weak muscle is either
an elevator of the right eye (right superior rectus/right inferior
oblique) or depressor of the left eye (left inferior rectus are left
2. Second step – hypertropia worse on right or left gaze?
The vertical rectus muscles (superior and inferior recti) have their
greatest vertical (and least torsional action) when the eye is abducted. The
oblique muscles (superior and inferior obliques) their greatest vertical action
(and least torsional action) when the eye is adducted.
So in each case.
i. Right hypertropia worse on right gaze (right inferior rectus/left inferior
oblique).
ii. Right hypertropia on left gaze (right superior oblique/left superior
rectus).
iii. Left hypertropia worse on right gaze (left superior oblique/right superior
rectus).
iv. Left hypertropia worse on left gaze (right inferior oblique/left inferior
rectus).
3. Third step - Is the hypertropia worse on head tilt to right or left?
a. The superior muscles (superior rectus and superior oblique) intort
the eye; the inferior muscles (inferior rectus and inferior oblique)
b. When the head is titled to the right, right eye will be intorted by
the contraction of the right superior rectus and right superior
oblique; these two muscles work together in effecting the
intorsion and neutralize each other’s vertical action (right
superior rectus is an elevator and right superior oblique is a
depressor).
c. If one of these muscles is the paretic muscle responsible for the
hypertropia, then the vertical action will not be neutralized and
the hypertropia will be worse on tilting the head to the right
ABDUCENS NERVE
It is an entirely motor nerve that supplies lateral rectus muscles of the
eyeball.
FUNCTIONAL COMPONENTS
i. SOMATIC EFFERENT – for lateral movement of the eye.
ii. GENERAL SOMATIC AFFERENT for proprioceptive impulses
from the lateral rectus muscle. These impulses ultimately reach
the mesencephalic nucleus of the trigeminal nerve.
NUCLEUS
Situated in the lower part of pons, close to the midline beneath the floor
of the IV ventricle. It is closely related to the fasciculus of the facial nerve. It
consists of two types of multipolar cells-large and small. The large multipolar
cells give rise to fibres of the abducens nerve, while the fibres of the small
multipolar cells relay in the oculomotor nucleus via the medial longitudinal
fasciculus. The small multipolar cells are believed to from the paraabducens
nucleus. Since the abducens nucleus belongs to the group of somatic efferent
nuclei, it lies in line with the nuclei of IV and III nerves above and the
CONNECTIONS OF THE NUCLEUS
1. Cerebral cortex
i. Motor cortex (precentral gyrus) through the afferent
corticonuclear fibres from both cerebral hemispheres.
ii. Visual cortex, through the superior colliculus.
iii. Frontal eye fields.
2. Nuclei of III, IV and VIII cranial nerves through the medial
longitudinal bundle.
3. Pretectal nucleus of both sides.
4. Horizontal gaze centre through the medial longitudinal bundle.
5. Cerebellum through vestibular nuclei.
COURSES AND DISTRIBUTION
It is divided into
i. The fascicular part
ii. The basilar part
iii. The intracavernous part and
THE FASCICULAR PART
It consists of efferent fibres which start from the nucleus, pass forward
traversing the medial lemniscus and pyramidal tract. These then emerge by 7-8
rootless from the junction of pons and medulla just lateral to the pyramidal
prominence of medulla. The rootlets join to form one nerve, at varying
distances from the origin.
THE BASILAR PART
The nerve then runs forwards, upwards and slightly laterally through the
cisterna pontis between the pons and the clivus. The nerve then runs upwards
on the back of petrous temporal bone near its apex. At the sharp upper border
of the petrous bone, the nerve bends forward at right angles under the
petrosphenoidal ligament through the Dorello’s canal and enters the cavernous
sinus by piercing its posterior wall at a point lateral to the dorsum sellae and
superior to the apex of petrous temporal bone.
THE INTRACAVERNOUS PART
In the cavernous sinus, the nerve runs horizontally forward, occupying a
position below and lateral to the internal carotid artery. The internal carotid
fissure through the annulus of Zinn. In the superior orbital fissure, the abducens
nerve lies inferolateral to the oculomotor and nasociliary nerves.
THE INTRAORBITAL PART
In the orbit the nerve runs forward and enters the ocular surface of the
lateral rectus muscle just behind its middle portion after dividing into three or
four branches.
CLINICAL FEATURES OF SIXTH NERVE PALSY
1. Deviation – In the primary position, the eyeball in convergent due to
unopposed action of the medial rectus muscle.
2. Ocular movements – Abduction is restricted.
3. Diplopia – Uncrossed horizontal diplopia occurs,
Worse towards the action of the paralysed muscle.
4. Head posture – The face is turned towards the action of the paralysed
LATERAL VIEW OF THE COURSE OF SIXTH NERVE
LOCATION OF CRANIAL NERVES IN THE CAVERNOUS
FEATURES OF OCULAR MOTOR NERVE
PALSIES IN DIABETES
• III nerve commonly affected
• More common in eldery
• Pupillary sparing
Because the peripherally situated papillary fibres supplied by the pial
plexus are spared whereas the centrally located fibres supplied by vasa
nervorum are affected.
• Usually recover spontaneously and completely in months.
• Can manifest as multiple episodes of transient ophthalmoplegia
affecting different muscles of either one or both eyes.
DIFFERENT DIAGNOSIS OF PAIN LESS OPHTHALMOPLEGIA
¾ Diabetes
¾ Hypertension
¾ Atherosclerosis
¾ Weber’s syndrome
¾ Tumours of orbit
DIFFERENT DIAGNOSIS OF PAINFUL OPHTHALMOPLEGIA
¾ Cavernous sinus thrombosis
¾ Tolosa Hunt syndrome
¾ Mucormyosis
¾ Nasopharyngeal carcinoma
¾ Herpes Zoster
TREATMENT OF OCULAR MOTOR NERVE PALSIES
Management of Diabetes mellitus mainly consists of
1. Life style modification
2. Tight glycemic control with insulin
Follow up of cases of ocular motor nerve palsy that do not need urgent
management, like the posterior communicating artery aneurysm must be at 6
weekly intervals till 6 months or till two consecutive 6 weeks follow-ups reveal
no change in motility. Every time diplopia charting, Hess charting, recording
of deviations in nine gazes is done. During the meantime, patient is greatly
disturbed by diplopia. So some nonsurgical modalities are practiced for
symptomatic relief. It no resolution occurs after about 8-12 months then
surgery is considered.
1. Prisms – are helpful in providing binocular vision as well as reducing
the changes of development of contracture, but are useful only in small
angle squints. Fresnel prisms are also used.
2. Botulinum toxin – the ipsilateral antagonist is paralysed by
chemodenervation. The effect lasts for about 2-3 months. If necessary
3. Occlusive prisms or opaque contact lens.
4. Surgery – mainly to weaken the antagonist, usually ipsilateral and
sometimes also the contralateral antagonist, in addition to
strengthening the paralysed muscle. The amount of recession
resection varies depending upon which eye habitually fixates
(secondary deviation or primary deviation needs to be corrected).
Another principle is to restrain the contralateral antagonist by
performing retroequatorial myopexy.
In the case of III nerve, the aim is to achieve diplopia free ocular
position in primary position and downgaze. The latter should never be
compromised for the upgaze. Anyway it is difficult because the III nerve
supplies most of the extraocular muscles except two. Moreover aberrant
regenerations alter the clinical picture. Each case has to be considered on an
individual basis.
In the case of IV nerve, either strengthening of superior oblique or
weakening of ipsilateral inferior oblique or contralateral inferior rectus is done.
The results of surgery for both congenital and acquired IV nerve palsy is
AIM OF THE STUDY
1. To study the ocular motor nerve palsy pattern in diabetes mellitus
2. To study the correlation of glycemic status in ocular motor nerve palsies
3. To study the association of diabetic retinopathy in case of ocular motor
nerve palsies.
MATERIALS AND
MATERIALS AND METHODS
The cases studied, included those patients with neurogenic ocular motor
nerve palsies who presented to the regional institute of ophthalmology and
Govt. ophthalmic hospital. All age groups and both sexes were included.
A complete ophthalmological workup was done.
INCLUSION CRITERIA
All infranuclear ocular motor nerve palsies with DM
EXCLUSION CRITERIA
All supranuclear, nuclear nerve palsies, myogenic and restrictive
REGISTRATION
Name
Age
Sex
Occupation
Address
HISTORY OF PRESENT ILLNESS
The common complaints were:
a. Double vision-whether uniocular /binocular, constant/intermittent,
fluctuating or not, more for near or distance, whether images were
horizontally or vertically separated, whether it is increased on any
particular direction, onset and progress.
b. Pain – headache / periorbital pain, location, nature, any radiation,
aggravating and relieving factors, any association with
nausea/vomiting.
c. Drooping of lids- unilateral/bilateral, total/partial
d. Defective vision- apart from double vision, any blurring or inability
to see due to drooping of lid.
e. Deviation of eyeball-right/left, eye, duration
g. Vertigo (sensation of rotation of self/surroundings)
Details of the progress from onset, the treatment undergone to the
present state is noted. Any other significant medical/surgical history is also
recorded.
PAST HISTORY
H/o diabetes, hypertension, tuberculosis, syphilis, AIDS, malignancy in the present or past.
H/o migraine or neurologic disease H/o xanthems and vaccination
PERSONAL HISTORY
Diabetes, smoking, alcoholism etc.
GENERAL EXAMINATION
General vital data like pulse, blood pressure, peripheral pulses are noted.
OCULAR EXAMINATION
• Head posture, facial symmetry are noted.
• Any deviation of eyeball is recorded. Under slit lamp, details of the
anterior segment from the lids to the lens are noted.
• Extraocular movements are noted down-both ductions and versions.
While looking for EOM, the aberrant innervation patterns are also
looked for.
• Pupil size, reaction, any anisocoria is noted.
• A dialted fundus examination and refraction is done. Ptosis and
proptosis if present are evaluated.
• Diplopia charting- is done in a dark room. Patient is asked to wear
goggles with red in front of the right eye and green before the left eye. A
torch light with a stenopaeic slit is used. The patient is asked to look at
the torch held 120cm away and then the torch is moved to various
positions. The false image is usually the fainter and farther one. Any tilt
of the image and variation in the distance between images at various
position is asked for.
• If a superior oblique palsy is suspected, Parks Bielchowsky’s 3 step
head tilt test is done.
• A forced duction test is performed in doubtful cases to rule out
restrictive etiology.
NEUROLOGIC EXAMINATION
Examination of other cranial nerves, Motor, sensory, cerebellar
symptoms and signs.
EXAMINATION OF THYROID
Any neck swelling is looked for
EXAMINATION OF SPINE & BACK
To look for congenital anomalies and neurocutaneous markers.
INVESTIGATIONS
Both right and left eyes (for all cases)
1. Vision a. uncorrected ( using Snellen’s charts at 6 metres)
b. best corrected.
2. Intra ocular pressure-with applanation tonometer after topical
anaesthesia.
3. Detailed slit lamp examination
o Lid
o Conjunctiva
o Cornea
o Iris
o Pupil
o Anterior chamber
o Lens/Pseudophakia/Aphakia
4. Fundus examination-any abnormalities, diabetic retinopathy etc.
5. Diplopia charting
6. Park 3 step test
7. Measurement of deviation-primary & secondary deviation, cover
uncover test in various gaze positions, for near and distance as well.
8. Hess charting
9. Exophthalmometry
BLOOD TEST: (For all cases)
¾ Total count
¾ Differential count
¾ Erythrocyte sedimentation rate
¾ Blood sugar – Fasting, Post prandial
¾ In doubtful cases, Glucose tolerance test/Hb A1c
¾ Mantoux intradermal test
¾ Serum cholesterol
¾ Blood VDRL
¾ Rheumatoid factor
RADIOLOGY (in indicated cases)
¾ X ray orbit – fractures/erosions
¾ X ray skull
¾ X ray chest – tuberculosis
¾ X ray PNS – (paranasal sinuses) – mucocoele, antral growth, sinusitis, orbit floor fractures.
¾ ORBITAL USG – (in indicated cases)
¾ NEURO IMAGING – (in indicated cases)
• CT
• MRI
• MRA
SPECIALIST OPINION (IN INDICATED CASES)
o Diabetologist
o Otorhinolaryngologist
o Neurophysician/Neurosurgeon
o Radiologist
FOLLOW UP
Recording of patient’s complaints-whether stable/improving/worsening.
‐ Vision
‐ Pupil assessment
‐ Extraocular movements
‐ Diplopia charting
‐ Fundus
‐ Examining for signs of papillary involvement or aberrant
regeneration in case of third nerve palsy
‐ Investigations
Blood sugar FBS and PPBS
Hb A1c
BP
Imaging studies, if necessary
RESULTS AND ANALYSIS
Table1:Mean of age distribution and glycemic status
The mean age of the study population was 55.34 with a standard
deviation of 8.92. The mean fasting blood sugar value of the study population
was 145.84 mg% with a mean HbA₁C of 7.73.
Variable Obs Mean Std. Dev. Min Max
Age 50 55.34 8.92 24 70
FBS 50 145.84 47.01 60 322
PPBS 50 219.20 72.49 110 486
58%
with nerve
% of the m
e palsy .In t
58 Wome Men Total SE Tab male patien
this study , SEX
en
EX DISTR
ble2:sex di
Chart1
nts and 4
, males we
Gend
F
RIBUTION
istribution
1:sex distr
42% of fem
ere more af
der
Freq. 21 29 50 N n ribution male patie ffected tha 42 Percen 42 58 100ents were a
an females.
Women
Men
nt
62%
in right ey
1 2 3 4 5 6 7 Tabl Char
% of the pa
ye. In this s
0 10 20 30 40 50 60 70 LAT LE RE Total le3:lateral rt2: lateral atients had tudy most LE TERALIT LATE
lity of ocul
lity of ocu
nerve pals patients ha
Latera
TY ERALITY lar motor ular motorsy in left ey
ad left eye
RE
ality
Freq. 31 19 50 nerve pals nerve palye and 38%
preponder Percen 62 38 100 sy lsy
% had nerv
In patients pr patients pr D Dr PRES COM Diplopia Droopin Total Chart3 this study resented w resented wi 0 Diplopia ooping SENTING MPLAINT a ng Table4 : 3: Distribu
64% of th
with droopin ith diplopia 20 COMPLA TS Distributi
ution of p
he patients
ng of the u
a. 40
Compla
AINTS OF Freq. 32 18 50ion of pre
resenting
s presented
upper eyel
60
aints
F NERVE
Percen 64 36 100 esenting co complaint
d with dipl
id. In this
80 E PALSY nt omplaints ts lopia. 36% study maj Diplopia Drooping
% of the
ority of
In t
and 16%
post PRP s
F FUN NAD MILD NP MOD NPD POST PRP HTR Total Table5 Chart
this study 5
have mod status.4% p 0 10 20 30 40 50 60 NAD FUNDUS I NDUS PDR DR P 5:Fundus t4: Fundus 52% patien erate NPD patients ha
D MILD
NPDR
IN DIABE
F
findings i
s findings
nts does no
DR and mi
d hyperten MOD NPDR P
FUN
ETES MEL Freq. 26 13 8 1 2 50in nerve pa
in nerve p
t have asso
ild NPDR
nsive retino
OST PRP H
NDUS
LLITUS Perce 52 26 16 2 4 100 alsy patien palsy patieociated ret
respective opathy. HTR ent 2 6 6 0 nts ents tinopathy. ely.2% pati NAD
[image:76.595.148.506.92.600.2]III
VI
III
To
In o
by VI ner
palsies. No 1 2 3 4 5 6 DI Nerve P NERVE I NERVE
& VI NER
otal
C
our study 4
rve palsy
one of the p
0 10 20 30 40 50 60
III NER
ISTRIBUT
Palsies
RVES
Tab
Chart5: D
40% of pa
and two p
patient is a
RVE VI N
TION OF ble6: Distr Distribution atients affec percent aff affected by
NERVE III &
Nerve
P
NERVE P Freq. 20 29 1 50 ribution of
n of nerve
cted by III
fected by
y IV nerve p
& VI NERVES
Palsies
PALSIES
Pe
1
f nerve pal
e palsies
I Nerve pa
both third
palsy in ou
V ercent 40 58 2 100 lsies
alsy, 58% a
d and sixth
ur study.
III NERVE VI NERVE
III & VI NERVE
affected
h nerve
It h complete r showed no ful No pa To
has been f
recovery o
o signs of r
[image:78.595.181.544.356.578.2]0 10 20 30 40 50 60 DISTRIB RECOV ll o recovery artial otal Table7: Chart 6: found out,
of the third
recovery. full BUTION O VERY Distribut : Distribut
, on follow
nerve pals par OF RECO Freq 30 5 15 50
ion of reco
tion of rec
w up, tha
sy, 30% ha
rtial No
Recove
VERY q. overy coveryat 60% of
ad partial r
o recovery
ery
Percent 60 10 30 100 the patien recovery an f p N nts had nd 10% full partialH <7 7.1 >9 To I HbA1C F
1 - 9.0 .1
otal 2
[image:79.595.102.539.146.492.2]In o than 7%, than 9.1% 7%, 48.28 than 9.1% with both 9.0%. 0 20 40 60 80 100 H III NERVE Freq P 7 8 5 20 Table8: Chart7: our study,
40% had H
%. 44.83%
8% had Hb
%. One amo
third and
III NERVE P
HbA1C LE E PALSY Percent 35 40 25 100 Distributi Distributi
35% of pa
HbA₁c bet
of patients
bA₁c betwe
ong the stu
sixth nerv
PALSY V
HbA1
EVEL IN N
VI NERV Freq 13 14 2 29
ion of HbA
ion of HbA
atients with
tween 7.1%
s with sixth
een 7.1% a
udy popula
ve palsies h
VI NERVE PALS
1C
in
Ne
NERVE P VE PALSY Percent 44.83 48.28 6.90 100
A1C level i
A1C level
h third ner
% and 9%
h nerve pa
and 9% an
ation , 2%
had a HbA
SY III & VI N
erve
Pal
ALSIES
Y III & Freq
0 1 0 1
in nerve p
in nerve p
rve palsy h
and 25%
alsy had H
nd 6.9% ha
% of patient
A₁c value b
NERVE PALSY
lsies
VI NERVE Per 0 10 0 10 palsies palsieshad HbA₁c
had HbA₁
HbA₁c of le
ad HbA₁c o
ts, who pr
between 7. E PALSY cent 0 00 0 00 of less
₁c more
ess than
of more
resented
1% and
<7
GLYCEMIC STATUS Vs RECOVERY
Full Recovery Partial Recovery No Recovery
Variable Mean Std. Dev. Mean Std. Dev. Mean Std. Dev.
N 30 15 5
Fbs 135.30 36.55 145.33 43.56 210.60 68.37
Ppbs 202.87 56.95 221.07 66.58 311.60 112.51
[image:80.595.144.486.361.586.2]Hbac 7.44 1.21 8.03 1.29 8.60 1.71
Table8:Glycemic status Vs recovery
Chart7: Glycemic status Vs recovery
0.00 50.00 100.00 150.00 200.00 250.00 300.00 350.00
Full Recovery Partial Recovery No Recovery
Recovery
Vs
Blood
Sugar
Levels
fbs
Chart8:Distribution of mean HbA1C according to recovery
The patients in this study who did not show any signs of recovery
following ocular motor nerve palsies, around 10 %, had a high HbA₁C levels of
8.6%
6.50 7.00 7.50 8.00 8.50 9.00
Full Recovery Partial Recovery No Recovery
Mean
HbA1C
according
to
Recovery
DISTRIBUTION OF MEAN BLOOD SUGARS AND HbA1C LEVELS
IN DIABET DIABETIC RETINOPATHY
Fundus NAD Mild NPDR MOD NPDR POST PRP HTR
Variable Mean Std. Dev. Mean Std. Dev. Mean Std. Dev. Mean Std.
Dev Mean Std. Dev.
N 26 13 8 1 2
Fbs 140.08 43.22 132.85 26.50 162.38 49.04 322.00 0.00 151.00 1.41
Ppbs 212.88 61.33 199.23 48.73 239.63 87.98 486.00 0.00 216.00 5.66
[image:82.595.140.493.410.660.2]Hbac 7.20 1.15 7.95 1.13 8.69 1.25 11.00 0.00 7.75 0.35
Table9: DISTRIBUTION OF MEAN BLOOD SUGARS AND HbA1C
LEVELS IN DIABET DIABETIC RETINOPATHY
Chart9: DISTRIBUTION OF MEAN BLOOD SUGARS IN DIABETIC
RETINOPATHY 0.00 100.00 200.00 300.00 400.00 500.00 600.00
NAD Mild NPDR MOD NPDR POST PRP HTR
Mean
Blood
Sugars
in
Diabetic
Retinopathy
fbs
Chart10: DISTRIBUTION OF MEAN HbA1C LEVELS IN DIABETIC
RETINOPATHY
In this study the mean FBS, PPBS and HbA₁C were estimated and their
correlation with the changes in the retina has been assessed. It has been found
that the patients with elevated glycated haemoglobin had more severe diabetic
retinopathic changes.
0.00 2.00 4.00 6.00 8.00 10.00 12.00
NAD Mild NPDR MOD NPDR POST PRP HTR
Mean
HbA1C
in
Diabetic
Retinopathy
DISCUSSION
1. AGE DISTRIBUTION AND GLYCEMIC STATUS
In our study, 50 cases of diabetes mellitus with ocular motor nerve
palsies were examined. Most patients with either third or sixth nerve palsies
were found to belong to 51 to 60 year age group. In a study of 22 cases of third
nerve palsy by Jack. E. Goldstein and David G. Cogan the average age was 62
years. The mean FBS of patients was 145.84mg%, mean PPBS was
219.20mg% and mean HbA₁C was 7.73 in this study. In a study conducted by
Vidhya chandran et al., on 61 diabetic patients with ocular motor nerve palsies,
the mean FBS was 116.61mg%, the mean PPBS was 179.62mg% and the mean
HbA₁C was 7.80.
2. SEX DISTRIBUTION
In our study,males were more affected as compared to female which is also
3. LATERALITY
The most common affected eye in our study was left eye. Bilaterality was
not found in our study. However the laterality has got no statistical significance
with the degree of nerve palsy or recovery from it in our study.
4. PRESENTING COMPLAINT
In our study 64% of the patients presented with complaints of diplopia
and the remaining with drooping of eyelids. Majority of patients had diplopia
in the study conducted by Saber et al on diabetes mellitus associated ocular
motor nerve palsies.
5. GLYCEMIC STATUS AND DIABETIC RETINOPATHIC CHANGES
Glycosylated haemoglobin was taken to assess the glycemic control of
the patient who presented with ocular motor nerve palsies. The American
diabetes association recommends the goal of diabetes therapy should be an
HbA₁c of less than 7%. In our study we analysed the retinal findings of all the
patients and their association with HbA₁c levels. It has been found that patients
who had normal retina had a mean HbA₁c of 7.2%. Those patients who
presented with mild NPDR and moderate NPDR had a mean HbA₁c of 7.95
with proliferative diabetic retinopathy had a HbA₁c of 11%. Thus there seems
to be a positive correlation between the HbA₁c levels and the degree of
retinopathic changes in diabetic patients who primarily presented to us with
ocular motor nerve palsies. These findings have also been observed by study
conducted by Vidhya chandran et al., on significance of HbA₁c in diabetic
ocular motor cranial nerve palsies.
6. OCULAR MOTOR CRANIAL NERVE INVOLVEMENT AND
GLYCEMIC STATUS
In our study involving 50 patients, 20 patients(40%) had third nerve
palsy where as 29patients(58%) presented with sixth nerve palsy. One patient
(2%) among 50 with both the nerve palsies. No one presented with fourth
cranial nerve palsy in our study. The most common nerve involved in a study
conducted on diabetic patients by Saber et al was the third nerve. 35% of
patients with third nerve palsy had HbA₁c of less than 7%, 40% had HbA₁c
between 7.1% and 9% and 25% had HbA₁c more than 9.1%. 44.83% of
patients with sixth nerve palsy had HbA₁c of less than 7%, 48.28% had HbA₁c
between 7.1% and 9% and 6.9% had HbA₁c of more than 9.1%. One among the
study population , 2% of patients, who presented with both third and sixth
7. RECOVERY OF NERVE PALSIES
Upon follow up of patients included in this study, 60% of the population
had complete recovery of nerve palsies who had a mean HbA₁c value of
7.44%. 30% of the population had partial recovery from ocular motor nerve
palsies who had a mean HbA₁c value of 8.03%. 10 % of the population showed
CONCLUSION
• The diabetic ocular motor nerve palsies occur in a wide range of age but
are more common in the age group of 51 to 60 yrs with mean age of
55.34 years.
• The males were more affected than females in our study
• Our study has shown more of left eye involvement
• The patient population presented more frequently with diplopia in our
study which was due to a slightly more common involvement of sixth
cranial nerve.
• Fourth nerve palsy associated with diabetes mellitus is less frequent than
third and sixth nerve involvements and no patient in our study presented
with fourth cranial nerve palsy.
• It has been found that the involvement of ocular motor cranial nerves in
diabetic patients has been associated with the glycemic status.
• There was a positive correlation between HbA₁c values and degree of
retinopathic changes in diabetes mellitus.
• Ninety percent of patients in our group had either a complete or partial
BIBLIOGRAPHY
1. Ashburg AK, Aldedge H, Hershberg R, Ficher CM, Oculomotor palsy in
diabetes mellitus a clinic – pathological study. Brain 1970; 93: 555-66.
2. Leslie RDG, Ellis C. clinical course following diabetic ocular palsy.
Post grad Med J 1978; 54 : 791 – 2.
3. Rucker CW. Paralysis of the third, fourth and sixth cranial nerves. Am I
Ophthalmol 1958; 46:787-794.
4. Acaroglu G, Akinci A, Zilelioglu O. Retinopathy in patients with
diabetic ophthalmoplegia. Ophthalmoplegica. 2008;222(4):225-8.
5. Jonathan D. Trobe. Neuro-Ophthalmology. 2007:11.
6. Kaushik U Dhume, Kiruba E Paul, Incidence of pupillary involvement,
course of anisocoria and ophthalmoplegia in diabetic oculomotor nerve
palsy, IJO 2013 Jan; 61(1) : 13-17.
7. Clare M Galtrey, Fred Schon, Arani nitkunan, Microvascular non –
artertic ocular motor nerve palsies – What we know and how should we
treat? Neurophthalmology. 2015 Feb; 39 (1) : 1-11.
8. Shahil Bhandari, Dayakar Yadalla, , Incidence of pupillary involvement,
course of anisocoria and ophthalmoplegia in diabetic oculomotor nerve
palsy, IJO 2013 Sep; 61(9) : 533-534.
9. Vidhya chandran, Neha Dhiware (2018) Significance of HbA₁c in
diabetic ocular motor cranial nerve palsies. J ophthalmol Eye care 1(1):
10. Rucker CW.The cause of paralysis of third, fourth and sixth cranial
nerve. Am J Ophthalmol. 1966; 61: 1293-98.
11. Trobe JD. Third nerve palsy and the pupil. Arch ophthalmol. 1988; 106:
601-2.
12. Jacobson DM. Pupil involvement in patients with diabetes associated
oculomotor nerve palsy. Arch ophthalmol. 1998; 116: 723-7.
13. Richards BW, Jones FR Jr, Younge BR, Causes and prognosis in 4278
cases of paralysis of the oculomotor, trochlear and abducens cranial
nerves. Am J ophthalmol 1992; 113: 489-96.
14. Harley RD. Paralytic strabismus in children Etiologic incidence and
management of the third, fourth and sixth nerve palsies. Ophthalmology
1980; 87: 24-43.
15. Rush JA, Younge BR, Paralysis of cranial nerves 3,4 and 6 causes and
prognosis in 1000 cases. Arch Ophthalmol 1981; 99: 76-79.
16. Siatkowski RM. Third, fourth and sixth nerve palsies In focal points;
Clinical modules for ophthalmologists 1996 ; 14-8.
17. Cox TA, Wurster JB, Godprey WA, Primary aberrant oculomotor
regeneration due to intracranial aneurysm. Arch Neurol 1979; 36: 570-1.
18. Schalz NJ, Sarino PJ, Corbett. JJ. Primary aberrant oculomotor
regeneration . A sign of intracavernous meningioma. Arch Neurol 1977;
34: 29-32.
19. Zorrilla E, Kozak GP. Ophthalmoplegia in diabetes mellitus: Ann intern
20. Weber RD, Daroff RB, Mackay EA. Pathology of oculomotor nerve
palsy in diabetes. Neurology 1970; 20: 835-838.
21. Jackson WPU. Ocular nerve palsy with severe headache in diabetics
BMJ 1955;408-409.
22. Waind APB. Ocular nerve palsy associated with severe headache BMJ
1956; 1.901-902.
23. King KP Paralysis of the extraocular muscles in diabetes. Arch intern
med 1959; 104: 318-322.
24. Eareekson VO Miller JM. Third nerve palsy with sparing of pupil in
diabetes mellitus a subsequent identical lesion of the opposite eye. Arch
Ophthalmol 1952; 47: 607-610.
25. Green WR. Hackett ER, Schizinger N.S. Neurophthlamologic
evaluation of oculomotor nerve palsy paralysis Arch ophthal 1964; 72:
154-167.
26. John K . Davidson. Clincal diabetes mellitus: A problem – oriented
approach. Diabetes. 2000:626.
27. Jack L. Leahy, Clarke NG, William T. Medical management of diabetes
mellitus.2000:483.
28. Aristidis Veves. Clinical management of diabetic neuropathy. 1998:
172.
29. John Dale Ward, Yoshio Goto. Diabetic neuropathy. 1990: 327.
30. Anderson H, Gries FA, Joseph C. Textbook of diabetic neuropathy.
31. Misra Usha Kant, Kalita Jayantee, Nair Pradeep P. Diagnostic approach
to peripheral neuropathy. Annals of Indian academy of Neurology.
2008: 11:2.
32. Bayrak AO et al. A case of bilateral simultaneous sixth cranial nerve
palsies secondary to diabetes mellitus. J Neurophthalmol. 2006.
33. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol
KEY TO MASTER CHART
M-Male
F-Female
RE- Right eye
LE-left eye
FBS- Fasting blood sugar
PPBS-Post prandial blood sugar
NAD- No abnormal diagnosis
MILD NPDR- Mild non proliferative diabetic retinopathy
MOD NPDR-Moderate non proliferative diabetic retinopathy
HTR-hypertensive retinopathy
N –Negative
P-Paralysis
PROFORMA
Name :
Age :
Sex :
Hospital No :
Occupation :
Date :
Complaints :
History:
1.Visual complaints
2.Diplopia
3.Nausea
4.Vomiting
5.Consciousness
6.Gait
7.Sphincter control
8.Weakness
9.Injury
EXAMINATION
Visual acuity :
RIGHT EYE LEFT EYE
VA
UA
Sph Cyl axis VA VA
UA
Sph cyl axis VA
DV
NV
Head position : Head tilt/face turn/chin/other skull Abnormalities
Eyelids : Ptosis, retraction, lid lag, macusgunn Phenomenon
Eye position : Deviation / proptosis
Movements : Uniocular & binocular, conjugate or disconjugate
Nystagmus : Present/absent
Right eye Left eye
Pupils:
Fundus:
Tension:
Central Nervous system
Higher Functions:
Right Left
Cranial nerves:
(1,2,5,7,8,9,10,11,12&3,4,6)
Motor system: tone, power,
wasting, involuntary movements
Sensory system: touch, pain,
temperature, position & vibration
Cerebellar system: co ordination,
tandem walking
Reflexes
Hess Screening, Diplopia charting (Separate Charts Attached)
Lab investigations
Blood routine
TC
DC
ESR
Urine routine
Mantoux
VDRL
Special investigations: (CT,MRI)
Treatment: