Copyright ©D 1994,AmericanSociety for Microbiology
Duck
Hepatitis B Virus Infection of Muscovy Duck
Hepatocytes
and Nature of Virus Resistance In Vivo
JOHN C. PUGH* AND HEIDI SIMMONS FoxChase Cancer Center, Philadelphia, Pennsylvania 19111
Received 1 December 1993/Accepted 17January 1994
To test the hypothesis that in vivo resistance to hepadnavirus infection was due to resistance of host hepatocytes,weisolatedhepatocytesfromMuscovy ducklings and chickens, birds that have been shown to be resistant to duckhepatitis B virus (DHBV) infection, and attempted to infect them in vitro with virus from congenitally infected Pekin ducks. Chicken hepatocyteswere resistant to infection, but we were able to infect approximately 1%of Muscovy duckhepatocytes inculture. Infection requires prolonged incubation with virus at37°C. Virusspread occurs in theMuscovy cultures, resulting in 5 to 10%DHBV-infected hepatocytes by 3 weeks afterinfection. Therelativelylowrate ofaccumulationofDHBV DNA in infectedMuscovy hepatocyte cultures is most likely due toinefficient spread of virus infection; in the absence of virus spread, the rates of DHBVreplication in Pekin and Muscovy hepatocyte cultures are similar.5-Azacytidinetreatment can induce susceptibilityto DHBVinfectionin resistantprimary Pekinhepatocytesbut appears to have no similareffect in Muscovycultures. The relatively inefficient infection of Muscovy duckhepatocytesthat wehave described mayaccount for the absence of a detectable viremia in Muscovy ducklingsexperimentallyinfected with DHBV. Animportantfactor indeterminingwhether a cell becomes
infected withagiven virusis theavailabilityofsuitable host cell receptors to facilitate virus binding and uptake. The impor-tanceof virusreceptors indetermining theveryrestrictedhost andtissuerangedisplayed by hepatitisBvirus (HBV) is largely inferred,as areceptorhasnotbeenidentified foranymember ofthehepadnavirus family. Duck hepatitis Bvirus (DHBV) is the bestcharacterized of the avian members of the hepadna-virus family. The hepadna-viruswasoriginally isolated from the domes-tic Pekin duck (Anas domesticus) but can be successfully transmitted to other closely related ducks and to geese (4). Marion and coworkers have shown that DHBV cannot be readilytransmitted to Muscovy(Cairina moschata) ducks or to chickens, as indicated by the absence of detectable DHBV DNA in the sera and livers of birds challenged with virus during the first few days after hatching (4). The Muscovyis a
species of domesticated duck that is distinct from all other domesticated ducks, which were originally derived from the mallard (Anas
platyrhzynchos).
In this study, we have asked whether resistance of Muscovy ducks and chickens to in vivo infection with DHBVcan beattributed solelytoresistance of hepatocytes toinfection.To determine resistance to DHBV, we isolated primary
hepatocytes from Muscovy ducklings and chickens and in-fected them in vitro with DHBV. We report that primary
hepatocytes isolated from Muscovy ducklings canbe infected with DHBV but that this infection is less efficient and the courseofinfection is retardedascomparedwith thatin Pekin duck hepatocytes. Chicken hepatocytes appear to be
com-pletely resistant to DHBV infection invitro,however. MATERIALSAND METHODS
Experimental animals,virusstocks, and cell culture. Pekin ducks were purchased as 1-day-old ducklings from Metzer Farms, Gonzales, California. Muscovy
ducklings
werepur-*Correspondingauthor. Mailingaddress: Fox Chase Cancer
Cen-ter, 7701 Burholme Ave., Philadelphia, PA 19111. Phone: (215) 728-4780.Fax:(215)728-3574.
chased as 1-day-old ducklings from Hoffman Hatchery, Gratz, Pa. Ducklings were tested for DHBV infection by dot blot hybridization ofserum, and any infected birdswere isolated.
The sourceofDHBV used for infections ofprimary hepa-tocytes was a pool ofsera isolated from 3-week-old ducklings
from
acongenitallyinfected flockmaintained by the Fox Chase Laboratory Animal Facility. The serum was stored in aliquots at -700C.
Primary hepatocytes were prepared from 1- to 2-week-old ducklings by collagenaseperfusion of the liver. Theprocedure
is based upon thatdescribed by Berry and Friend (la) and is described in detail elsewhere (6, 13). Approximately5 x 106
cellswereplated oneach60-mm-diameter dish(4mlofa 1%
[vol/vol] suspension of cells). Cellswere maintained in Liebo-vitz-15 (L-15) medium (Gibco BRL) supplementedwith insu-lin (Sigma), hydrocortisone-hemisuccinate (Sigma), HEPES
(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic
acid), and antibiotics, as previously described (13), but without fetal bovine serum,dimethyl sulfoxide, oradditional sodium bicar-bonateorglucosesupplements unlessotherwise indicated(6).Fresh mediumwas added tocellsevery 1 to 2 days. 5-Azacy-tidine (5-aza)(Sigma)wasaddedtoculturesfrom asterile0.1 Mstock inwater. Suramin
(Bayer)
wasaddedtocellsatafinalconcentrationof 100
[Lg/ml.
Hepatocytes were infected for 16 h at 370C with a 1:5 dilution of DHBV duck serum
(approximately
10i
DHBVDNA-containing particles per ml) in L-15, unless otherwise stated. No attempt was made to remove the great excess of noninfectious surface antigen
particles
from the duck serumused for infections.
Stainingfor DHBVcoreandenvelope
proteins
by immuno-fluorescence. DHBV coreprotein
was detectedby
staining
cells fixed on tissue cultureplastic
withethanol-glacial
acetic acid (95:5), by using aspecific
rabbit antiserum(a
gift
of William Mason)followedbyfluorescein isothiocyanate-conju-gated goat anti-rabbitimmunoglobulin
G(Cappell) (13).
Forstaining of DHBV
envelope
proteins,
cells were fixed in methanol-acetone (1:1) and stained with a mixture of twomouse monoclonal
antibodies,
1H.1 and7C.12,
that arespe-cific for the
pre-S
and S domains of the DHBVenvelope,
2487on November 9, 2019 by guest
http://jvi.asm.org/
1:5
DHBV
1:500
DHBV
Day 8p.i.
FIG. 1. Titration ofinfectivityofaDHBVserumstockon Pekin duckhepatocytes. Primary hepatocyteswereplatedon60-mm dishes and infected2daysafterplatingwith 2 ml ofa1:5or1:500dilutionof duckserumin L-15 mediumfor 16 h at37°C.Infected cultures were maintained
in L-15medium for 8 days and then fixed and stained for DHBVenvelope protein (see Materials and Methods).The bar in therightpanel
representsapproximately 200 urm.p.i.,postinfection.
respectively (5a). Antibody binding was detected by using a
fluoresceinisothiocyanate-conjugated goat anti-mouse immu-noglobulinG(Cappell).CellswerephotographedwithaNikon
Diaphot fluorescencemicroscope.
Assayofinfected hepatocytecultures for DHBV DNA. Total cellular nucleic acids were isolated from infected hepatocyte
culturesasfollows. Afterbriefwashingof themonolayerwith phosphate-buffered saline(PBS), 1 mloflysis buffer(20mM Tris-hydrochloride [pH 7.5],10 mMEDTA,0.2 MNaCl,0.2% sodiumdodecyl sulfate (SDS), 0.5mgofpronase perml)was
addedtoeach 60-mmdish,and cellswereincubated for 1 hat
37°C. Thelysatewas extracted oncewith an equalvolume of
phenol, and nucleic acidswereprecipitated with 2 volumes of
100% ethanol. The nucleic acid precipitate was dissolved in 100
RI
of TE (10 mM Tris-hydrochloride [pH 7.5], 1 mM EDTA). A third of each DNA sample was loaded onto individual lanes of a 1.5% (wt/vol) agarose gel, and after electrophoresis DNA was denatured and transferred to aHybondN(Amersham) nylonfilter. DNAwasimmobilizedby
UVcross-linkingof thefilter inaStratageneStratalinker1800, and DHBVDNAwasdetectedby usinganinvitro-synthesized 32P-labelled RNA complementary to DHBV minus strand DNA(13).
To prepare nucleic acids enriched for DHBV covalently closed circular (CCC) DNA, cells were lysed in the buffer describedabove (excluding pronase)for30 min at37°C, and then KCl was added to a 0.5 M final concentration. The
mixturewasvortexedbrieflyandstored at roomtemperature for 30 min. The protein-detergent complex was removed by
centrifugation, and the supernatant, which contains CCC DNA,wasextracted withanequal volume of phenol. Nucleic
acids were recovered by ethanol precipitation, and DHBV CCC DNA was detected by Southern blot hybridization as
describedabove (10, 13).The hybridization standardonall gels
was10pgoflinear clonedDHBV DNA. RESULTS
Optimal conditions for DHBV infection of Muscovy duck
hepatocytes. In order to determine whether Muscovy duck hepatocytes could be infected in vitrowith DHBV,weinitially
used conditionswhich had
previously
been shownto result in maximalinfection ofprimary Pekin duck hepatocyte cultures(6). Optimalinfection of
hepatocytes
requires
incubation with ahigh-titervirus inoculum(approximately109 DHBVparticles
per ml) for several hours at 37°C. Staining of Pekin duck cultures for DHBVenvelope
protein 8 days after infection underthese conditions reveals thatalmost all cellsareinfected (Fig. 1, leftpanel [1:5 dilution]).To compare the relativeamountsof infection in Pekin duck and Muscovy duck hepatocyte cultures following infection under identical conditions, hepatocytes isolated from both birdswereinfectedwithequivalentamountsof DHBVat4and 37°C. The relativeamountsof DHBVDNAincultures 8days
after infection were determined by Southern hybridization (Fig. 2).The much greateramountof DHBV DNA in infected Pekin cultures suggests that many more hepatocytes become
infected in the Pekin than in the Muscovy duck cultures
following incubation with the same amount of virus. The results also indicate that prolonged incubation at 370Cwitha
high-titer virus inoculum isrequired foroptimal infection of
Muscovyduckhepatocytes. Suraminwasaddedtocells
imme-diatelyafter infection in theseexperimentstolimit thespread
of virus infection. However, it appears that uptake of bound virus is sensitive tothe action of suramin and thatincubation for several hoursfollowingvirusadsorptionisrequiredtoallow virustobypass the suramin-sensitive step (5). This accounts for the absence of detectable DHBV DNAafterinfection at40C,
atwhichtemperature nouptake of bound virus should occur, and for the much larger amount of DHBV DNA in cultures incubated with virus for 5 hat37°C(Fig. 2, lanes 3) compared with 1 h (Fig. 2, lanes2) before addition of suramin.
Kinetics ofDHBV replication following infection of Mus-covyduckhepatocytes.The appearance of CCCDHBV DNA was assayed following infection of Muscovy and Pekin duck hepatocytes under the conditions described above (Fig. 3). The number of CCC DNA molecules in DHBV-infected hepato-cytesistightlyregulated (10, 12); thus, CCC DNA serves as an indicator of the relative number of infected cells. CCCDNA could be detected in cells 24 h after addition of the virus inoculum to Pekin duck hepatocyte cultures, indicating that most cells in the culture had taken up andconverted at least
on November 9, 2019 by guest
http://jvi.asm.org/
[image:2.612.82.544.79.262.2]Pekin Muscovy 1 2 3 1 2 3 hs
RC - t
s
FIG. 2. Relativesusceptibilityof Pekin andMuscovyduck
hepato-cyteculturesto DHBV infection.Primary hepatocytecultures(60-mm dishes) prepared from 1- to 2-week-old ducklings were exposed to
equivalentamountsof DHBV (1:5dilution of stockserum)for 1h at
4°C (lanes 1), 1 h at 37°C (lanes 2), or 5 h at 37°C (lanes 3). After
removal of thevirusinoculum,cellswerewashedbrieflywith PBS and
then maintained in L-15 containing suramin at 100 p.g/ml. Nucleic
acidswerepreparedfrom cultures 8days afterinfection, and equiva-lent amounts of DNA from each culture were loaded on a 1.5%
agarosegel. DHBV DNAwasdetectedbySouthern blothybridization (see Materials and Methods). RC and SS, relaxed circular and
single-stranded DHBVDNA, respectively; hs, hybridizationstandard
of 10pgof cloned linearDHBV DNA.
one molecule of virus DNA. The level of CCC DNA in
infected Pekinduck hepatocytecultures increased until6days after infection, after which time no significant increase was
observed (Fig. 3).This resultwould be expected if all suscep-tible cells in the culture were infected by the original virus
inoculum. CCC DNA could not be detected 24 h after
infection ofMuscovy duckhepatocyteswith the same amount
ofvirus, indicatingthat eitheruptake and conversionof DHBV
DNAwasdelayedorthatarelativelysmall numberof cells had
Muscovy
dayp.i. 1 3 101316 2024hs
RC_ ___Z
ccc -4
Pekin
dayp.i. 1 3 4 6 8 10 1316hs
RC
FIG. 3. Kineticsof DHBVreplication inMuscovyand Pekin duck
hepatocyte cultures following infection with equivalent amounts of virus. Cultureswere infectedwith ahigh titerof DHBV (1:5 dilution
of stockserum)for 16 h at37C.After removalof the virus inoculum,
cells were maintained in L-15 medium. Cultures were harvested for nucleic acids atthe timesindicatedpostinfection (p.i.) byaprocedure
that enriches for CCC DHBV DNA molecules (see Materials and
Methods). Equivalent amounts of DNA were loaded on a 1.5%
agarose gel,and DHBVDNAwasdetectedbySouthernblot
hybrid-ization (see Materials and Methods). RC and hs are defined in the
legend to Fig.2.
been infected. CCC DNAwasnotdetected until 10
days
afterinfection,
and thisamountincreasedtenfold between 13 and16days
after infection.Staining
forDHBVcoreantigen (DHBcAg)
confirmedthatonly
approximately
1%
of cells in theMuscovy
duckhepato-cyteculturewereinfected
(Fig. 4)
underconditions in which80to
100%
of Pekin duckhepatocytes
become infected(Fig.
1,
leftpanel).
Staining
also revealed that the increase inDHBV DNAinMuscovy
duckhepatocyte
cultures thatoccurredat16days
postinfection
was due tospread
of virus infection in the culture, as foci ofDHBcAg-positive
cellsappeared
concomi-tant with the increase inDHBV DNA
(Fig. 4).
Fociwere notdetected when suramin was maintained in the medium after
infection
(Fig.
4).
Suramin is known toprevent
thespread
of DHBV infection in vitro(5).
Comparison
of the rateof DHBVreplication
in Pekin andMuscovy
duckhepatocyte
cultures whenequal
numbers of cells are infected. Thehighly
efficient initial infection ofprimary
Pekin duckhepatocytes
made itdifficulttodetermine whether virusspread
was in fact any more efficient than inMuscovy
duck cultures. In order to compare the relativecapacity
of the twoculturesystems
tosupport
DHBVreplica-tion,
the virus titer in the inoculum wasadjusted
such thatapproximately
equal
numbers ofcellswere infected. Titrationof virus
infectivity
on Pekin duckhepatocytes
showed that a1:500 dilution of a
DHBV-positive
duck serum resulted inapproximately
1%infected cells after 8days
(Fig.
1,
right panel
[1:500
dilution]).
Infection ofMuscovy
duckhepatocytes
witha1:5 dilution of the same serum also resulted in
approximately
1% infected cells
(Fig. 4).
Thus,
having
established conditionsfor
infecting equivalent
numbers of cells in Pekin andMuscovy
duck
hepatocyte
cultures,
wecompared
the kinetics ofDHBVDNA
replication
in thesecultures(Fig. 5).
The appearance ofsingle-stranded,
minus strand DHBV DNA in both culturesconfirms that de novo DHBV DNA
replication
takesplace
following
infection. Aproportion
of the relaxed circularDHBV DNA
signal
iscontributedby
infecting
virusparticles
that remain associated withcells; therefore,
single-stranded
DNA,
which is notpresent
in maturevirusparticles, provides
a morereliable marker ofDHBV DNA
replication.
Theresultsindicate that the rate ofaccumulation of DHBV DNA
repli-cationintermediates inMuscovy
duckhepatocytes
wasslightly
slow
compared
with that in Pekinhepatocyte
cultures(Fig. 5).
Single-stranded
DNAcanbedetectedatday
6postinfection
inthe infected Pekin
hepatocyte
culture but not until 15days
after infection in theMuscovy
duck culture(Fig. 5).
We also
compared
the kinetics ofDHBV DNAreplication
inPekin and
Muscovy
duckhepatocyte
culturesinthe absence of virusspread (Fig. 6).
Approximately
1% of cells in bothPekin and
Muscovy
duckhepatocyte
cultures were infectedwith
DHBV,
asdescribedabove,
andsuraminwasaddedtothe cultures Iday
after infection(5).
The cultureswereprepared
independently
from thoserepresented
inFig.
5,
which mayaccount for the small differences in infection
efficiency.
Suraminwasmaintainedinthe culturemedium until cellswereharvested for DHBV DNA
analysis
at the times indicated.Maximum levels of intracellular DHBV DNA were attained
approximately
14days
after infection in both Pekin andMuscovy
duckhepatocyte
cultures.Hence,
whenonly
asingle
round of infection was allowed to take
place,
it was notapparent
that the kinetics of DHBV DNAreplication
weresignificantly
differentinMuscovy
versusPekinduckhepatocyte
cultures. Themore
rapid
accumulationofDHBV DNAinter-mediatesininfected Pekin duck
hepatocytes
inthe absence of suramin(Fig.
5)
may,therefore,
be due to more efficienton November 9, 2019 by guest
http://jvi.asm.org/
[image:3.612.123.229.79.213.2] [image:3.612.121.238.473.631.2]plus
suramin
minus
suramin
Day
15
p..
Day
26
p.i.
I
FIG. 4. Spreadof DHBV infection inMuscovyduckhepatocytesinfected in vitro.Muscovyduck cultureswereinfected with DHBV andstained
for DHBVcoreproteinat 15 or26daysafterinfection (seeMaterials and Methods). Following infection,cellsweremaintainedin L-15plus
suramin(100,ug/ml)orin L-15 mediumalone,asindicated. The barrepresentsapproximately 120 pum. p.i., postinfection.
spread of DHBV in Pekin duck
hepatocyte
cultures rather thanto anincreasedrate of DHBV DNAreplication.Passage ofDHBVfrom infected Muscovy duckhepatocyte
cultures. Analternative explanation for the
apparently
morerapid rate ofaccumulation of DHBV DNAintermediates in Pekin duckhepatocytes mightbe that virus is released more
efficientlyfromMuscovy duckhepatocytes.To testthis hypoth-esis, we monitored the release of infectious DHBV from
Muscovyduck cellsbytransferring virustohighly susceptible
cultures of Pekin duckhepatocytes. Figure7Ashows therate atwhich DHBV DNAaccumulated in the infected Muscovy duckhepatocytecultures. DHBVwasrecovered from medium harvested from the Muscovy duck cells and used to infect
freshlyprepared Pekin duckhepatocytes. The Pekin duck cells wereharvested 8 days after infection, and the relative amount of DHBV DNA in infected cultures was determined by Southern hybridization (Fig. 7B). The results show that the
maximum amount ofinfectious DHBV is released from the
Muscovy duck cells between 16 and 19 days after infection
(Fig. 7B). For reasons that are not clear, single-stranded DHBV DNAwas not recovered efficiently from the infected Pekin duck cultures in this experiment. Maximal release of
infectiousvirus coincides withamarkedincrease in the amount
Muscovy Pekin
dayp.L. 2 4 6 810 13 15 171820hs 2 4 6 8 10
RC
-
SS-FIG. 5. KineticsofDHBVreplicationinMuscovyandPekinduck hepatocyteculturesfollowing infectionofequivalentnumbers ofcells. Primary Muscovy andPekin duck hepatocyte cultureswere infected for 16 h at 370Cwith a virus inoculum determined to infect equal numbers(approximately 1%)ofcells in both cultures(a1:5dilutionof DHBVduckserumforMuscovyduckhepatocytesanda1:500dilution for Pekin duck hepatocytes). Cells were maintained in L-15, and infectedcultureswere harvested fortotal nucleic acids atthe times shownpostinfection(p.i.).DHBV DNAwasdetectedbySouthernblot hybridization(seeMaterials and Methods andFig.2). RC, SS,andhs aredefined in thelegendtoFig.2.
on November 9, 2019 by guest
http://jvi.asm.org/
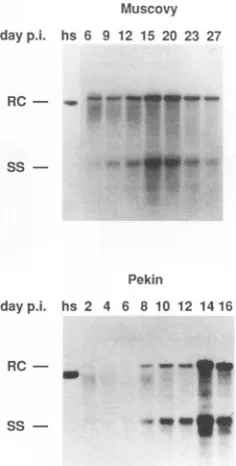
[image:4.612.60.553.80.448.2] [image:4.612.315.550.516.629.2]Muscovy dayp.i. hs 6 9 12 15 20 23 27
Pekin
dayp.i. hs 2 4 6 8 10 12 14 16
RC- _
[image:5.612.371.499.78.293.2]SsS
FIG. 6. Kinetics ofDHBVreplicationinMuscovyand Pekin duck
hepatocytes when virus spread is inhibited by suramin. Primary Muscovy and Pekin duck hepatocyte cultures were infected as de-scribed in the legend to Fig. 5 in order to infect approximately equivalentnumbers of cells in both cultures. After removalof the virus inoculum, cells were maintained in L-15 containing suramin at 100
p.g/ml
to inhibit spread of virus infection. Total nucleic acidswereprepared from cultures at the times shown postinfection (p.i.), and DHBV DNAwasdetectedbySouthernblothybridization (see Mate-rials and Methods andFig. 2).RC, SS,and hsaredefined in thelegend
toFig. 2.
of intracellular DHBVDNA
(Fig. 7A),
whichwe have shown is associated with the appearance of virusspread
in the cultures(Fig. 4).
Maximal release ofDHBVfrom Pekin duckhepatocytes
is also coincident withanincrease inintracellular DHBV DNAandspread
of virus infection(data
notshown).
Thus, it appears that DHBV is released fromhepatocytes
isolated from both Pekin andMuscovy
ducksat similarstagesduring
the virus infectiouscycle.
The results also indicate thata
single
round ofreplication
inMuscovy
duck cells does notappear to reduce the
infectivity
of DHBV on cells isolated from the normalpermissive
host.When virus released into the medium from a DHBV-infected
Muscovy
duckhepatocyte
culture waspassaged
toasecond
Muscovy
culture, a time course similar to that in theoriginal
infected culture was observed(results
notshown).
Very
few cellswereinfected, however,presumably
because of both therelatively
low amounts of virus released from theoriginal
infected culture and the small number ofsusceptible
cells.Hence, it appears that in vitro infection ofMuscovyduck hepatocytes hasnotselectedfor rare DHBV variantswiththecapacity
to infect these cells, as infection at the second passmight
thenbeexpected
tobe moreefficient.Chicken
hepatocytes
are resistant to DHBV infection in vitro.Wewereconcernedthatinfectionof thesmall number of cellsintheMuscovy
duckculturemight
result fromuptake
of virus by some route that did not involve aspecific
virus-receptor
interaction. Ifthiswere the case, we wouldexpect
asimilar
proportion
ofprimary
chickenhepatocytes
to besus-ceptible
to DHBVinfection. Chickens are resistanttoDHBVA
dayp.l. 1 3 6 9 1215 202327 hs
RC
-
Ss-B
dayp.i. 3 5 7 9 12 14 1619 21 hs
RC
-
Ss-FIG. 7. Time course to assay release of infectious DHBV from in vitro-infectedMuscovy duck hepatocytes (p.i., postinfection). Primary Muscovyduckhepatocytes were infected with a 1:5 dilution of DHBV duckserumin L-15for 16 h at 37°C. One set of infected cultures (nine 60-mmdishes)wasusedfor nucleic acid analysisto assay the accumu-lation of DHBV DNA intermediates in infected Muscovy duck cells (A). A pair of infected Muscovy duck cultures (100-mm dishes) infected under the same conditions was used to assay the release of virus. Mediumwasharvested from thesecells every 2 to 3 days after infection and stored at -70°C. Virus was concentrated from the medium by precipitation with 10% (wt/vol)PEG 8000(Sigma), and the viruspellet wasdissolvedin 2 ml of L-15 medium and used to infect 60-mm dishes ofPekin duck hepatocytes for 16 h at 37°C. Eight days after infection (p.i.), total nucleic acids were prepared from the infectedPekinduckcultures, equivalentamountsofDNA wereloaded on a 1.5% agarosegel, and DHBV DNA was detectedbySouthern hybridization (B). RC, SS, and hsaredefinedinthelegendtoFig.2.
infection (4),butacell line derivedfrom transformed chicken hepatocytes has been shown to support highly efficient
repli-cation ofDHBV(2). Hence, if DHBV DNAcanbe delivered
tothe nuclei of chicken hepatocytes,replication mightinitiate
efficiently. We prepared chicken hepatocytes and infected
them underconditions that gave rise to maximal infection of
primary Pekin duck hepatocytes. We were unable to detect DHBVreplicationbySouthernhybridizationorbystainingfor DHBcAg in cultures harvestedat48-h intervals between 2 and 20days after infection(Fig.8).Hence,weconclude that fewer than0.001% ofprimarychickenhepatocytesweresusceptible
toDHBV infection. This result suggests that chicken
hepato-cytesare resistant to DHBVbecause ofthe absence of virus receptorsand that thelimited infection weobserved in Mus-covy duck cultureswas indeed receptor mediated. The pres-ence of DHBV relaxed circular DNA indicates that virus remained associated with cells for the 20 daysfollowing
infection. Whether this represents trapping of virus at the plasma membrane or specific binding to ahepatocyte
cell surface molecule isnotclear.5-aza cantransformprimaryPekin duckhepatocytesfroma
DHBV-resistant to a DHBV-susceptible phenotype. We have
previously shown that primary Pekin duck
hepatocytes
pro-gressivelylose
susceptibility
toDHBVinfectioninculture;
this effect is especiallypronounced
in cultures maintained in medium supplemented with fetal bovine serum(6).
Theon November 9, 2019 by guest
http://jvi.asm.org/
[image:5.612.123.241.80.313.2]2492 PUGH AND SIMMONS
Chicken
dayp.l.
RC
-
Ss-FIG. 8. Timecourse ofDHBVinfection ofprimarychicken
hepa-tocytes. Primaryhepatocytesprepared fromaweek-old chickenwere plated in 60-mm dishes and infected 2 days afterplatingwitha 1:5 dilutionofDHBVduckserumin L-15. Cellswereincubated with virus for 16 h at 37°C. Total nucleic acids were prepared from infected cultures at the times shown postinfection (p.i.), and equivalent amountsofDNA wereloadedon a1.5%agarosegel.DHBV DNAwas detectedby Southernblothybridization (see Materials andMethods andFig.2). RC, SS, and hsaredefined in thelegend toFig.2.
Pekin
5-azapre- 5-aza
post-Infection Infection
0153060120 0153060120
RC
-Ss
-L15alone
Muscovy
5-aza pre-Infection 0 20
.^-40 hs
-k
_*
___w
_s%ms X
__Xi
_w
_w s
o..w.
sls,ls. v .S.; Q
01530601200153060 120 hS
RC- U
pearance ofresistancetoDHBV infectionprobablycorrelates with the gradual loss of liver-specific gene expression in hepatocytes maintained in culture.
5-aza has been shown to induce expression of avariety of quiescent genes (3, 8). Wewere interested, therefore, to see whether the gradual appearance of virus resistance in Pekin
hepatocytecultures could bereversedbytreatmentwith5-aza. Cultures maintained for 12 days after plating in either L-15 aloneorL-15 plus 5%fetal bovineserum
(Fig.
9, leftpanels)were treatedwith various concentrations of 5-aza for
approx-imately 16 h before infection with DHBV. Cultures were maintainedfor6to8days after infectionandthenanalyzedfor the presence of DHBV DNA(Fig. 9; see also Materials and
Methods).Drugtreatmentresulted in a5- to 10-foldincrease
in the amount of virus DNA in the cultures. Staining of cultures for the presence of DHBVcore protein showed that this increase in the amount of DHBVDNA corresponded to anincreasein thenumber ofinfectedcells(Fig. 10).Treatment of cultures with 5-aza after infection did not result in the marked
increase
in DHBVDNAthat was observed when thedrugwasadded beforeinfection(Fig.9).5-azahadnoeffect on thelevel ofDHBV DNAinprimary hepatocytes isolated from
ducklings congenitally infected with DHBV (results not
shown),suggesting that the drug does not affect DHBV DNA
replicationdirectly.
TreatmentofprimaryMuscovy duck hepatocytes with 5-aza does notinduce susceptibility to DHBV infection. The results
described aboveindicate that 5-aza may induce expression of one ormoreproteins required early during DHBV infection of Pekin duck hepatocytes. We hypothesized that the relative resistance of Muscovy duck hepatocytes to infection with DHBVmight be due to repressed expression of host cell genes required for DHBV infection. Hence, we hoped that treatment with 5-aza might remove the block to expression and result in arelativeincrease in the number of cells susceptible to DHBV infection. However, we found that 5-aza added at
concentra-tions that induce susceptibility to infection with DHBV in Pekin duck hepatocyte cultures (Fig. 9, left panels) did not enhance the susceptibilityof Muscovy duck cells, as indicated
by the absence of any detectable increase in the amount of DHBV DNA in treated versus untreated cells (Fig. 9, right
panel).
ss
-FIG. 9. 5-aza induces susceptibility to DHBVinfection in Pekin duck hepatocytes. Pekin duckhepatocytes weremaintained in L-15 medium (top panel) or L-15 plus 5% (vol/vol) fetal bovine serum (bottom panel)for 12days,atwhich time cells wereincubated with0, 15, 30, 60,or 120 p.M5-aza in L-15 mediumfor 16 h at37°C.After removalof5-aza, cultureswereinfected witha1:10 dilution ofDHBV duck serumin L-15 for 2h at37°C.Aduplicatesetof infectedcultures were incubated with 5-aza immediately after infection, asdescribed above.Cultureswereharvested 8 days after infection for nucleicacid preparation,andDHBV DNA wasanalyzed by Southernblot hybrid-ization (see Materials and Methods). Duplicate 60-mm dishes of Muscovy duckhepatocytes (top right panel)weremaintainedinL-15 mediumfor 12days before incubation with 0,20, and 40 ,uM 5-azain L-15 for 16 h at37°C.Cultureswere infectedimmediately following 5-azatreatment, asdescribed above.Tendays afterinfection,cultures wereharvestedfor DHBVDNA analysis (see MaterialsandMethods). RC, SS,andhs aredefined in thelegend toFig.2.
DISCUSSION
Hepadnavirusesarecharacterizedbya
very
limitedhost and tissue range. Experiments involving transfection of liver cell lines with clonedhepadnavirus DNA have shown that hepad-naviruses will replicate efficiently in liver cells isolated from hosts that are not the normal hosts for virus infection (2, 7). However, no cell line has been clearly demonstrated to be susceptible to infection with anyhepadnavirus.Arecent report indicates that HepG2 cells can be infected with HBV under certain conditions, however(1). Resistance to HBV infection exhibitedbycellsthat are competent to support HBV replica-tion suggests thattheprincipal factor limiting host range acts at the level ofvirusuptake.The present study has investigated the nature of resistance toDHBVinfection in the Muscovy duck and the chicken. Our initial hypothesiswasthat resistance of Muscovy ducklings to infection could be explained by resistance of hepatocytes to virus infection.Wehaveshown, however, that virus resistance in vivo cannot be fully accounted for by the presence of
J. VIROL.
on November 9, 2019 by guest
http://jvi.asm.org/
[image:6.612.328.542.77.362.2] [image:6.612.110.242.93.194.2]A
FIG. 10. 5-aza inducessusceptibility to DHBV infection in Pekin duck hepatocytes. Primary Pekin duck hepatocytes were maintained for 12 days after plating before infection with DHBV. Cells were
incubated for 16 h before infection with 20 jiM 5-aza in L-15 (B)or
with L-15 medium alone (A). Cultures were infected with a 1:10 dilution of DHBV duckserumin L-15for 2 hat370C.Eight daysafter
infection, cellswerestained for the presence ofDHBcAg(see Mate-rials andMethods).The barinpanelArepresentsapproximately 100
m.
resistant hepatocytes, as we are able to infect a significant
proportion of primary hepatocytes isolated from Muscovy ducklings. Virus infection spreads to neighboring cells in the
Muscovy duck cultures, indicating that most cells are suscep-tibletoinfection but that virus in the inoculum binds with low
efficiency. Wewere notabletoinfectprimary chicken
hepato-cytes in vitro, which indicates that resistance of chicken
hepatocytes to DHBV is at the level of virus adsorption and
uptake.Moreimportantly,resistance of chickenhepatocytesto
DHBV infection~indicates that the infection ofMuscovy duck
hepatocytes we have described takes place via specific
virus-receptor interactions.
We have shown that 5-aza can transform DHBV-resistant Pekin duckhepatocytes toasusceptible phenotype.The prin-cipalmechanism ofaction of 5-aza istopreventmethylationof
newly replicatedDNAand thus allowexpressionof genes that
arerepressed by methylation (3, 8).Itisnotclear howthedrug
maybeactinginthisinstance, asprimaryduckhepatocytesdo
not actively divide after a few days in culture. However, we
wouldpostulatethat 5-aza isinducingexpressionofa
hepato-cyte protein(s) that is essential for virus infection. The
resis-tance ofMuscovyduckhepatocytes tothisinduction indicates
that the low level of DHBVinfection in these cells is not due to repressed expression of a virus receptor gene that can be induced with5-aza.
The following two models could bothadequately account for the limited infection of Muscovy duck hepatocytes which we have described. According to the first model, Muscovy duck hepatocytes may express the same receptor for DHBV as is present on Pekin duck hepatocytes, though at much reduced levels. Our results would support a model in which very few unoccupied receptors are present on Muscovy duck hepato-cytes and infectious DHBV mustcompete foravailable recep-tors with the huge excess of noninfectious DHBV surface antigen particles. The second model proposes that DHBV infects Muscovy duck hepatocytes via binding to a receptor distinct from that present on Pekin duck hepatocytes. If this receptor had a much lower affinity for binding to DHBV, it might account for the prolonged incubation at 37°C in the presence of virus required to achieve successful infection of Muscovy duck cells.
The rate atwhich replicative DHBV DNA accumulates in infectedMuscovy duckhepatocytes and the period before virus is released from infected cells are delayed compared with the course of virus replication in Pekin duck hepatocytes. How-ever, infection of equivalent numbers of cells in Pekin and Muscovy duck cultures, under conditions in which spread of virus infection was inhibited, indicated that the kinetics of DHBV DNAreplication were similar inhepatocytes from both birds. Hence, itwould appear that areduced rateofspread of virus infection in Muscovy duck hepatocyte cultures may account for the relatively low rateof accumulation of DHBV DNA.
We should consider how we define resistance to in vivo infection forhepadnaviruses. Experimental infection of Pekin ducklings usually leads tomassive infection, withvirus titers as high as1010DNA-containing DHBV particles per ml of serum. If hepadnavirus infection fails to give rise to a detectable viremia, weconclude that infection did notbecome established and that the hostanimal may be consideredresistant. Infection of 1-day-old Muscovy ducklings with DHBV doesnotproduce
a virus titer that can be detected in serum by dot blot hybridization 1 to 3 weeks after infection. It must be
empha-sized that this assay will fail todetectvirus if there are fewer thanabout 106 DHBVparticles per ml ofserum.It is
possible,
inlight of our results, thatinfection of Muscovyducklingswith DHBV does result in infection of a small numberof cells in the liver but that this infection is insufficient to give rise to adetectable level of viremia. Alternatively, the
relatively
ineffi-cient infection of Muscovy duck hepatocytes may allow the developing immune system in youngducklings torapidly
clear DHBV-infected cells from the liver, such that a viremia is never achieved.In contrast to DHBV infection of Pekin ducklings, in vivo infection of ground squirrelsorchipmunks with
ground
squir-rel hepatitis B virus results in a latencyperiod
of several months before viremia can be detected(9, 11).Ourresultswith the Muscovy duck may provide apossibleexplanation
for how such a long latent period could occur. Ifonly
a very minor subset of hepatocytes become infectedby
the initial virus inoculum and spread of virus to other cells in the liver is restricted because of inefficientbinding
to available virus receptors, or perhaps because of low receptordensity,
then along latent period before detectable viremia
might
be pre-dicted.on November 9, 2019 by guest
http://jvi.asm.org/
[image:7.612.67.301.81.413.2]ACKNOWLEDGMENTS
This work was supportedby USPHS grants CA-40737 and CA-06927 and by anappropriationfromthe Commonwealth ofPennsylvania.
We thank Maureen Climaldi for secretarial assistance, the Fox ChaseSpecial Services department forphotographic work, and Jeff Saputelli for helpwith the ducks. Weare gratefultoWilliamMason and Jesse Summers forhelpfulcomments onthemanuscript.
REFERENCES
1. Bchini, R., F. Capel, C.Dauguet, S. Dubanchet, and M. A. Petit. 1990. Invitro infection ofhuman hepatoma (HepG2) cells with hepatitis B virus. J. Virol. 64:3025-3032.
la.Berry, M. N., and D. S. Friend. 1969. High-yield preparation of isolatedratliverparenchymal cells. J.Cell Biol.43:506-520. 2. Condreay, L. D., C. E.Aldrich, L. Coates, W. S. Mason, and T. T.
Wu.1990. Efficient duck hepatitisBvirusproduction byanavian liver tumor cell line. J. Virol. 64:3249-3258.
3. Jones, P. A., and S. M. Taylor. 1980. Cellular differentiation, cytidine analogs andDNAmethylation. Cell 20:85-93.
4. Marion, P. L., J. M. Cullen, R. R. Azcarraga, D. M. Van, and W. S. Robinson. 1987. Experimental transmission of duck hepatitis B virustoPekin ducks and todomestic geese. Hepatology 7:724-731. 5. Petcu, D., C. Aldrich, L. Coates, J. Taylor, and W. Mason. 1988. Suramin inhibitsinvitroinfection by duck hepatitis B virus, Rous sarcomavirus,andhepatitis Delta virus. Virology 167:385-392. 5a.Pugh,J. C.Unpublished data.
6. Pugh, J. C., and J. W. Summers. 1989. Infection and uptake of duck hepatitis B virus by duck hepatocytes maintained in the presenceofdimethyl sulfoxide. Virology172:564-572.
7. Pugh, J.C., K. Yaginuma, K.Koike,andJ. Summers.1988.Duck hepatitisBvirus(DHBV) particles produced by transient expres-sion ofDHBV DNA inahumanhepatomacell lineareinfectious in vitro. J.Virol.62:3513-3516.
8. Razin, A., and A. R. Riggs. 1980. DNA methylation and gene function.Science210:604-610.
9. Seeger, C., P. L.Marion,D.Ganem, and H. E. Varmus. 1987. In vitro recombinantsofground squirrel and woodchuck hepatitis viral DNAsproduceinfectiousvirusin squirrels. J. Virol. 61:3241-3247.
10. Summers, J., P. M. Smith, and A. L. Horwich. 1990.Hepadnavirus envelopeproteins regulate covalently closed circularDNA ampli-fication.J.Virol.64:2819-2824.
11. Trueba, D., M. Phelan, J. Nelson, F. Beck, B. S. Pecha, R. J. Brown, H. E. Varmus, and D. Ganem. 1985. Transmission of ground squirrel hepatitis virusto homologous and heterologous hosts.Hepatology5:435-439.
12. Tuttleman, J.S., C. Pourcel, and J. Summers. 1986.Formation of thepool of covalently closed circularviral DNA in hepadnavirus-infected cells. Cell47:451-460.
13. Tuttleman, J. S., J. C. Pugh, and J. W. Summers. 1986. Invitro experimental infection of primary duck hepatocytecultures with duck hepatitis B virus. J. Virol. 58:17-25.