Certificate from the DEAN
This i
s to certify that this dissertation entitled “CLINICAL STUDY ON
CARCINOMA ESOPHAGUS
IN GOVERNMENT RAJAJI HOSPITAL,
MADURAI”
is the bonafide work of
Dr.
M.Udayakumar
.,
in partial fulfillment of
university regulations of the Tamil Nadu Dr. M.G.R. Medical University, Chennai,
for
M.S General Suregry Branch I
examination to be held in
April 2015.
Captain
Dr.B. SANTHAKUMAR , M.Sc(F.Sc) , M.D(F.M).,
PGDMLE., Dip.N.B (F.M) .,
THE DEAN
Madurai Medical College and Government Rajaji Hospital,
Madurai.
INCIDENCE, PATHOLOGICAL PATTERN AND MANAGEMENT OF
Certificate from the HOD
This is to certify that this dissertation entitled
“CLINICAL STUDY ON
INCIDENCE, PATHOLOGICAL PATTERN AND MANAGEMENT OF
CARCINOMA
ESOPHAGUS
IN GOVERNMENT RAJAJI HOSPITAL
,
MADURAI”
is the bonafide work of
Dr.
M
.
UDAYAKUMAR
.,
in
partial fulfillment
of university regulations
of the Tamil Nadu Dr. M.G.R. Medical University,
Chennai, for
M.S General Surgery Branch I
examination to be held in
April 2015.
Dr.A.Sankaramahalingam, M.S.
Professor and HOD,
Department Of General Surgery,
Government Rajaji Hospital,
Madurai Medical College,
Madurai.
Certificate from the GUIDE
This is to certify that this dissertation entitled
“CLINICAL STUDY ON
INCIDENCE, PATHOLOGICAL PATTERN AND MANAGEMENT OF
CARCINOMA
ESOPHAGUS
IN GOVERNMENT RAJAJI HOSPITAL,
MADURAI”
is the bonafide work of
Dr.
M.UDAYAKUMAR
.,
in partial fulfillment of
university regulations of the Tamil Nadu Dr. M.G.R. Medical University, Chennai,
for
M.S General Surgery Branch I
examination to be held in
April 2015
Dr.N.Vijayan, M.S
Professor of Surgery ,
Department Of General Surgery,
Government Rajaji Hospital,
Madurai Medical College,
Madurai.
DECLARATION
I ,
DR.
M.UDAYAKUMAR
, solemnly declare that
this
dissertation
“CLINICAL STUDY ON INCIDENCE, PATHOLOGICAL PATTERN AND
MANAGEMENT OF CARCINOMA
ESOPHAGUS
IN GOVERNMENT
RAJAJI HOSPITAL, MADURAI”
is a bonafide record of work done by me at
the Department Of General Surgery, Government Rajaji Hospital , Madurai , under
the guidance of
Dr.N.VIJAYAN, M.S,
Professor , Department of General
Surgery, Madurai Medical college , Madurai.
This dissertation is submitted to The Tamil Nadu Dr. M.G.R Medical
University, Chennai in partial fulfillment of the rules and regulations for the award
of
M.S Degree General Surgery Branch- I
; examination to be held in
April
2015.
Place: Madurai
Date:
Dr.
MUDAYAKUMAR
,
ACKNOWLEDGEMENT
I would like to thank
Captain
Dr.B. SANTHAKUMAR , M.Sc(F.Sc) ,
M.D (F.M)., PGDMLE., Dip.N.B (F.M) .,
Dean Madurai Medical College and
Government Rajaji Hospital, for permitting me to utilize the facilities of Madurai
Medical College and Government Rajaji Hospital facilities for this dissertation.
I wish to express my respect and sincere gratitude to my beloved teacher and
Head of The Department,
Prof. Dr.A.SANKARAMAHALINGAM, M.S.,
Professor of Surgery for his valuable guidance and encouragement during the study
and also throughout my course period.
I would like to express my deep sense of gratitude, respect and thanks to my
beloved Unit Chief and Professor Of Surgery,
Prof. Dr.N.VIJAYAN, M.S.,
for
his valuable suggestions , guidance and support throughout the study and also
throughout my course period .
I am greatly indebted to my beloved Professors ,
Dr. NASHEER AHMED SYED
, M.S., Dr. SELVA CHIDHAMBARAM, M.S., Dr. MARUTHUPANDIAN,
M.S., Dr. LAKSHMI , M.S., Dr. SYED IBRAHIM , M.S., and
Dr. DHAMODHARAN ,M.S.,
for their valuable suggestions throughout the
course of the study.
I am extremely thankful to Assistant Professor of Surgery of my Unit,
Dr.K.SARAVANAN,
M.S.,
Dr.M.MANIKANDAN,
M.S.,
Dr.P.MUNIASAMY, M.S.,
for their valid comments and suggestions.
I sincerely thank the Professor of Surgical Gastroenterology,
Prof.Dr.
S.PADMANABHAN, M.S. M.Ch (SGE)
&
the Professor of Surgical Oncology,
Prof.Dr. S.S.SUNDARAM, M.S. M.Ch (Surgical Oncology)
for their guidance
and suggestions in my dissertation work.
I sincerely thank all the staffs of Department Of General Surgery and Department
Of Surgical Gastroenterology & Surgical Oncology for their timely help rendered
to me, whenever needed.
I extend my thanks to all my friends, batch mates, any senior and junior colleagues
who have stood by me and supported me throughout my study and course period.
Finally, I thank all my patients, who form the backbone of my study, for their
patience and co-operation .I pray god for their well-being and their speedy
recovery.
VIII
L
LI
IS
ST
T
O
OF
F
A
AB
BB
BR
RE
EV
VI
IA
AT
TI
IO
O
NS
N
S
A
A →→ AAvveerraaggee
AC → Adenocarca iinnoomam
AG → Agricuulturl iists
B
B →→ BBuussiinneessssppeerrssoonn
B. Pneu → Bronchhooppnneumonie aa
C
Caa →→ CCaarrcciinnoommaa
CE → Carrcicnnoommaa Esophagus
D → Depennddente
D
DOOAA →→ DDaatteeooffAAddmmiissssiioonn
F → Femmalea
G
G →→ GGoooodd
HW → Housewe ifei
I
IPPNNOO.. →→ IInnppaattiieennttNNuummbbeerr
M
M →→ MMaallee
RLNN → Recurrec ntn LLaryngeaal Nerve
S
IX
A
AB
BS
ST
TR
RA
AC
CT
T
N
NEEEEDDFFOORRSSTTUUDDYY
Bennigni ttumoorrssoofftthe esoopphhagusa area rare anndd arreeuusuas llyl more bothher-e soms eethant
harrmful.m TheT mmoosstt coommmonm tyyppee ofbbenigne ttumoriissaa leiome yyoommawhiw cchh occcuurrssini peoopplel
b
beettwweeeenn 3300 aanndd 6600 yyeeaarrss ooff aaggee.. OOtthheerr ttuummoorrss ccoonnssiisstt ooff ffiibbrroovvaassccuullaarr ppoollyyppss aanndd
Schwannommasa .. Bennigni tumorst ooff esophas gguuss is vverye rrarea compprriissingi ooff 0.5 tto 00..88%% ooff
e
essoopphhaaggeeaallttuummoorrss..
Carrcicnnoommaa ooff esophas gguuss is thet ninthh mmoosts coommmon canncec rr inn the wworldd..
N
Nuummeerroouussssttuuddiieesshhaavveeddeemmoonnssttrraatteeddtthhaattiinnddeevveellooppiinnggccoouunnttrriieesscciiggaarreetttteessmmookkiinnggaanndd
alcl oohhooll cconsuumptim oonn are thet mmoosstt imppoorrtantt pprredispose ingi faccttor for esophas gguuss
c
caarrcciinnoommaa..
Barrrrette ’’ssese oopphhagusa issaa conseqquueenncec ooff chroonniiccggaassttrroo esophas ggeeala rrefle eexx disordes rr
w
whhiicchh iiss tthhee mmoosstt iimmppoorrttaanntt rriisskk ffaaccttoorr ffoorr aaddeennooccaarrcciinnoommaa ooff eessoopphhaagguuss.. SSeevveerraall
esophas ggeale mmotiillittyy disorders havvee beenn impli iicattedd iinn the ddeevellooppmment of esophageala
c
caarrcciinnoommaa..
Carrcicnnooggeennici efeffeecttssooff tobaccoc anda allcoholc isi farmmoorree pronouncede forsquams oouuss
c
ceellllccaarrcciinnoommaatthhaannffoorraaddeennooccaarrcciinnoommaa..
Recentc epie ddeemiolm ooggici aall studit ese hhavea found thhaat oobbeessiitty (meeaasuurred ass body masa ss
i
innddeexx)) iiss aannootthheerr ssttrroonngg rriisskk ffaaccttoorr ffoorreessoopphhaaggeeaall ccaarrcciinnoommaa ssqquuaammoouuss cceellll ccaarrcciinnoommaa
XI R
REESSUULLTTSS
A tootat ll of3300ppataiiente sswew rree operraatet ddwiwtht aaggeeggroupr ((4400--8877yyrrss))wwiitht 2200malm ese anndd
1
100 ffeemmaalleess ((rraattiioo 22::11)) ssqquuaammoouuss cceellll ccaa ((7700%%)),, AAddeennooccaarrcciinnoommaa ((3300%%)) ccaarrcciinnoommaa ooff
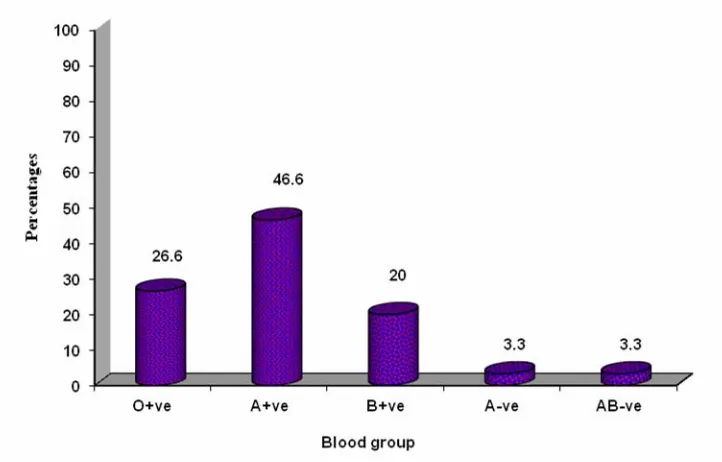
Loowew rrtthhiirdesophaguse isi moorreecomc mmoonn..((>70%)> of ppaatiieentss beloonnggede tot bbllood groupAA
(
(4466..66%%)) oorr OO ((2266..66%%)).. TToobbaaccccoo SSmmookkiinngg//cchheewwiinngg,, aallccoohhooll ccoonnssuummppttiioonn,, cchhrroonniicc
i
irrrriittaattiioonnaannddpprree--eexxiissttiinnggeessoopphhaaggeeaallccoonnddiittiioonnssaarreessttrroonnggrriisskkffaaccttoorrss..
C
COONNCCLLUUSSIIOONN
Toobbacca oo,,sms ookking/ci hhewe ingi and allcoholc ccoonnssuummpptitoonnaannddpprreee xxiissttingi esophageala
c
coonnddiittiioonnssaarreessttrroonnggrriisskkffaaccttoorrss..DDiisseeaasseesshhoowwsspprreeddoommiinnaanncceettoommaallee,,mmoorreeccoommmmoonniinn
5
5thh&&66tthddeeccaaddeeoofflliiffee..SSqquuaammoouusscceellllccaarrcciinnoommaaiissmmoorreeccoommmmoonntthhaannaaddeennooccaarrcciinnoommaa
Differrente surgis calc aapproachh has no effffecte oonn durationi of hospitaal stsaay, mortaaliitty or
s
suurrvviivvaall..
K
Keeyywwoorrddss
XII
T
TA
AB
BL
LE
E
O
OF
F
CO
C
ON
NT
TE
EN
NT
TS
S
S
Sl..
N
No. Topic
Paaggee Noo..
1
1.. INTRODUCTU IIONO 1
2
2.. AAIIMMOOFFTTHHEESSTTUUDDYY 33
3
3.. HISTOT RRIICCALA ASPA ECTSE 4
4
4.. REVIEV WW OFF LIITERATURET 7
5
5.. SSUURRGGIICCAALLAANNAATTOOMMYY 1144
6
6.. GGEEOOGGRRAAPPHHIICCDDIISSTTRRIIBBUUTTIIOONN 2222
7
7.. ETIOT PATHOP GEG NEN SISS 24
8
8.. TTRRAANNSSHHIIAATTAALLEESSOOPPHHAAGGEECCTTOOMMYY 2288
9
9.. MMEETTHHOODDOOLLOOGGYY 4433
1
100.. RESULTS SS 50
1
111.. DISCCUSSU IIONO 77
1
122.. CCOONNCCLLUUSSIIOONN 8899
1
133.. SUMMAM RRYY 92
1
144.. BIBLIIOGRAO PHYP 94
A
ANNNNEEXXUURREESS
•
• PPrrooffoorrmmaa 110077
•
• MMaastter Charrtt 116
1
155..
XIII
L
LI
IS
ST
T
O
OF
F
T
TA
AB
BL
LE
ES
S
T
Taabbllee N
Noo.. TTooppiicc PPaaggeeNNooss..
1
1.. Age distsrributii oonnooffppatia entse studiet dd 5500
2
2.. Genderrddisi tributt ioni of patienti ss studiede 5522
3
3.. Occuuppatia oonnooffppaatientse studiet dd 5533
4
4.. DDuurraattiioonnooffddiisseeaasseeiinnmmoonntthhss 5544
5
5.. CClliinniiccaallffeeaattuurreess 5555
6
6.. Habitst 5566
7
7.. Comoorrbbidi conditionst 5577
8
8.. H/o respis rratorya ailmi eents preoperratia vvelye 5588
9
9.. AAnneemmiiaa 5599
1
100.. HHeemmoogglloobbiinn((%%)) 6600
1
111.. AAllbbuummiinn 6611
1
122.. Blooooddggrroouupp 6622
1
XIV
1
144.. DDuurraattiioonnooffhhoossppiittaallssttaayy((ddaayyss)) 6644
1
155.. Surgicala proceduree 6655
1
166.. Compplicl atia oonnss durinnggsurges rryy 6666
1
177.. PPoossttooppeerraattiivveeccoommpplliiccaattiioonnss 6677
1
188.. PPoossttooppeerraattiivveeRRTTaannddCCTT 6688
1
199.. OOuuttccoommee 6699
2
200.. Correlatl ioni ooff cclilnniicac ll vvaariar bbllese wiwtht oouutcomt ee ((PPost--oopp
coommppllicatc ionsi /del/ ayeda coommpplilcatc ioni ss/M/ oorrtalt ity)i 7700
2
211.. Tablea showings peaka ageiinciddencee bbyyvvaarrious authhoorrss 8800
2
222.. MMeeaannAAggeeiinnYYeeaarrss 8800
2
233.. MMaalleettooFFeemmaalleerraattiioo 8811
2
244.. PresentStSuuddyy--AngiA ooggrraphica LeveL ll of Diiseasee 8844
2
255.. Blooooddggrroouupp 8844
2
266.. Comppaarison studiet ss histsooppathola ooggicai ll behavviouri oofftumt oorr 8855
2
277.. CCoommppaarriissoonnssttuuddiieessbbllooooddlloossss 8866
2
XV
L
LI
I
ST
S
T
OF
O
F
F
FI
IG
GU
UR
RE
ES
S
F
Fiigguurree N
Noo.. TTooppiicc
P
Paaggee
N
Nooss..
1
1.. Surgicala annatoma yyooffese oopphhagusa 1155
2
2.. Arterriali bbloodl suupppplyl of esophagus 1177
3
3.. Venous drainnagea of esophas gguuss 1188
4
4.. LLyymmpphhaattiiccddrraaiinnaaggeeooffeessoopphhaagguuss 1199
5
5.. NNeerrvveeSSuuppppllyyooffEEssoopphhaagguuss 2211
6
6.. Mobilisi atia oonnoofftthe stomacha 4411
7
7.. Preparratia oonnooffggaastrict conduitc 4411
8
8.. Mobilisi atia oonnini thennecke 4422
9
9.. AAnnaassttoommoossiissiinntthheenneecckk 4422
1
100.. BBaarrggrraapphhsshhoowwiinnggaaggeeddiissttrriibbuuttiioonn 5511
1
111.. Pieggrrapha shhoowiwnnggggendere distsrributii oonn 5522
1
XVI
1
133.. Barrggrrapha shhoowiwnnggdduurratia oonnooffddisi ease ee innmmoonnthst 5544
1
144.. Barrggrrapha shhoowiwnnggmodem of preses nntatt iioonn 5555
1
155.. BBaarrggrraapphhsshhoowwiinngghhaabbiittss 5566
1
166.. PPiieeaannddBBaarrggrraapphhsssshhoowwiinnggccoommoorrbbiiddccoonnddiittiioonnss 5577
1
177.. PPiieeggrraapphhsshhoowwiinngghhiissttoorryyooffrreessppiirraattoorryyaaiillmmeennttss 5588
1
188.. Pieggrrapha shhoowiwnngganema iai 5599
1
199.. Barrggrrapha shhoowiwnngghhemogle oobbini (%) 6600
2
200.. BBaarrggrraapphhsshhoowwiinnggsseerruummaallbbuummiinn((%%)) 6611
2
211.. BBaarrggrraapphhsshhoowwiinnggbbllooooddggrroouuppaammoonnggppaattiieennttssssttuuddiieedd 6622
2
222.. PPiieeggrraapphhsshhoowwiinngghhiissttooppaatthhoollooggiiccaalleexxaammiinnaattiioonn((HHPPEE)) 6633
2
233.. Barrggrrapha shhoowiwnnggdduurratia oonnooffhhoospis talt stsaya (days) 6644
2
244.. Pieggrrapha shhoowiwnnggcomplc icai tionst during surgery 6666
2
255.. PPiieeggrraapphhsshhoowwiinnggppoossttooppeerraattiivveeccoommpplliiccaattiioonnss 6677
2
XVII
2
277.. Pieggrrapha shhoowiwnnggoouutcomt ee 6699
2
288.. Ennddooscs ooppici picturec showings superficiac ll malliignannttesophagee aall caancerr 7711
2
299.. EEnnddoossccooppiiccppiiccttuurreesshhoowwiinnggpprroolliiffeerraattiivveeggrroowwtthh 7711
3
300.. EEnnddoossccooppiiccppiiccttuurreesshhoowwiinnggmmaalliiggnnaannttssttrriiccttuurreeooffeessoopphhaagguuss 7722
3
311.. EEnnddoossccooppiiccppiiccttuurreesshhoowwiinnggmmaalliiggnnaannttcciirrccuummffeerreennttiiaalluullcceerr 7722
3
322.. CTffilmi sshowing Camiddlm ee thirrddese oopphhagusa 7733
3
333.. CTffilmi sshowing Calowl ere thirrddese oopphhagusa 7733
3
344.. EEssoopphhooggoo--ggaassttrreeccttoommyyssppeecciimmeenn 7744
3
355.. MMaalliiggnnaannttssttrriiccttuurreeccaauussiinngg8800%%nnaarrrroowwiinnggoofflluummeenn 7744
3
366.. MMaalliiggnnaannttttuummoouurrooffmmiiddddllee--tthhiirrddeessoopphhaagguuss 7755
3
377.. Malignanti tumourt of loowew rr--thirdt of esophas gguuss 7755
3
388.. Histopathologit calc slsidei showiing adennooccarca iinnoomam 7766
3
1
INTRODUCTION
Tumours of the esophagus are among the most challenging problems
confronting the surgeons.1
Benign tumors of the esophagus are rare and are usually more bothersome than
harmful comprising of (0.5-0.8%) all esophageal tumors.2
Esophageal cancer represents one of the most lethal malignancies affecting the
mankind.3 It is the 9th most common carcinoma of all carcinomas.4 Adenocarcinoma of
the esophagus is increasing in incidence at a rate exceeding that of any other neoplasm.5
Once the overt symptoms appears the average survival rate without treatment is
9 months.6
Most of the patients in our setup presents in the late stages. Considering the rate
of blockage of stents and cost of procedure more emphasis in laid on the surgical
procedure which gives long term relief compared to other procedures.
After several studies controversy still exist regarding the operative management
of carcinoma esophagus even in Indian setup.
Surgical therapy remains the mainstay therapy for patients with respectable
2
Palliation is the primary goal for patient with locally advanced cancers and those
with metastasis. Primary goal of palliation is restoration of swallowing, relief of plain and
local control of the disease.8 To achieve this surgical resection gives best results in all
forms of esophageal cancers.2
Transhiatal esophagectomy can be performed with minimal morbidity and is the
desired operation of choice5and it is better tolerated physiologically.4 It also confers the
advantage of a radical approach and incorporates near total esophagectomy and cervical
anastomosis.9,10
There are many studies reported in the literature which have student the
transhiatal esophagectomy and its complications. However, there is necessity to evaluate
he complications more precisely and group them in form of operative postoperative
complications. Also, comparing such type with mode of presentation and other pre
existing systemic illnesses. There is a need for a new study to evaluate the complication
4
HISTORICAL ASPECTS
2500 BC: “Smith Surgical Papyrus” described repair of the “gullet” after
perforation (no indication malignancy was involved)
AD 0-1: Chinese described esophageal dysphagia from carcinoma.
Circa 2nd Century: Galen described fleshy growths causing obstruction of the gullet.
1849: Long described first use of sulfur either as an anesthetic.
1868: Kussmaul is the first to pass a lighted tube through the entire
esophagus into the stomach.11
1869: Trendelenburg employs the endotracheal route for administration
of anesthesia.12
1871: Billroth successfully resected and reanastomosed the cervical
esophagus in dogs.13
1897: Czerny is the first to successfully resected the cervical esophagus
for carcinoma in humans.14,15
1989: Roentgen published his early investigations on the use of “x-rays”.
1898: Rehn attempted resections of an esophageal carcinoma via right
5
1901: Dobromysslow successfully performed the first intrathoracic
resection and reanastomosis of the esophagus in dogs.
1905: Beck described formations of the gastric tube from the greater
curvature of the stomach, based on the gastroepiploic artery.
1907: Wendell described transpleural resection of an esophageal
carcinoma of the lower esophagus with lateral
esophagogastrostomy in a lumen (patient dies the following day).
1908: Volecker successfully resected a carcinoma of the gastresophageal
junction with primary esophagogastrostomy via laparotomy.
1913: Zaaijer successfully resected a carcinoma of the cardia via an
abdominothoracic approach.16
1913: Denk described the “blunt” or “blind” esophagectomy.17
1913: Turner described the thoracic abdominal approach for blindly
mobilizing the resecting the thoracic esophagus.18
1933: Oshawa resected the thoracic esophagus for carcinoma with
immediate esophagogastrotomy (8 of 18 patients survive).19
1938: Admas is the first surgeon in the United States to perform
transthoracic esophageal resection with immediate
6
1946: Ivor Lewis introduced esophagectomy and esophagogastrostomy
through a right thoracotomy.20
1947: Sweet completed 212 resections for esophageal carcinoma
(17% operative mortality and 8%, 5- year survival).
1963: Logan described 853 resections for esophageal carcinoma
(29% operative mortality).
1978: Orringer and Sloan revived the technical of Gray Turner’s
“esophagectomy without thoractomy”.21,22
1984: Leichman et al at Wayne State University combine 3,000 cGy with
two cycles of 5-FU and cisplatin preoperatively in 21 patients with
squamous cell carcinoma of the esophagus (37% pathologic
complete response; operative mortality 27%).23
1997: Multiple phase III randomized trials fail to show significant
7
REVIEW OF LITERATURE
Esophageal cancer represents the third most common gastrointestinal malignancy
and ranks among the ten most common cancers worldwide.24 U.S. mortality data from
1990-1994 have revealed a steady increase in age adjusted mortality in males as well as
females subjects, due to this malignancy.25
The combined incidence of adenocarcinoma of the esophagus and cardia in
currently estimated at 5.8 per 1,00,000 ranking this tumor among top 15 cancer of white
men in United States.26
In India squamous cell carcinoma is more common and accounts for nearly 75%
of cases. The incidence of esophageal cancer in women is much lower.27,28
Through cultural as well as dietary practices contribute to esophageal cancers,
tobacco and ethanol are believed to be primary risk factors.25,29,30,31
A five to ten fold increase in esophagus squamous cell cancers has been noted
smokers relative to non-smokers and risk of esophageal carcinoma correlates with extent
of tobacco exposure.29,32,33
A multicentric study by Gammon et al as well as additional clinical studies have
indicated that the risk of esophageal cancer is approximately two fold higher in smokers
8
Alcohol abuse has been associated with increased risk of esophageal cancers and
the risk increases with the amount of alcohol consumed.35,36
Vaughan TL and Brown LM demonstrated in two different studies that obesity
may be related to risk of esophageal cancer, particularly adenocarcinoma.37,38
Esophageal squamous cell carcinomas have been associated with nutritional
deficiencies; a low intake of fruits and vegetable may influence the esophageal cancer
risk two fold.25,39,40 Deficiencies in carotene, vit, E, and selenium may also increase the
risk of squamous cell carcinoma in underdeveloped areas.
Dietary practices including drinking hot beverages or ingestion of fermented
vegetables may contribute to increased cancer risk.41,42
Esophageal cancer should be suspected in any patient complaining of dysplasia
and weight loss. A through history should be ascertained focusing on preexisting
conditions as well as tobacco and ethanol abuse which are known to be associated with
cancer risk.
The most common symptom of esophageal carcinoma at the time of presentation
is dysphagia3 palliation is the primary goal for patient with advanced local cancers and
9
Dysphagia is the most common symptom at the time of presentation.43 This
unfortunately is a late sign because the esophageal wall is easily distensible due to lack of
serosa.44 Dysphagia leads to various problems like weight loss, malnutrition and
aspiration pneumonia.45 Anorexia malnutrition, loss of weight cardiovascular and
pulmonary problems will have direct impact on morbidity and long term survival.46,47
Age is also an important prognostic factor as patients over age 70 undergoing
esophagectomy have a postoperative morality of 13%.48
Surgery represents the best chance for cure and best palliation for dysphagia and
local control of the disease.8 Surgical resection gives the best results for all forms of
esophageal cancers.4
The incidence of esophageal stricture is very less in surgery when compared to
radiotherapy.7,49
Most surgeons favour transhiatal resection of esophagus which involves posterior
mediastinal blunt dissection without throracotomy.7,50
Transhiatal esophagectomy can be performed with minimal morbidity and is the
desired operation of choice and its is better tolerated physiologically.4 It also confers the
advantage of radical approach under direction vision and incorporate near total
10
The main goals of this procedure are to resolve dysphagia, to achieve a operative
mortality of less than 10%, to prevent the complications and morbidity (e.g. Infection,
Stricture, reflux and aspiration).8,50
This procedure is preferred in patients of respiratory functions impairment who
are not fit for thoracotomy.7 Regardless of level of tumor the maximum vertical surgical
margin possible is obtained minimizing suture line tumor recurrence. Postoperative
deaths from mediastinitis and sepsis resulting from anastomotic disruption is vertically
eliminated also clinically significant gastresophageal reflux seldom occur.9
A study by Randini-Martini et al showed no apparent difference in postoperative
morbidity in Ivor-Lewis when compared with transhiatal esophagectomy and
Ivor-Lewis procedure had more mortality rate.51
Morbidity in form of blood loss, operating time and fewer days in ICU is very less
despite being performed in older patients.52
Hand sewn anastomosis (oesophago-gastric) are better than the stapled ones in
esophagectomy as demonstrated by Laterza et al.53
The choice of the operation for carcinoma esophagus depends upon many factors
viz., location of tumor, preference of surgeon, body habits, prior operation, condition of
11
Most of the evidence accumulated from random studies suggests that the use of
various modalities of induction therapy provide little or no advantage over primary
surgical resection alone.56
Orringer et al57 reported on 800 patients with cancer of the intrathoracic
esophagus and cardia treated with transhiatal esophagectomy, adenocarcinoma was
present in 69% of cases, where as 28% had epidermoid cancer. Hospital mortality was
4.5%, and morbidity was 27%. Major complication including anastomotic leak (13%),
recurrent laryngeal nerve injury, (7%) wound infection (3%), pulmonary complications
(2%), bleeding (1%) and chylothorax (1%). More than 90% of the patients were
discharged within 21 days of hospitalization. There was an overall statistically significant
survival with adenocarcinoma (24%, v/s 17%).
The study by the University of Michigan group represents the largest experience
with transhiatal resection for carcinoma and survival rate and morbidity were quite
consistent with those reported by other surgeons who practice a similar approach.57,58
Gelfand et al59 reported on 160 patients who underwent transhiatal
esophagectomy, the operative mortality and 5 year survival rates were 2% and 21%
respectively.
Gertsch and Colleagues60 described their experiences with 100 patients, hospital
12
Vigneshwaran et al61 reported on 131 patients who underwent transhiatal
esophagectomy with 2% operative mortality.
Parker SL, Tong et al have demonstrated pulmonary complications are most
common problems encountered in esophagectomy patients. Pneumonia being the
commonest smoking cessation preoperatively, adequate pain control with aggressive
physiotherapy and early ambulation are cornerstones of preventive therapy.
Gastric advancement is without doubt the best esophageal replacement when
esophagectomy in performed for palliation of cancer. The extent of operative dissection
and the resultant physiologic insult are less when preparing the stomach for advancement
compared with colon.
The hospital recovery and time to return of unrestricted alimentation are also
shorter in patients undergoing gastric pull up.62
There was a general belief earlier that a vagally denervated stomach acts like a
denervated tube63,64 and the emptying depends on the gravity65 but recent scintigraphic
studies ambulatory gastric manometry and video fluoroscope have shown that its motility
remains unchanged and the transposed stomach tube acts as a “dynamic conduit”.
Erythromycin is known to enhance the early postoperative contractivity of
13
Bardini et al have reported anastomotic leak of around 12% regardless of method
of suturing. Conduit necrosis is due to ischemia. Anastomotic stricture required dilatation
in upto 4% of patients.
Advances in perioperative care including anaesthetic techniques and nutrition
have enabled mortality rates for resection of esophageal cancer to fall to between 5% to
14
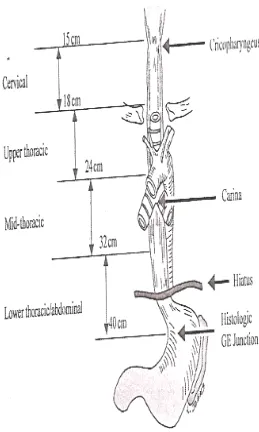
SURGICAL ANATOMY
4-8It’s a hollow muscular tube extending from the pharynx to the stomach i.e. from
C6-T11. The length is approximately 25 cm.
It commences in the midline, slightly deviates to left upto the root of the neck,
returns to the midline at the level T4-T5, at T7 deviates again to the left and pierces the
diaphragm at T10.
1. Cervical constriction: 15 cms from incisor teeth at the level of cricopharyngeus.
This is the narrowest part in the GIT.
2. Aortic arch: 22.5 cms from incisor teeth.
3. Left main bronchus: 27 cms from incisor teeth.
4. Diaphragm: 40 cms from incisor teeth.
Anatomical divisions
1. Pharyngesophageal
2. Cervical
3. Thoracic
15
Fig. 1: Surgical anatomy of esophagus
Pharyngesophageal: It extends from inferior pharyngeal constrictor to upper
esophageal sphincter.
Killian’s dehiscence: It is a potential point of weakness in this segment.
Site pharyngesophageal / Zenker’s diverticulum
16
Cervical Esophagus: Extends up to the beginning of T1 measuring 5-6 cms in
length. Surgically, more approachable through the left neck incision, as it tends to course
more towards left of trachea.
Thoracic esophagus: Passes through the superior and posterior mediastinum.
Below the tracheal bifurcation esophagus curves to the right and lies adjacent to the right
pleural cavity. Therefore perforation leads to right pleural cavity. Therefore, perforation
leads to right pleural effusion. Below level of T7, esophagus curves towards that left. So
perforation of the lower third leads to left pleural effusion.
Abdominal esophagus: Measures 1-2-5 cms in length. IT passes through
diaphragmatic hiatus along with vagus nerve and branches of left gastric artery. It is
covered with peritoneum on the anterior and lateral surface. It takes a sharp turn to the
left on entering the abdomen.
Esophagus is divided into 4 principle regions according to American Joint
Committee for Cancer (AJCC) staging:
• Cervical esophagus
• Upper thoracic
• Middle thoracic
17
Most surgeons divide the esophagus into ‘thirds” as follows”:
• Upper third: Cricopharynx to superior portion of aortic arch.
• Middle third: Aortic arch to inferior pulmonary vein.
• Lower third: Inferior pulmonary vein to gastroesophageal junction.
BLOOD SUPPLY
Cervical esophagus: By superior and inferior thyroid artery.
Thoracic esophagus: Mainly supplied by 4-6 aortic esophageal branches.
Lower esophagus: By esophageal branches of left gastric and left inferior phrenic
[image:34.612.187.465.372.658.2]artery.
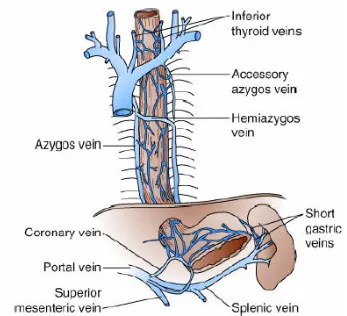
18 VENOUS DRAINAGE
Cervical esophagus: Mainly to inferior thyroid vein.
Thoracic esophagus: Left side – left hemiazygos.
Right side – azygos
[image:35.612.152.499.279.595.2]Abdominal: Left gastric vein which empties into portal vein.
19 LYMPHATIC DRAINAGE
There are 2 lymphatic plexuses in the:
1. Mucosa
2. Mucosal layer
Flow of lymphatics
1. Upper 2/3- upwards
2. Lower 1/3- downwards
Lymph nodes
There are 3 parallel inter connected chains:
1. Paraesophageal
2. Periesophageal
20
Fig. 4: Lymphatic drainage of esophagus
NERVE SUPPLY
1. Cervical esophagus
• Sympathetic- fibres from superior and inferior cervical sympathetic
ganglia.
• Parasympathetic- recurrent laryngeal nerve.
2. Thoracic esophagus
• Sympathetic – upper thoracic and splanchnic nerves
• Parasympathetic – vagus.
3. Abdominal esophagus
• Sympathetic-celiac ganglia
• Parasympathetic–vagus.
The recurrent laryngeal nerve, also supplies cricopharynx, so if there is weakness
of the recurrent laryngeal nerve. Patients tend to aspirate while swallowing.
In addition, Meissners and Auerbach plexus provide an intrinsic autonomic
nervous system within esophageal wall.
21
2. Auerbach plexus-connective tissue between longitudinal and circular
[image:38.612.103.557.184.554.2]layers.
24
ETIOPATHOGENESIS
Any study of cancer cannot be done without detailed study of causative factors.
The causative factors can be obtained from a detailed study of diet habits, environmental
agents, infectious agents and other factors. The etiologic factors play a very important
role in the pathogenesis of upper gastrointestinal tract cancer.
[1] TOBACCO SMOKING AND ALCOHOL
Ernest Wydner and colleagues carried out several studies since 1957 and have
shown that the extensive use of alcohol significantly increases the risk of esophageal
carcinoma in tobacco users/although alcohol itself does not seem to increase the risk of
this cancer.74
But later on Pottern and Colleagues identified tobacco and alcohol as important
independent risk factors for esophageal cancer and also established that apart from being
independent risk factors, they also exert and synergistic action by potentiating each
other.75 The risk is said to increase with increasing number of cigarettes and duration of
smoking habit.76
Ribeiro et al in his Study said that the risk of esophageal carcinoma varied with
the type of alcohol consumed suggesting that the risk spirits is usually more than twice
than from beer and the risk from wine being intermediate between that for beer and
25
Similar studies carried out in South India by Chitra et al identified Alcoholism,
smoking, and chewing of tobacco as important predisposing for esophageal cancer.78
Studies carried out by Devisor et al indicate that cigarette smokers have 2 to 3 times
increased risk of esophageal and proximal gastric center.79
BETELNUT AND BETEL LEAF
Observatory regarding the association of esophageal cancer with betel nut claims
were made as early as the 1970s by Stephne et al.80 The chewing of betel nut and betel
leaf with or without tobacco is or common practice in certain parts of India. In a study
conducted by Nayar et al at the All India Institute of Medical Sciences, the risk of
developing esophageal carcinoma increased by 3.16 times with the daily habit of clewing
betel leaf and tobacco.28
DIETARY FACTORS
A variety of dietary factors have been implicated in the development of
esophageal cancer, because of their distinct variations in incidence and mortality,
between countries in different socio-economic groups and in migrants and their
offspring’s. The role of diet has been extensively investigated but with in conclusive
results.
Nitrosamines
They have been implicated in the development of esophageal carcinoma.
Khuroo et al in their study, found that the Kashmir Province of India is a high risk area
26
compounds and comprises of Salt tear, dried fish, vegetables (especially brassica
abesecar), red abilities and spice cakes.25
Fruits & Vegetables
Rensburg in his study suggested that or chronic low intake of several
micronutrients, together with an inadequate protein intake, increases the be disposition of
the esophageal epithelium to neoplastic transformation. Diet deficient in vitamins A, C,
E, Niacin, Riboglasin and Zinc have been suggested as risk factors.81
Infectious Agents
The infectious agents may be in the form of viral, bacterial or fungal organisms.
1. Viral Agents: The Viruses that have been implicated is Human Papilloma
virus (HPV) for esophageal cancer. Toh et al found an association between
HPV infection and development of esophageal cancer.82
2. Fungal agents: These have been implicated as etiological agents in
malignancies of esophagus. Bhatior et al in their study isolated fungi in
75% cases of Carcinoma esophagus and the most common species over
candida albicans, species.83
Genetic Factors
The risk of esophageal cancer is found to be more among first degree relatives.
Pour et al in their study found that the risk of developing esophageal cancer among blood
27 Low Socio-economic Status
Several studies, carried out by Enzinger et al proved that low socioeconomic
status is associated with an increase risk of esophageal.
Predisposing lesions
The risk for SCC esophageal Carcinoma include; Achalasia, Caustic injury to
esophagus, tylosis, plumervision syndrome84 and Barrett’s esophagus.
Clinical Course and Prognosis
The clinical course and prognosis of esophageal cancer depends chiefly on the
extent of local strand, lymph node involvement and metastasis.
Esophageal Cancer
The overall prognosis for esophageal carcinoma is very poor with 5 year survival
of around 10% 15%. In esophageal carcinoma, Metastasis to lymph node is very common
because of the rich lymphatic supply of the esophagus, especially to the periesophageal
areas, below the diaphragm and upward to the cervical nodes.
Distant metastasis can occur to lung, liver and adrenal glands.85 Esophageal
cancer is classified according to the 2002 American Joint Committee on Cancer – tumor
node metastasis (TNM) Classification system, which takes into account the
characteristics of the Binary tumour, regional lymph node. Metastasis and digital
metastasis more than 50% of patients have unresectable or metastatic disease at the time
28
TRANSHIATAL ESOPHAGECTOMY
Operative steps
In an otherwise, fit patient subtotal resection of esophagus with esophageal gastric
anastomosis at the neck level should be the sensible, approach of achieving best form of
palliation. For this removes the near total esophagus, reducing the recurrence rate and
restoring their swallowing act. This goal is achieved with admirable success rate by
Transhiatal esophagectomy (THE) as compared to other approach, which is known for
higher morbidity and mortality. If there is an anastomotic leak patients do not end up with
mediastinitis. It is easily managed by drainage and wound care. The patient will be fit for
discharge within 2 weeks after THE. Few cases are also gifted with a bonus of cure.
Transhiatal esophageal resection
It is usually done synchronously by 2 teams of surgeons, one operating per
abdomen and the 2nd surgeon exposing the cervical esophagus.
Procedure
Incision from xiphisternum to 2-3” below the umbilicus. Liver, retrogastric celiac
nodes, spleen, stomach and the entire intestine are seen and palpated. In growth,
29 Steps by the abdominal surgeon
1) The greater curvature of the stomach is freed from its omental attachment by
dividing the omentum outside the gastro epiploic arch. Short gastric vessels are
ligated midway between stomach and the spleen, curved fundic dome is released
by diathermy coagulation applied well clear of the viscera.
2) Posterior wall of stomach is exposed and any adhesions are diathermy divided
under vision.
3) Lesser omentum is divided upto the hiatus, preserving the right gastric artery.
4) With the assistant firmly retracting the pancreas the left gastric vessels are made
taught and double ligated individually. The posterior dissection is carried
proximally upto the hiatus.
5) Duodenum is Kocherised.
6) The hiatus is widened by dividing the crura of the diaphragm keeping in mind the
proximity of IVC and hepatic veins.
7) The index and middle finger are insinuated into the posterior mediastinum, the
growth proper is palpated, limits ascertained and nodes if any palpated. The
esophagus is freed from the vertebrae by gentle finger dissection. Posterior vagus
hooked and divided. Next the esophagus is freed on the right and left sides. The
anterior vagus is hooked and divided, the fingers are carried anteriorly and the
distal esophagus with the growth is mobilized all around. When the major part of
esophagus is mobilized one should carefully palpate the tracheal bifurcation and
the main bronchi and avoid injuring them. At this stage the surgeon’s right hand is
30
During mobilization of thoracic segment of esophagus there is likely to be venous
return impediment, and fall in BP or even arrhythmia. The anaesthetist is to monitor the
BP and Pulse, whenever the systolic BP falls below 60 mm of Hg, the hand is withdraw
and within a few minutes the BP recovers to everyone’s delight. Thus blunt mobilization
of thoracic esophagus is an intermittent procedure, the timing dictated by the anaesthetist.
Also at the time of blunt mobilization the anaesthetist switches onto hand ventilation so
that any inadvertent injury to the tracheobronchial tree is immediately recognized.
Once the abdominal surgeon begins to mobilize the esophagus and gives the go
ahead sign another surgeon being to expose the cervical esophagus.
By the time abdominal surgeon have mobilized upto the tracheal tree the other
surgeon would have mobilized the cervical esophagus and upper thoracic esophagus by
blunt dissection.
9) At this state it is easier for the abdominal surgeon to put his left index of
middle finger from above and feel his right hand fingers from below.
Thus, he is confident of good all around mobilization.
10) Once the esophagus is divided at the neck, the entire esophagus with the
growth is pulled down through the laparotomy wound.
11) The specimen and the stomach are carefully palpated. The stomach is
transected 6 cm distal to the lesion along a line extending from low down
31
12) The stomach is reconstructed into a conduit with staplers. Careful
attention is paid in maintaining the vascularity of the gastric conduit
especially at the angle of sorrow in the lesser curve.
13) The loose thread lying in the posterior mediastinum is stitched to the
stomach and gently pushed up the post mediastinum rather than being
pulled by the silk at the neck end.
14) Feeding jejunostomy (Witzel type) is done as a routine about 12 cm distal
to the D. J. junction.
Stomach
Stomach is the referred viscus to replace esophagus because of:
1. Single anastomosis
2. Easy mobilization
3. Reliable and consistent blood supply
4. Physiological function of receptive relaxation and adaptive
accommodation retained
32 Steps in mobilization of cervical esophagus
1. Incision along the anterior border of left sternomastoid muscle.
2. Greater auricular and accessory nerves are preserved.
3. Middle thyroid vein is ligated and divided.
4. Thyroid gland pushed medially by the assistant fingers rather than by a retractor
and the greater vessels are retracted laterally.
5. Recurrent laryngeal nerve safeguarded.
6. Esophagus mobilized by blunt dissection and tape passed around it.
7. With traction on the tape the distal part is gently mobilized by blunt finger
dissection, keeping in mind the proximity of brachiocephalic vein, left common
carotid artery and aorta.
8. After all round mobilization the esophagus is transfixed with a long thick silk as
low down as possible and divided proximal to the stitch. The distal cut end of the
esophagus is pushed down the mediastinum for the abdominal surgeon to pull out
the specimen per abdomen. After the stomach is pulled down the long silk will
remain in the posterior mediastinum with the free end lying in the neck. This is
used to pull up the reconstructed gastric tube as the case may be.
9. Once the reconstructed gastric tube pulled upto the neck the surgeon ascertains
that the viscera is not rotated and its vascularity is well maintained.
10. An end to side single layered interrupted anastomosis is done 3 0’ PDS. The
33
Complications: Variety of iatrogenic complications of vital structures in close
proximity to the esophagus can occur. Some of them e.g., tracheal tear, aortic tear, may
end up in death on table. Knowledge of anatomy, avoiding aggressive mobilization of
esophagus by keeping fingers close to the esophagus (Ryle’s tube inside the esophagus
helps) and willingness to perform thoracotomy in difficult mobilization will reduce the
rate of complication. Acute myocardial problem may also result in death on table.
PREOPERATIVE
A. Neck level
1. Injury to recurrent laryngeal nerve
During mobilization of cervical esophagus recurrent laryngeal nerve (RLN) may
be injured either by traction or transaction. The result may vary from hoarseness,
coughing, vocal cord paralysis which may lead to aspiration. Bilateral vocal cord palsy
may need re-intubation or tracheostomy. Using finger of the assistant instead of retractor
to retract the thyroid will reduce the incidents of injury to RLN. Since the esophagus lies
a little to the left of midline, left side exposure will reduce the injury to RLN. By keeping
close to the esophagus while passing the tape right nerve will be safe guarded.
2. Injury to blood vessels
34
B. In chest cavity
1. Injury to tracheobronchial tree
Preoperative bronchoscopy is mandatory before esophageal surgery to rule out
any infiltration. Tracheobronchial tree injury occurs more commonly after THE. The
finger dissection should be kept close to esophagus. The volar aspect of the left index
finger passed through the neck guards the trachea, while the right fingers passed through
the hiatus dissects the esophagus from the tracheobronchial (TB) tree. The tear may also
occur due to over insufflation of the cuff. Injury can be recognized by anaesthetist by the
fact of tightness of the bag. Injury will result in inadequate ventilation and drop in
saturation and blood pressure. Instead of over ventilation the tubes should be carefully
advanced into the left bronchus and patient prepared for thoracotomy. Small injuries can
be directly sutured whereas loss of tracheal tissue may be buttressed with pleural flap or a
pericardial flap. Postoperatively ventilation has to be monitored.
2. Tear of azygos vein
After ligation of the left gastric artery and inferior thyroid artery, esophagus
receives its blood supply from small branches from aorta which form sub mucosal plexus.
When these vessels are torn the vessels contract and the bleeding stop. If during
mobilization in THOR, there is a continuous bleeding and steady fall in BP, azygos vein
tear should be suspected and the mediastinum should be packed immediately and the
35
3. Opening of pleural cavity
This invariably occurs in THE and sometimes can result in tension pneumothorax
and if so immediate chest drainage tube should be positioned.
4. Injury to aorta
It is rare injury but it can result in awesome bleeding and could end up in death on
table before thoracotomy could be done.
C. Abdominal cavity
1. Inadequate blood supply to the transposed viscera
When the stomach is used as the conduit the blood supply is maintained by right
gastric and gastroepiploic artery. These vessels should not be subjected to twisting or
extrinsic compression which may result in poor fundal perfusion, venous congestion and
possibly anastomotic break down. The conduit should be transposed into the posterior
mediastinum by a gentle push from the abdomen rather than pull at the neck level.
Volvulus of the transposed stomach can occur. After transposition the stomach lies in the
mediastinum with 180 degree rotation, the greater being rotated anteriorly. If the stomach
rotates through 360 degree than gastric outlet obstruction are even sloughing of the
conduit occur.
Before transposing the stomach venous congestion of the site to the anastomosed
at the neck should be eliminated for this may later interfere with arterial perfusion and
36
In the postoperative period unexplained fever, tachycardia, leucocytosis and
discharge from the neck should alert the physician of breakdown in anastomosis. If
confirmed excision of the sloughed graft and cervical esophagectomy is the choice. Later
substernal colonic transposition may be considered.
2. Injury to spleen, liver and pancreas
Splenic injury could be prevented by avoiding undue traction during greater
curvature mobilization. Small tear from adhesions may be controlled with packing.
Bigger lacerations better dealt with splenectomy. Liver and pancreatic injuries are less
often encountered and easily preventable.
3. Hiatal herniation
Herniation of the abdomen content through the divided hiatus can occur even after
many years. Uncomplicated hernia can remain asymptomatic. When the patient develops
chest pain respiratory distress, fever, leucocytosis, flat but rigid abdomen, obstruction or
strangulation should be suspected. Immediate surgery either by abdominal or thoracic
route should be undertaken.
If at operation hiatus is considered to be very wide it may be sutured to the
37 Postoperative: Early complications
Hoarseness: Due to recurrent laryngeal nerve involvement and usually improves
in 6 weeks time. Intubation trachitis may also cause temporary hoarseness, short course
of steroid will help.
Anastomotic leakage: The incidence of anastomotic leaks various widely and has
been reported upto 53%. Vigneswarn et al classified anastomotic leakage into 3 types.
Type 1: Silent clinically or discovered by the contrast study, usually heal
spontaneously without any stricture formation.
Type 2: Leak with systemic disturbances like fever, pain, leucocytosis, discharges
from the wound. Given time it will heal with wound care and antibiotics. May result in a
stricture.
Type 3: Leak due to ischemia and necrosis at the anastomotic site. May require
exploration and cervical oesophagostomy and later colonic transposition.
Bruce et al in their review article proposed to use the definition as suggested by
38
Definition of leak as adopted from the surgical infection study group87
Leak Definition Treatment
Radiological No clinical signs No change in management
Clinical minor Local inflammation, cervical wound X-ray contained leak (thoracic anastomosis) Fever, WBC, CRP
Drain wound Delay oral intake antibiotics
Clinical major Severe disruption on endoscopy, Sepsis
Change management CT guided drainage (Reintervention)
Conduct necrosis Endoscopic confirmation Reintervention
Absence of serosal coat, tension at the anastomotic site due to imperfect or
inadequate mobilization of the stomach conduit, compression of the conduit at the
substernal level which may affect its vascular integrity, are the local factors contributing
to anastomotic leak. Systemic disease like diabetes, tuberculosis, cirrhosis of the liver,
preoperative irradiation etc., may also contribute to poor healing and anastomotic
leakage.
Leakage in the mediastinum after intrathoracic anastomosis have high morbidity
and mortality. Immediate drainage of the chest cavity should be done along with
appropriate antibiotic. Reoperation to establish the continuity of the GI tract have high
39 Chylothorax
Uncommon complication but can result in nearly 50% mortality if left
unrecognized. Patients who had neoadjuvant RT and patients with the adherent tumour
the thoracic duct is more like to be injured. If there is an excessive discharges of milky
fluid from the ICD chylothorax should be suspected. Estimation of triglyceride and
chylomicron in the drainage will clinch the diagnosis. Excessive drainage for long time
result in nutritional deficiency. Once recognized re-exploration and the ligation of the
duct should be done.
Aspiration: While swallowing liquids in the early stages. This may lead to
fulminate pulmonary complication.
Necrosis of the transposed stomach in the mediastinum: The patient is usually
toxic with respiratory distress, leucocytosis etc. early recognition and re operation is the
only change of survival. At surgery the gastric conduit is dismantled, vascular integrity
ascertained and if the conduit if found to be ischemic that segment is transected and
substernal colonic bypass done.
Pulmonary complications: More common with transthoracic resection.
Complications may range from pneumonia to respiratory failure. Patients with
preoperative uncompromised respiratory diseases are more prone to develop pulmonary
complications. Preoperative radiotherapy, chemotherapy also pre dispose to pulmonary
40
Reflux of gastric contents: This may lead to aspiration and pulmonary
complications. Reflux of gastric contents is more common after intrathoracic anastomosis
compared to cervical anastomosis. The addition of pyloric dilation helps to certain extent
in preventing the reflux. Keeping the Ryle’s tube in the early post operative period and
aspiration also minimizes chances of aspiration.
Late complications
Stricture at anastomotic site: This may present early or even after few months or
years. Early anastomotic leak and wound healing by fibrosis predispose to stricture
formation. The incidences are more common after stapled anastomosis compared to hand
sewn anastomosis. Quite often these patients can be managed with regular dilatation
using a balloon dilator. With delayed occurrence of stricture one should suspect
recurrence at the anastomotic site.
Recurrence of tumour
Anastomotic recurrence
With T4, N1 status with venous permeation, in intrathoracic anastomosis when
less then 10cms of the esophagus is removed and missing skip lesions recurrence is more
common.
41
With adherent tumour and incomplete clearance at esophageal bed predispose to
loco regional recurrence. Such patients are in a poor state of health not suitable for
[image:58.612.145.504.166.439.2]chemoradiation.
42
Fig. 7: Preparation of gastric conduit
[image:59.612.143.505.392.667.2]44
Patients were selected from those presenting to the OPD and those referred from
elsewhere.
As mentioned earlier, all fresh patients biopsy proven carcinoma of the esophagus
were included in the study. The following patients were however excluded from the
study:
(a) Inoperable patients,
(b) Patients with history of previous chemotherapy, radiotherapy or
esophageal surgery,
(c) Non-ambulatory patients, and
(d) Patients having malignancy of upper third of esophagus.
1. The cases of carcinoma esophagus which were confirmed by relevant
investigations were subjected to surgery. Operative findings and
postoperative findings were recorded.
2. There were 30 patients of which 20 were male patients and 10 female
patients with age ranging from 40 to 87years.
3. The indication for surgery is mainly palliation to relieve pain and
dysphagia.
4. Depending upon the operability, stage of the disease cases were selected.
5. Preoperative evaluation included history and physical findings. UGI
45
6. The evaluation criteria used were selected on the basis of availability of
resources in our setup, with the aim of using the minimum possible
investigation to achieve the diagnosis and provide optimum treatment.
7. Most of the patients were shifted to postoperative wards except for few
patients who were shifted to ICU immediately after surgery.
8. Laboratory investigation done in each case included:
a. Complete haemogram
b. Coagulation profile
c. Base line renal function serum creatinine, electrolyte, urine routine
and microscopy.
d. Routine chest roentgenograms were done in all the cases.
Diagnostic Investigation done included:
1. Upper GI Endoscopy: The Olympus flexible video endoscope was used
for all patients for diagnostic purposes.
2. Endoscopic biopsy was done for all the patients and the diagnosis was
confirmed by histopathological examination.
3. U/S abdomen was also done in all the patients.
4. C-T abdomen: To know the infiltration into surrounding structures, the
46
5. Pulmonary function tests: In case of patients with pulmonary disease and
in smokers and tobacco chewers to plan anaesthesia and for better post
operative rehabilitation.
Surgical treatment
All the patients underwent elective surgery after the correction of anemia,
improvement of nutritional status, and after thorough preoperative preparation. Vigorous
chest physiotherapy by experts was given in the preoperative and postoperative phases.
Board spectrum antibiotics were given to all patients preoperatively,
47 Operative steps
In an otherwise fit patient subtotal resection of esophagus with esophageal gastric
anastomosis at the neck level should be the sensible approach of achieving best form of
palliation. For this removes the near total esophagus, reducing the recurrence rate and
restoring their swallowing act. This goal is achieved with admirable success rate by
Transhiatal esophageal resection (THE) as compared to other approach, which is known
for higher morbidity and mortality. If there is an anastomotic leak patients do not end up
with mediastinitis. It is easily managed by drainage and wound care. The patient will be
fit for discharge within 2 weeks after THE. Few cases are also gifted with a bonus of
cure.
Compulsorily corrugated drains were kept for all the patients in the neck near the
anastomosis.
Postoperatively patients were given intense monitoring, and all were closely
monitored for development of complications.
Intercostals drain were kept in all the patients in whom pleura was damaged.
Monitoring vital signs of temperature, pulse, respiratory rate, blood pressure,
ECG, monitoring fluid balance, acid base balance, biochemical and hematological
48
Postoperative chest X-ray film to check position of inter costal drainage tubes,
central venous pressure line and to know lung expansion.
Adequate pain control, using narcotic analgesics through either, intravenous,
intramuscular or epidural route and non steroidal anti-inflammatory drugs.
Ventilator care and endotracheal tube care with intermittent suctioning to prevent
aspiration of bronchial secretions.
After checking lung expansion and if the acid base studies and biochemical
parameters are within acceptable limits patients is extubated.
Nasogastric tube is maintained to minimize gastroesophageal reflux and
aspiration and is removed when it is no longer required, usually on the fourth
postoperative day.
Maintenance of intercostal drainage tube in proper position with underwater seal
or negative suctioning (usually the drains are removed after the drainage becomes
minimal)
Neck drain is removed after starting the patient with oral liquids to prevent wound
infection and to detect leak.
Maintaining good oral hygiene to prevent oral infection, which can cause
49
Maintaining good general hygiene and proper positioning to prevent pressure
sores.
Total parenteral nutrition supplementation according to the requirement of the
patient.
Jejunostomy feeding
Usually started after 48 hours after surgery when the bowel starts moving
actively. Feeding is started with clear fluids and gradually changed over to liquid diet.
Commercial formula preparations available can also be used, the usual requirement being
around 2500 kcals per day.
Venous thromboembolism
Prophylaxis with administration of subcutaneous heparin or low molecular weight
heparin, anti-embolism stockings and early ambulation.
Physiotherapy
Physiotherapy with postural drainage, percussion and vibration with breathing
exercises to improve the lung function and encouraging the patient to cough out bronchial
secretions was done by an experts.
• Passive leg exercises to prevent Deep Venous Thrombosis.
• Wound care.
• Patient is monitored in the postoperative intensive care ward for 24 hours
50 Late postoperative care
Implying the importance of maintaining good general hygiene.
Oral feeding is started with soft solids in sitting position dietary intake is
gradually liberalized with emphasis being placed on small frequent feeds as patient will
experience fullness in early stages after operation.
Jejunostomy tube is usually retained in situ till the completion of chemotherapy
for maintaining nutrition as the patient may experience vomiting during this period.
Jejunostomy wound care.
Discharge
The criteria for discharge are:
1. Adequately healed abdominal and neck incisions.
2. Maintenance of normal vital parameters
3. Repeat U.G. scopy after 12 days and if there are no anastomotic
52
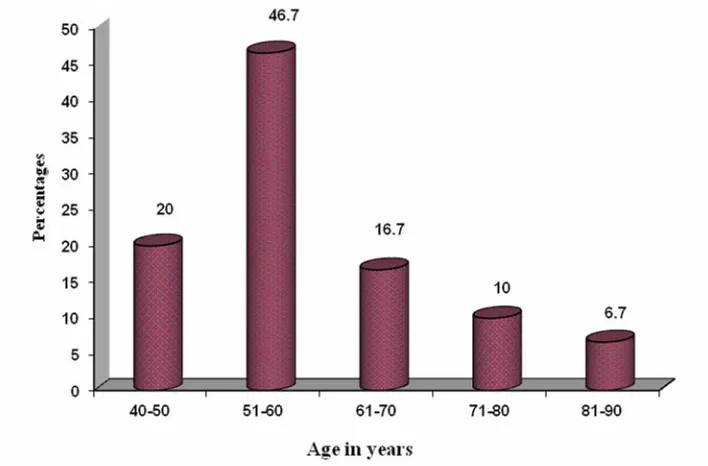
Fig. 10: Bar graph showing age distribution
The highest incidence was observed in 5th and 6th decade of life, patient aged
66
Fig. 21: Bar graph showing blood group among patients studied
75
Fig. 28: Endoscopic picture showing superficial malignant esophageal cancer
[image:92.612.143.509.410.680.2]76
Fig. 30: Endoscopic picture showing malignant stricture of esophagus
[image:93.612.144.508.411.681.2]77
Fig. 32: CT film showing Ca middle third esophagus
[image:94.612.145.507.409.680.2]78
79
Fig. 35: Malignant stricture causing 80% narrowing of lumen
[image:96.612.145.508.430.702.2]80
Fig. 37: Malignant tumour of lower-third of esophagus
81
Figure


Related documents
The results of this study indicated a high prevalence of behavioral risk factors (smoking and alcohol consumption), obesity, hypertension, diabetes and
To demonstrate that transepithelial transport was mediated by HIV-1 gp120 and not by receptors from the H9 cell line used to grow the virus, fluorescent polystyrrol microspheres
Infection with carbapenem resistant A.baumannii significantly increased the risk of mortality in the patients included in the study. Thus it proves the fact that infections with
74 E A+ve Antral wall thickening Antrum Adeno ca Antral Growth fixed to Pancreas, LN ascites..
Group A received 2mg/ kg of bupivacaine in 60ml of normal saline immediately after creation of pneumoperitoneum.. Group B received 2mg / kg of bupivacaine in 60ml of
Jejunal mucosa, axillary (Ax-LN) and mesenteric (Ms-LN) lymph nodes, and blood samples were collected at necropsy from SIV-infected animals during the primary acute (n 5 3) and
In the present study, we have analyzed in detail the cellular immune responses induced by influenza vi- rus nucleoprotein (NP) DNA and have established that both CD4 1 T cells
However the equilibrium in this reaction usually lies in favour of the free carbonyl group and amine, so that azeotropic distillation or use of dehydrating agent such as