Rochester Institute of Technology
RIT Scholar Works
Theses Thesis/Dissertation Collections
8-1-1982
An Investigation of the effect of an infrared
post-exposure latent image in a 3M dry silver film
Thomas J. CardinaliFollow this and additional works at:http://scholarworks.rit.edu/theses
This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contactritscholarworks@rit.edu.
Recommended Citation
AN INVESTIGATION OF THE EFFECT
OF AN INFRARED POST-EXPOSURE ON THE
LATENT IMAGE IN A 3M DRY SILVER™ FILM
by
Thomas J. Cardinali
A thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Science in the School of Photographic Arts and Sciences in the College of Graphic Arts and Photography of the Rochester Institute of Technology
August, 1982
Thomas J. Cardinali
Signature of the Author. -_ .
Photographic Science and Instrumentation David A. Morgan
Certified by , .
Thesis Advisor Ronald Francis
Accepted by -- .
ROCHESTER INSTITUTE OF TECHNOLOGY
COLLEGE OF GRAPHIC ARTS AND PHOTOGRAPHY
PERMISSION FORM
Title of Thesis AN INVESTIGATION OF THE EFFECT OF AN INFRARED
POST-SXPOSURE ON THE LATENT IMAGE IN A 3M DRY SILVER™ FILM
I Thomas Cardinali prefer to be contacted
each time a request for reproduction of this thesis is made
and to consider each request individually. I can be reached
at the following address.
International Business Machines, Corp.
River Road, Essex Junction, Vermont, 05452
AN INVESTIGATION OF THE EFFECT
OF AN INFRARED POST-EXPOSURE ON THE
LATENT IMAGE IN A 3M DRY SILVERTM FILM
by
Thomas J. Cardinali
Submitted to the
Photographic Science and Instrumentation Division in partial fulfillment of the requirements
for the Bachelor of Science degree at the Rochester Institute of Technology
ABSTRACT
An investigation to determine the effect of an
infrared post-exposure on the latent image in blue sensitive
3M Dry Silver film 7842 was performed. The film was first
given a step tablet exposure using the unfiltered output of
a tungsten source at 2856 K. A Wratten #87C filter was used
with the same source to provide the second, infrared exposure
over one half of the first exposure area. Absolute exposure
and exposure reciprocity were tested. Processing was by
immersion into a bath of 3M fluorocarbon FC-43 maintained at
127C/260F.
The original objective, to cause Herschel effect
bleaching of latent image, was not achieved. All IR exposures
density change. A quanta ratio of 3*106 between the infrared
and actinic exposures was obtained using an IR exposure time
of 96 hours. The lack of any density decrease due to the
Herschel effect apparently gives evidence to the theory that
the Herschel effect is a rehalogenation process as reported
by Farnell and Birch. The presence of halogen acceptors in
the emulsion is believed to prevent the loss of latent image
silver atoms due to rehalogenation. Further, the emulsion
carrier and developing agents are IR absorbers and reduce the
ACKNOWLEDGMENTS
I wish to express my thanks to David Morgan and his
staff at 3M for their hospitality and assistance with this
project during my trip to St. Paul on 3/16/82. I would like
to especially thank Doyle Strong, Roger Shaw and Bob Hawksford
for their help in obtaining the processing fluid and films
required for this project. I would also like to thank Greg
McCarney for showing me 3M's Dry Silver research laboratories
and for suggestions in establishing and maintaining process
control with both heated roller and fluid bath processing
schemes.
I would also like to thank Bill Dahlen and Erich
Florentine of Sperry-Univac for allowing me to spend a day
consulting with my advisor during my plant interview in
St. Paul.
I would like to thank the Central Intelligence
Agency for their financial assistance in this project.
TABLE OF CONTENTS
Page
LIST OF TABLES iv
LIST OF FIGURES v
INTRODUCTION 1
I The Herschel Effect 1
II 3M Dry Silver 4
III Aim of Research 7
EXPERIMENTAL 9
DISCUSSION OF EXPERIMENTAL 12
SUMMARY & CONCLUSIONS 19
LIST OF REFERENCES 22
APPENDIX A 27
APPENDIX B 30
VITA 35
LIST OF TABLES
Page
Table 1. 3M Dry Silver Films 28
Table 2. Comparisons of Quantum Exposures 29
LIST OF FIGURES
Page
Figure 1. Dry Silver Development 5a
Figure la. Dry Silver Developing Agents 5a
Figure 2. RLF Sensitometer 10
Figure 3. Refrigeration Test 16
Figure 4. Oven Test 17
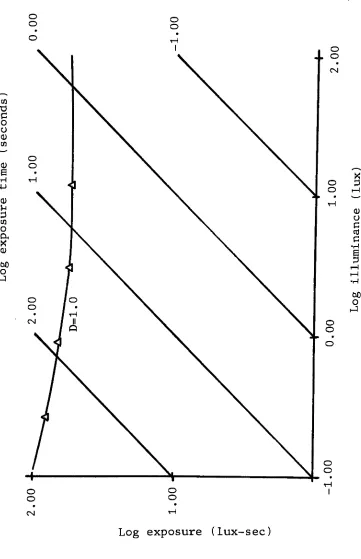
Figure 5. Reciprocity Curve 7842 31
Figure 6. Spectral Sensitivities 32
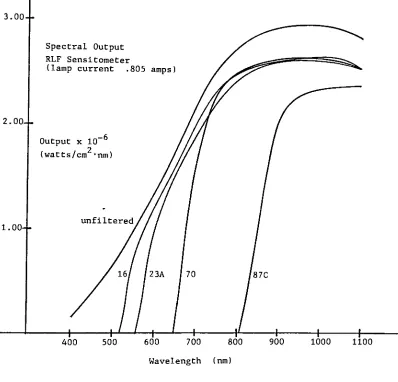
Figure 7. Spectral Output 33
Figure 8. Heat-Developing Dry Silver 34
INTRODUCTION
I The Herschel Effect
The Herschel effect generally refers to the
reduction of density of an exposed silver halide emulsion by
the destruction of surface-latent image by a post-exposure to
red or infrared radiation. This was discovered in 1840 by
Sir John Herschel as he projected a spectrum onto silver
chloride paper which was simutaneously exposed to diffuse
daylight. He observed the printout of photolytic silver in
2
all areas except where the spectrum was "full and firey red."
Herschel effect is now used to describe the destruction of
3
latent-image silver clusters rather than printout silver.
Two different theories were found to best describe
published experiments. The first involves a redistribution of
4
developable latent images to an undevelopable site. This is
best illustrated by a special case of the Herschel effect
mechanism, called the Debot effect. This can be shown by
silver halide crystals which have been given an actinic
exposure to produce both surface and internal latent images.
After exposure the film is treated with a chromic acid solution
and washed. This acid treatment will bleach any surface latent
images, leaving only internal sites. If the film is now
will result. If the acid treated film is exposed to red
radiation before processing an increase in density will be observed as compared to areas which received no red exposure.
This is explained as a redistribution of latent image silver
atoms from undevelopable internal sites to surface sites
where they can be developed. This redistribution is caused
by the red radiation.
The mechanism is as follows. Upon exposure to
actinic radiation, the absorbtion spectrum of the film shifts c.
toward longer wavelengths as more and more silver metal
latent-images are formed. Their absorbtion spectrum lies further toward the red end of the spectrum than the silver
halide alone. If a photon is absorbed by a latent image,
a silver atom will be lost. Given sufficient exposure, that
latent image will be destroyed. Though absorbtion of any
photon by the latent image will cause the loss of a silver
atom, red and infrared photons are unique in that they will not contribute to the formation of a latent image should it
strike the crystal instead; unless of course the crystal was
deliberately sensitized to those wavelengths. This theory
maintains that the atoms comprising an exposed latent image
will be involved in the reformation of a latent image at another
location in the crystal. If the crystal has no surface latent
images due to a chromic acid treatment, upon red exposure some
internal latent images should be redistributed to the surface,
developer.
This same mechanism operates to cause the Herschel
effect. Because the surface region of the crystal is a small
fraction of the total crystal volume, when an exposed,
unbleached crystal is exposed to red radiation, there is a
greater probability of surface sites being moved inside than
for internal sites being moved to the surface. Upon develop
ment with a surface acting developer, a density decrease is
Q
realized. This is true only if the trap to which the silver
atom is moved is equivalent to or deeper than the one from
which it originated.
The second theory proposes the rehalogenation of
9
the Herschel bleached silver latent image. If the halogen
acceptance of the carrier medium is low, the silver ion
produced when a photon is absorbed by a latent-image cluster
can recombine with the positive holes resulting in an image 10
loss. When halogen acceptors are present in the film, the
recombination is prevented and the latent image remains intact.
The amount of quanta required to achieve the
Herschel effect bleaching of a latent image has been estimated
to be to 1010 times the number of quanta as it took to
1 1
form it. One interpretation of this value may be that it
12 represents the ratio of the crystal area to latent image area.
The quanta of each exposure can be calculated from the spectral
output of the source using suitable a
thus the actual quanta ratio as well.
3a
Many different photographic imaging effects have
been produced using the Herschel effect and conventional 1 /
silver-halide films. By altering the exposure sequence,
it is possible to produce reversal imaging, and sensitization
II 3M Dry Silver
3M Dry Silver is a negative-working silver halide
based film system using incorporated chemistry and thermal
activation of development. The process was developed to
provide a rapid access system which did not require aqueous
processing chemistry. Dry Silver was evolved from taking
a thermally induced silver producing reaction and making it
16 light sensitive (UV sensitive as well).
Dry Silver consists of a light-sensitive silver
halide in catalytic proximity to the source of the image
17
forming silver compound which itself is insensitive to light.
The silver halide is used only to produce latent images in
exposed areas which catalyze the thermally initiated reaction
between a silver soap and a developing agent. The silver
halide is not significantly consumed in the production of the
silver image. The image silver is supplied by the silver soap,
18
specifically silver behenate. In most respects this product
19 has characteristics similar to equivalent, conventional films.
The silver halide emulsion can be precipitated by
three different methods. The two-coat method forms the silver
halide at the interface between a coating of silver behenate
20
and a coating of potassium bromide. The preform method adds
a separately precipitated silver halide to an emulsion of
9n
used in film manufacture. In this method, the silver halide
is precipitated by the addition of potassium bromide directly
7^
to an emulsion containing an excess of silver behenate.
After development of the film, the silver halide remains
intact; no conventional fixation is accomplished. This
necessitates the use of very small silver halide crystals,
0/
<0.5 \im in diameter, to maintain a low D-min in the processed
film. The use of such small crystals also limits the sensi
tivity of the film and helps maintain good resolution
25
(-300 lp/mm) . Dry Silver may also be dye sensitized to
9 ft provide desired spectral sensitivities.
The preferred carrier for Dry Silver emulsions is
polyvinyl butyral. The use of this material helps maintain
27
the dimensional stability of the image due to softening.
Cellulose acetate is used as a protective overcoat to prevent
the loss of reagents or the diffusion of harmful substances,
such as oxygen, into the film. Film treatments which rely
on the diffusion of aqueous reagents into the emulsion are
also prevented, such as the conventional fixation of silver
halide and the latent image experiment used to illustrate the
28 Debot effect.
The development of Dry Silver is represented in
29
Fig. 1. The temperature required for standard development
30
is 127C/260F. Developers are hindered phenols, weak anti
oxidants composed of double and triple benzene rings (see
5a
*-K _ ,.
+ R'-OH - Ag + R'=0
N0 Ag+
<Ag>
Figure 1.
Dry Silver Development
Example 1
CH:
I
, OH i
CH-1
tC-1
CH:.
9
CH
Example 2
CH3 OH
OH CH3
I I
-CHtj^j-C-CH;.
ch3
CH3
OH OH OH,
CHtC ^N -CHT ^s -CHT
^^|
-C-CH,I I | CH3
CH3 CH3 CH3
Figure la.
[image:16.502.59.344.223.641.2]in order to control the activity of the developer at the
temperatures encountered by the film. Hindered phenols
derive their stability from the great difference between the
temperature of development and normal storage conditions
(dark storage, 22C/72F, 50% RH).31'32
At the temperature
33
of development, the developing agents are quite reactive.
The proportions and concentrations of the developing agents
used directly affect the sensitivity and stability of both
processed and unprocessed material.
Development is accomplished by heating the film
34
using one of several methods. The most common are a
heated roller and high temperature fluid. The heated roller
offers a dry, simple and convenient means of processing. Its
performance, however, is inadequate for use in a rigorous
sensitometric investigation. Problems encountered with
cross-web uniformity and frequent destruction of small film
35
strips made it unsuitable for use in this project. The
high-temperature fluid offers the best means of development
and is the method used by 3M for testing Dry Silver materials
This method requires a fluid which boils at a temperature far
above the desired processing temperature and does not react
37
with the film's emulsion. The fluid used by 3M is their
own product, FC-43, an inert fluorocarbon with a sufficiently
low vapor pressure to remain stable at development temperature
38 (actual boiling point unspecified).
8
It may be possible to use the Herschel effect to erase latent
images from the silver halides and retain sensitivity so that
another image may be exposed and developed in the undeveloped
regions of the first image.
A reversal image would be produced by prefogging
a piece of film just to D-max and then giving a red exposure
in an image-wise fashion. By exposing just to D-max the
maximum tonal range of the film will be utilized. The minimum
exposure required to produce D-max should be used because the
additional, unnecessary latent images produced would require
additional exposure to bleach a given amount of density.
Regions receiving exposure will decrease in developed density
due to the destruction of surface latent image sites. Upon
42
EXPERIMENTAL
Dry Silver film 7842 (see Table 1.) was exposed in a modified Kodak 101 Process sensitometer. A Kodak #2 step
tablet was held in contact with the film emulsion. An exposure
time of 5 sec. at an illuminance of 878 lux at the first film
plane (see Fig. 2.) resulted in an open gate exposure of
4390 lux-sec or LogH = 3.64. This
exposure in combination
with the #2 step tablet produced both D-min and D-max on one
film sample over 21 steps.
The film was processed by immersion into a bath of
3M fluorocarbon FC-43 held at a temperature of 127.0C/260. 6F.
Development time was 30 seconds. The film was handled under
recommended safelights throughout the experimentation.
Densities were measured using a Macbeth TD-504 diffuse,
trans-44
mission densitometer.
Herschel effect exposures were accomplished by
covering half of the sensitometric exposure and replacing the sample into the sensitometer without the step tablet. The desired filtration was placed in the sensitometer and the
shutter was held open for the required exposure time. Exposure
reciprocity was tested by locating the film sample at different distances from the source to vary illuminance and adjusting the
45
Ill Aim of Research
Little research has been performed to determine how
Dry Silver materials respond to unusual photographic effects
often observed in conventional silver halide materials.
Research and development has been limited primarily to the
characterization and perfection of the system for its intended
39
use as a copying, data recording and graphic arts material.
Any additional research into this system is desireable and
could have practical application. With this system, the
Herschel effect could be used to produce some useful latent
image changes. They include the erasure of fog, add-on of
an image and the production of a reversal image.
The erasure of fog would be accomplished by treating
a fogged emulsion with red radiation before the imaging
exposure. The red exposure would decrease fog by breaking
up small fog latent image centers. Fog latent images, how
ever, may not be directly interchangeable with latent images
caused by an exposure which results in the same density after
processing. This is because fog latent images are formed over
a long period of time and are more stable than those caused
by an actual exposure to radiation.
The add-on of an image requires a material which
does not remove the light-sensitive material after the
10
Figure Z.
11
The output of the RLF sensitometer was calibrated
using an EG&G Photometer-radiometer set up for both illuminance
and irradiance measurements. This was repeated with the
filters in place to quantify their spectral transmittances.
A spectrosensitometer was used to determine the spectral
sensitivities of the films investigated. All exposures in
12
DISCUSSION OF EXPERIMENTAL
The actual experimentation performed differed
radically from that originally planned.47
This was a result
of unanticipated difficulties encountered with both materials
and equipment involved with the project. Only the blue
sensitive (7842) Dry Silver film was used for all the exper
imentation. This choice was based on measurements of the
spectral sensitivities of the three available films and the
spectral transmittances of the required filters. All of the
films exhibited residual sensitivities for building latent
images upon post exposure to the long wavelengths intended
48
for Herschel effect bleaching. Experimentation was first
performed using the blue sensitive film because it had the
least sensitivity to the red exposures and should therefore
exhibit the Herschel effect more easily than the other films.
The blue sensitive film, 7842, showed no sensitivity
to wavelengths longer than 620nm under the test conditions,
as seen from the spectrosensitometer exposures. The green
and panchromatic films, 7859 and 7869 respectively, both
exhibited sensitivities to wavelengths beyond the long wave
length limit of the spectrosensitometer (>720
-near IR) .
The Wratten filters #16 and #23A both transmit significant
13
respectively and were thus unsuitable for the Herschel effect
exposures with any of the films.
The Wratten filters #70 and 87C seemed most ;
appropriate for the Herschel effect exposures. However, most
experimentation used the 87C filter, as most exposures using
the 70 filter would result in density increases.
The RLF sensitometer was used for both actinic and,
with suitable filtration, Herschel effect exposures. The
output of the tungsten source is mostly in the red and infra
red region of the spectrum. With filtration, it provides a
51 convenient, repeatable infrared source.
The set up of a suitable process for Dry Silver film
required the greatest effort. Initial experimentation using
two different heat shoe/drive roll type dry processors showed
that development uniformity was inconsistent. This was
primarily due to variations in the speed of the roller
transport. This processing method isn't wholly unuseable;
however, the results obtained are less than satisfactory for 52
use in a rigorous sensitometric investigation.
On recommendation from Morgan of 3M, an effort
was made to set up a process utilizing an inert
high-temper-ature fluid. This mode of processing is used in the Imaging
Research Laboratories of 3M. One gallon of fluorocarbon FC-43
was originally provided for this project. Unfortunately, the
temperature control bath available required a minimum of two
14
to function properly. Because the FC-43 was quite expensive,
an effort was made to set up using one gallon or less of the
fluid.
The temperature control bath was filled with
ethylene glycol (boiling point 192C) and a beaker of FC-43
was immersed in the fluid. The FC-43 required circulation to
insure the repeatability and uniformity of development.
A pneumatic-magnetic stirrer and a propellor stirrer were
tried and required special film handling procedures. Good
results were obtained with this set-up. However, it was very
inconvenient and tedious to use.
At this point, an additional two gallons of FC-43
were provided, and the temperature control bath was filled
with this liquid. This allowed the use of a simple, easy to
use film holder and eliminated the noxious fumes of the
ethylene glycol and the ungainly apparatus required previously
The actinic exposure was chosen to produce both
D-min and D-max on the same film sample. The Herschel effect
exposures were determined empirically. Initially, investigation
of the significance of both actinic and IR exposure reciprocity
showed that longer exposure times produced apparently better
results at a fixed exposure level. The shorter exposure times
caused the IR exposure to result in a density increase. As
the exposure time was increased, at the same exposure, the
film showed a decrease in the amount of density build-up
after the IR exposure. The exposure time was lengthened until
15
a time was used which did not cause a density increase.
At this point, the IR exposure was increased
by lengthening
the exposure time at a fixed irradiance. The exposure was
increased in two-stop increments, and an exposure time of
96 hours was used at the project's
termination.56
The design of the experiment yielded test strips
separated by many hours, and, in several instances, days.
Because of this, it was impractical to process immediately
after the termination of an exposure. Frequent operation of
the temperature control bath caused significant losses of
FC-43 due to evaporation. From the beginning of the experi
ment all unprocessed films, both exposed and unexposed, were
stored under refrigeration for processing together at a later
time. A test was devised to investigate any latent image
changes which may have resulted from such storage. At the
same time the effect of storage at both room temperature and
elevated temperature on test exposures was determined.
Standard sensitometric exposures were made over 5 consecutive
days and a portion was stored at 4.5 C, 20 C and 50 C. All of
the strips were then processed in a random order in one
58
session.
The refrigeration test indicated no significant
change in film latent image during 5 days of storage (see
Fig. 3.). The test at 50 C showed a significant decrease in
film speed and an increase in contrast over the same length
16
D E N
S I T
2.8 -r
2.4__
2.0
--1.6
--1.2 -"
0.8
--0.4
--Refrigeration Test
Dry Silver 7842
C - Control 1-21 hrs II - 45
III - 73
IV - 94.5
4.5C
III
0.80 1.20 1.60 2.00 2.40 2.80
Log exposure (lux-sec)
3.20 3.60
Figure 3.
[image:27.502.40.449.226.583.2]17
2.8
->-Oven Test 50C
2.4 -- Dry
Silver 7842
C - Control 1-21 hrs II - 45 III - 73 IV - 94.5 V - 117
0.80 1.20 1.60 2.00 2.40 2.80
Log exposure (lux-sec)
3.20 3.60
Figure 4.
[image:28.502.36.418.228.576.2]18
blue sensitive film indicates normal latent image stability.
The lack of Herschel effect bleaching could not be attributed
to an extraordinary latent image stability exhibited only by
59
this film. These tests were required to provide justifica
tion for waiting until several Herschel effect exposures had
been collected before operating the high-temperature bath.
This resulted in a minimal loss of FC-43 during the
. 60,61
experiment.
19
SUMMARY & CONCLUSIONS
The objective of this project was never achieved.
No detectable Herschel effect bleaching of latent image was
ever accomplished. On the contrary, the only post-exposure
change due to the infrared exposure was a density increase.
This was a result of the conversion of sub-latent images to
full latent images by the infrared exposure. A ratio of
3><106, red to actinic quanta, was achieved in this experiment,
ft *?
using a 96 hour exposure time ! Significant increases in
this ratio would have required prohibitively long exposure
times and were thus not investigated. Doubling the ratio
would require a doubling of the exposure time. All exposures
were made at room temperature. No variation of the film
temperature during the exposure was investigated.
The Herschel effect does not operate in this silver
halide system. The redistribution theory of the Herschel
effect as reported by Debot, Falla,, Hautot, '
Eggert and
67
Heimann, was used as the theoretical basis for this project.
According to them, the redistribution operates within an indi
vidual silver halide crystal and is independent of the emulsion
composition. The incident radiation acts to move a developable
surface latent image to an undevelopable internal site.
20
rehalogenation of the latent image silver as reported by
6R
Farnell and Birch. This theory adequately accounts for the
imaging effects observed in this project. The presence of
halogen acceptors in the Dry Silver films would prevent the
rehalogenation from occuring, leaving the latent image intact.
Heating this same emulsion would release the holes, promoting
latent image fade at high temperatures. Both of these obser
vations are consistent with the experimental findings of this
project.
The emulsion composition was looked at to determine
the hole trapping ability of its constituents. The developing
agents used were found to be strong hole traps. The benzene
ring structure is a strong halogen acceptor and the hindered
71
phenols used as developing agents include this structure.
The hindered phenols must be included in the emulsion in such
concentrations as to provide fast, uniform and complete
development of the film. These same concentrations would be
sufficient to absorb significant quantities of photo-released
u 1 72
halogen.
The long chain hydrocarbons used as emulsion
carriers, though not halogen acceptors, are strong absorbers
73
in the infrared. The hindered phenols are IR absorbers as
well, and, together, they act as an infrared filter, reducing
the number of infrared photons available for latent image
destruction. Together these two factors combine to effect
21
image in Dry Silver films.
In conclusion, the theoretical basis for this experi
ment was faulty. This film was chosen for its potential to
exhibit the useful effects of reversal, add-on, and fog
erasure simply by altering the exposure sequence. The compo
sition of the film, however, keeps the Herschel effect from
operating and preventing the desired film response. Though
the objectives of this project were not achieved, the results
are consistent with the recent work done in this field by
Farnell and Birch. This experiment adds further evidence
to their theory of the Herschel effect as a rehalogenation
process the loss of latent image is the result of a
photo-induced break-up of the latent image and subsequent rehalogen
22
23
LIST OF REFERENCES
1. Farnell, C.G., D.C.
Birch, "The Herschel Effect as a
Rehalogenation Process,"
J. Photo. Sci, 27, 145, (1979).
2. Hillson, P.J., "Literature Review of the Herschel Effect,"
J. Photo. Sci, 10, 182, (1962).
3. ibid.
4. Saunders, V.I., R.W. Tyler, W. West, "Herschel Effect in Single Crystals of Silver Bromide,"
J. Chem. Phys,
16, 206, (1966).
5. James, T.H., The Theory of the Photographic Process,
4th ed, MacMillan, New York, 1977,
p 185.
6. Hillson, p 182.
7. James, p 185.
8. James, p 184.
9. Farnell, p 145.
10. ibid.
11. Hillson, p 183.
12. Francis, R. , Conversation, 5/5/82.
13. Francis, R. , Lecture in R.I.T. course 0907-421, 9/10/80.
14. Hillson, pp 182-186.
15. Morgan, D.A. , 3M's Dry Silver Technology, Address to
Photoscientists of Japan, 3M Co., St Paul, Minnesota, p 1.
16. ibid.
17. Barry, D.G., Dry Silver Technology, 3M Microfilm Products
Division, 3M Co., St Paul, Minnesota, p 1.
18. Morgan, p 6 .
24
20. Morgan, D.A. , 3M's Dry Silver Technology, Presented at
Meeting of the institute of Image Electronics Engineers
of Japan, 3M
Co., St Paul, Minnesota, 9/5/80, p 2.
21. ibid.
22. Morgan, D.A., Conversation
during visit to 3M Imaging Research Division, St Paul, Minnesota, 3/16/82.
23. Morgan, p 2.
24. Francis, R. , Lecture in R.I.T. course 0907-532, 12/8/81.
25. 3M Data Sheet for Dry Silver Product 7842.
26. Barry, p 1.
27. Morgan, p 6.
28. Mees, C.K.& T.H. James, The Theory of the Photographic
Process, 3rd ed, MacMillan, New York, 1966, pp 158-9.
29. Barry, p 2.
30. Morgan, p 6.
31. Morgan, D.A., Telephone conversation, 2/8/82.
32. 3M Data Sheets for Dry Silver Products.
33. Morgan, telecon, 2/8/82.
34. 3M Data Sheet, "Developing 3M Brand Dry Silver Films
and Papers," January 1978.
35. Cardinali, T., Laboratory Notebook, 3/20/82, pp 9-10.
36. Morgan, conversation, 3/16/82.
37. ibid.
38. ibid.
39. Morgan, telecon, 10/2/82.
40. Mees, p 342.
41. Francis, R. , Lecture in R.I.T. course 0907-531,
9/23/82-9/24/82.
25
43. Laboratory Notebook,
4/16/82, p 31.
44. Laboratory Notebook,
4/12/82, p 26.
45. Laboratory
Notebook, 4/18/82, p 40.
46. Laboratory Notebook, 3/19/82,
p 7.
47. Cardinali, T., "The Herschel Effect
Bleaching of Latent
Image in 3M Dry Silver Films,"
Proposal for Research,
10/19/81, pp 9-14.
~
48. Laboratory Notebook, 4/6/82,
p 20.
49. Laboratory Notebook, 3/31/82-4/2/82,
pp 13-18.
50. Laboratory Notebook, 4/19/82, pp 42-45.
51. Francis, R. , Conversation, 5/13/82.
52. Laboratory Notebook, 3/20/82, pp 9-10.
53. Morgan, Conversation, 3/16/82.
54. Laboratory Notebook, 4/12/82-4/22/82, pp 25-48.
55. Laboratory Notebook, 4/28/82, p 50.
56. Laboratory Notebook, 5/13/82, p 74.
57. Laboratory Notebook, 5/4/82, p 57.
58. Laboratory Notebook, 5/10/82, p 63.
59. laboratory Notebook, 5/11/82, pp 69-70.
60. Laboratory Notebook, 4/7/82, p 23.
61. Hawksford, R. , Telephone conversation, 4/23/82.
62. Farnell, p 145 .
63. Laboratory Notebook, 5/13/82, p 74.
64. Hillson, p 184 .
65. ibid .
66. ibid .
67. ibid .
26
69. Farnell, p 182.
70. Farnell, p 184.
71. Morgan, p 6 .
72. Haist, G., Conversation at the 35th S.P.S.E. Convention,
Rochester, New York, 5/13/82.
73. Westbrook, J., Conversation, 5/17/82.
74. Hardesty, R. , Telephone conversation, 5/17/82.
27
3M DRY SILVER FILMS
28
Film
(Lot number)
7842
001x94001-15
7859
C002x43024
7869
31-1425
Spectral
sensitivity blue green pan
D-max 2.6 2.3 3.5
D-min 0.12 0.10 0.20
Gamma 1.7 1.3 3.0
Resolution
cycles/mm
300 250 300
Log E Range
ALogE from(Dmin+.04)
to(90% of Dmax)
1.8 1.6 1.7
Safelight filter 1A or low OC 1A 1A or low OC
Table 1.
29 r^ vo co ll cu X 4J o o to
o 00o
i-H
H
I-l T-l tH
pj m CO 04 X X ro cO + o cu CO 00 c
O 1 ai
H X 4J
C 3 u
CU CM
H i-H cO
U e J-) !-i
D o O CM S
CO ^* ctl r^
o co ^D r
CX C <r >
X o u; 4-> M
cu -< 4J r.
o II
o o O .O a
1-1 i-H X! T-t X o
cu a ^ P H
Si X X o i-i U
o CH H
co o O vO CO
^1 00 00 10 o
CU .
CU <r a
X r-
r-CO o
X CO
Vi
CM a O 3
cu E X CU o
Vi o cu CO XI
p ^-.
co CO E o ^>
o C 3 CN O
a o u co
X " u 2 C
w o o o CTJ
r-t Si tH 3 1
u a cr 1 1
H X X CO 1
e CU CU CU
I-l m in Si M cu >-l
4-> ro ro 4-1 D u 2
O CO a CO
< CNI CM CW o CO o
O X o a, a X o cu X cu w
O co I-l i-i
is I-l CU o cu
C cu V-i.fi I-l Si
CU -U o CO o c o
acu . a,co I-l CO O 00 ro E ^ J-l Vj
o cu o CU
+ O X < X
Table 2.
[image:40.502.42.288.106.608.2]30
31
o
o oo
co C O O CU co cu E i-i u cu 0 CO o a X cu 00 o pj X 3 cu o CO a H 00 o pj CN
Log exposure (lux-sec)
Figure 5.
[image:42.502.63.424.93.652.2]32
-- 10.0
8.0
Relative Spectral Sensitivities
of Dry Silver films
360 400 440 480 520 560 600
Wavelength (nm)
640 680 720
[image:43.502.32.485.264.506.2]Figure
33
3.
00--Spectral Output
RLF Sensitometer
(lamp current .805 amps)
2.
00--Output x 10 2
(watts/cm -nm)
1.00--400 500 600 700 800
Wavelength (nm)
900 1000 1100
Figure 7.
[image:44.502.61.459.178.560.2]34
aO ^"^"^"^qO
y
/
<?
/ SAME CURVES
.^Oo^
LOG E
Figure 8.
[image:45.502.145.360.185.497.2]36
VITA
Thomas Cardinali is a U.S. citizen born on January
22, 1960 in Fulton, N.Y. to Robert and Virginia. The youngest
son of a Cornell graduate in chemistry, turned professional
photographer, an early intrest in both fields was fostered.
In 1977, he spent the summer with a family in Pasching, Austria
on an American Field Service scholarship. Tom graduated with
honors from the G. Ray Bodley H.S. in June 1978 and entered the
Photographic Science and Instrumentation program at the Roch
ester Institute of Technology that fall. He felt this would
be an ideal combination of his intrests as well as preparing
him for a career in the microelectronics industry. There he
chose electives within the Photoscience Division and maintained
a 3.30 G.P.A. In 1982, Tom was chosen to receive the Fuji
Scholarship for academic achievement by a Photoscience senior.
Tom was employed by the Photo Cage at R.I.T. and
during the summer of 1981 by E.I. DuPont in Rochester, N.Y. as
a Quality Control Technician in the Test Methods Division.
Tom is presently employed by the International Business Machines
Corporation, in their General Technologies Division in Essex
Junction, Vermont as an engineer in the Mask House.
Tom is an avid motorcyclist and photographer, enjoys