JOURNALOFVIROLOGY, OCt. 1968,p.1191-1199 Copyright (D1968 American Society forMicrobiology
Vol.2,No. 10 Prin7tediniU.S.A.
Morphology of the Nucleoprotein
Component
of Rabies
Virus
KLAUSHUMMELER, NATALE TOMASSINI, FRANTISEK SOKOL, ERNST KUWERT, AND
HILARY KOPROWSKI
Divisioni of Experimental Pathology, TheChlildreni's HospitalofPlhiladelphiai, Phliladelphia, Pennsylvania 19146,
Departmenit ofPediaitrics, School of Medicinte, Uiiiversity ofPennsylvania, antd The Wistar Ilnstituite of
Aniatomy and Biology, Philadelphia, Pennsylvania Receivedforpublication 28June1968
Theintracytoplasmic ground substance, ormatrix, associated with the develop-ment ofrabies virus and the nucleocapsid ofthe virus were investigated. The fila-ments of the matrix were identified as virus-specific by means of ferritin-labeled
antibodies. Inthinsections, the diameter was 15 nm and the strands seemed tobe incorporated into virions during morphogenesis of the virus. The nucleocapsid
was isolated frompurified viruspreparations andwas studiedin negative contrast.
The rabies nucleocapsid appeared as a single-stranded helix with a diameter of
16nmandaperiodicity of7.5nm;itslengthwasinexcessofI,im.
Themorphogenesis of rabies virus is inevitably associated with the formation of an
intracyto-plasmic
ground substance or matrix. This has beenobserved inbrain tissue
of mice (14, 23, 24, 29)aswellasin tissue cultures (3, 8).This filamentous matrix constitutes the
ma-terial
of theNegri
body in the infected brain of animals (25), and its development precedes the formation ofvirus particles, as studied in tissue cultures(13).
Fluorescent antibody studies haveyielded
indirect evidence that the substanceof
the Negri body, aswell as thesamematrixininfected tissuecultures,
isspecific
for the rabies virus(2,
10). From the
foregoing
evidence itwassuggest.d that the strands which constitute the matrix are thenucleoprotein
of the virus(13).
A method has
recently
beendeveloped
which allows forproduction
ofhighly
purified
rabies virus (32). With this method, animalsinjected
with the
purified
virus preparationsdevelop
high concentrations
ofspecific
antibody
in their sera. These sera, afterconjugation
withferritin,
provide
direct evidence that the strands in the matrices are indeedvirus-specific
and thus give someinsight
into the role of these strands in themorphogenesis
of rabies virus. Furthermore, isolation of thenucleocapsid
frompurified virus,
containing
all of the viral ribonucleic acid (RNA),permits
thedeterminationofitsstructureand its identification with the strands of the matrix.
MATERIALS AND METHODS
Vir-us.
Thechallenge virus standard (CVS), Flury high egg passage (HEP), and Pitman-Moore (PM) fixed strains of rabies virus were used, as indicated below.Vir-us-tissueculture systems. (i) In the ferritin
con-jugateexperiments, monolayers ofBHK-21 cells (22) were maintained in Basic Medium Eagle (BME) in Hanks' solution supplemented with 10%'- fetal calf serum. Afterremoval ofthemedium, the cell mono-layers were washed and exposed for 1 hr at 37 C to CVS-virus at a multiplicity of5. The virus was sus-pended in maintenance medium consisting of
2c%,
fetal calfserum in BME to which 50 ,ugof diethyl-aminoethyl dextran per ml had been added (15). After virus adsorption, the cell monolayers were washed, maintenance medium was added, and the cultureswereincubatedat 34C. (ii) To prepare puri-fied virusforimmunization, thePM strainwasgrown in Nil-2 cells(9) and processed as described previ-ously (32). (iii)Topreparepurifiedvirus for the isola-tion of thenucleoprotein component, theHEP-Flury strainwaspropagatedinBHK-21cells in thepresence of 3H-uridine and was purified and concentrated as before (32).Immunle
seruim. Thepurified viruspreparation hadaproteincontentof300,pgper mlasdeterminedbythe Lowry method (19). This material was used for im-munization of adult rabbits (WhiteNewZealand).
Theanimals were first immunized subcutaneously withadoseofabout 0.03 pg ofprotein;2weekslater they received 100 pg of protein, emulsified in equal
parts of complete Freund's
adjuvant,
injected
intra-dermallyinto thefootpadsandat twosites in the chest region. After this, boosterdosesof100pg ofproteinperdose, withouLt adjuvant, weregiven at 1-, 2-, and 5-week intervals bytheintramuscular route.
1191
on November 11, 2019 by guest
http://jvi.asm.org/
Nil-2cells, BHK-21 cells, and bovineserumalbumin used as a protein supplement in the tissue culture medium. Againstthehomologous virus,the absorbed serumhadaneutralizing antibodytiter of 1:60,000in the 50% plaque reduction test (40 plaque-forming units) and a complement-fixing titer of 1:2,000 as
measuredagainst 10complement-fixing antigen units of the CVS strain.
Ferritin conjugation. Serum globulin was precipi-tated from the above antiserum bymeansof sodium sulfate andwassubsequently conjugatedto recrystal-lized ferritin (1).
Stainingprocedure. Four days after infection, the cellmonolayers were washed with
PBS,
fixedbriefly
with 4% Formalin, and frozen in situ by means of dry ice. After rapid thawing, the antibody-ferritin conjugate, diluted 1:10 in PBS, was addedand the monolayerswerestained for 1 hrat roomtemperature. After repeated washing with PBS, the cells were
re-moved byscraping;thentheywerefixed in3%
glutar-aldehyde,washedagaininPBS,andpelleted.The cell pelet waspostfixed in osmic acid, dehydrated, and embeddedinepoxy resin.
Isolation of the
ribonucleoproteini
component. The purified virus preparation was mixed with an equal volume of2%sodiumdeoxycholateindistilledwater.The mixturewaskeptat4 Cfor2min.Subsequently, 1.4mlof the mixturewaslayeredon alinear10 to30% sucrose density gradient (32). The
gradient
wascen-trifugedinanSW 25.1 rotorina
Spinco
modelLcen-trifuge for 1 hr at 50,000 X gat 4 C. Thenarrow
bandcontainingthelabelwascollected.
Electron microscopy. Thin sections were stained with uranylacetate and leadcitrate and wereviewed in a Siemens Elmiskop electron microscope at a
magnification of 10,500 X. For demonstration in negative contrast, a drop of the previously dialyzed gradient bandcontaining the nucleoprotein component was transferred to a carbon-covered Formvar grid bymeans ofa platinum loop. A drop of phospho-tungstic acid (pH 6.8) wasadded to this, and the ex-cessfluidwasremovedby filter paper. The preparation was transferred into the electron microscope while still moist and was viewed at a magnification of 50,000 X.
particles
and the absence of suchlabel
on cellconstituents demonstrated
thespecificity
of the reaction.The distribution of the ferritin on singleparticles
wasof interest.
Longitudinal
sections through aparticle (Fig. 4a) or above it (Fig. 4b andc) gave theimpression of ferritin
attachment on thespikes of
theviruses,
following
thehoney-comb
array of thespikes (Fig. 4c) which
has beendescribed
previously (13).
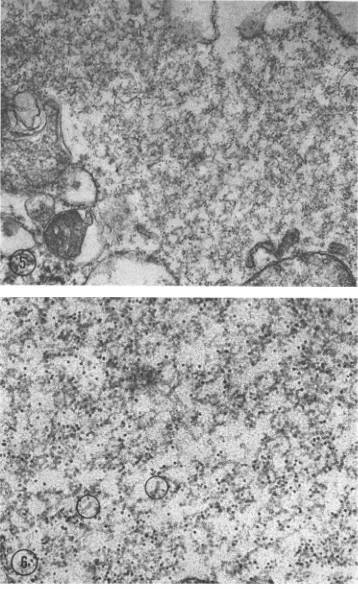
The
matrices
werefound
tobe labeled
heavily, totheexclusion of all
othercell
constituents (Fig. 5). At ahigher magnification,
the label wasre-stricted
to the strands of thematrix
(Fig. 6). Measurementsof these strandsvaried,
depending on theplane of sectioning
and possibly theembedding
procedures used. In areas indicating proper longitudinal sections, the diameter of the strands wasabout
15 nm.The
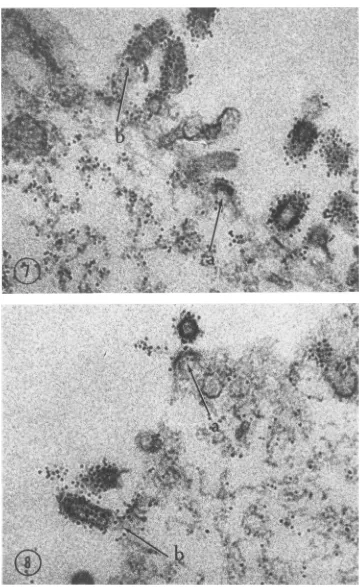
relationship
of thestrands to the buddingvirus particles
can be seen inFig. 7. The labeledstrands
are near the cellsurface,
some of themseemingly reaching into
thevirus
particles form-ing on themembrane.
This isalso evident in Fig.8;
in thisfigure, thematrix material
bears a close relationship to the virus particle which has beensynthesized
onthemembrane. The early budding process is shown in both figures. A thickening of themembrane
isevident with
labeled filaments as aninner
lining.
The nucleoprotein component obtained from
purified
viruspreparations
isillustrated
in Fig. 9. Thesingle
stranded helix had an average outsidediameter
of 15.6 nm and a periodicity of 7.5 nm. The single strand itself was about 3 nm wide. These measurements must be considered asap-proximations,
because the coils exhibited anirregular
periodicity. This lability may be an inherent property of the rabies nucleocapsid or anartifact
resulting from the procedures used. Most of the strands were tangled, making an accurate measurement of their length difficult.on November 11, 2019 by guest
jt*
v4
ik
r'
{t+S.
*4..'%S..e* A.
.
FIG. 1. Extracellular rabies virus labeled with ferritin. X 105,000.
FIG. 2.
Idenitification
ofrabies virus in cytoplasmic vacuole. No attachment offerritin to surrounding cellular material. X105,000.1193
on November 11, 2019 by guest
http://jvi.asm.org/
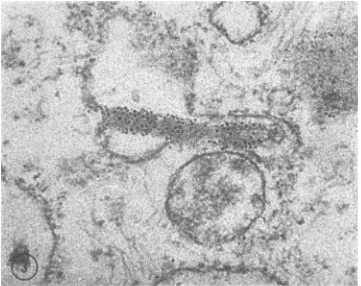
[image:3.489.62.423.25.609.2]FIG. 3.
Long.form
ofrabiesvirusincvtoplasmic
vacuole,labeledspecifically. AsinFig.
2,1toferritill
labelonl
cellcontstituentts. X 105,000... 1.-, :'
y
¢ ...
E L *'
.. t; t;
''t, t t
o .
Wt
FIG. 4. Distributiolnof
ferritinl attcached
to virusparticles.(A)
Sectionzthrouiglh
aparticle;
(B) sectioni
sliglhtl/
above;
antd
(C)sectionzaboveaparticle. Theferritiin
conjugate
seemingly
attachesottthespikes
ofthe virus(A
antd
B),
antd
thesurface view(C)
inidicatestheregular
oltlinie
ofthespikes.
X 152,000.on November 11, 2019 by guest
[image:4.489.73.434.75.361.2] [image:4.489.56.450.408.564.2]FIG. 5. Cytoplasmicmatrixislabeled distinctly. O0 FIG. 6. Highermagnzificationt of thematrixshIownI i
labelisconfinedto them. Measutrements ini selectedG
lim. X 105,000.
S
6,'o', @ b 44+v} '*
w
'A
~~~~~~~~~~I
i.. IF'
thei cellcomponelntsarefreefromferritint. X 52,500.
if the previousfiguire. Thestrandsarevisible,andthe fe, ireas, indicatedby circles, yielded a diameter of aboi
1195
?rritin tIt IS
on November 11, 2019 by guest
http://jvi.asm.org/
[image:5.489.69.427.32.621.2]4 * 1
ti
FIG. 7and 8. Outeredge ofadisintegratedcell. Thevirusparticlesand matrixstrandsnearthem are
specifi-callylabeled. Thzeconnection ofthestrandstoemerging virusparticles can be seen. Theearly buddingprocess
(arrow
a)and thecompletevirusleavingthecelllimits(arrow b) indicateapossibleroleofthematrixstrandsin themorphogenesis ofrabies virus. X 105,000.1196
on November 11, 2019 by guest
[image:6.489.66.426.23.610.2]NUCLEOPROTEIN COMPONENT OF RABIES VIRUS
FIG. 9. Pcart of theniiieleocaipsidofiabies irus. Asiiiglestrciudlecdhelical structure is evident. X 300,000.
It can be stated, nevertheless, that they were at least 1 ,um long, onthe basis ofmeasurements in areaswhere measurement was feasible.
DISCUSSION
The identification of complete rabies virions by means of
ferritin-conjugated
antibodies has been described by Atanasiu et al. (4). The main interest of the present investigation centered on the nature of the fibrils which constitute thecharacteristic
matrixand their roleinthe morpho-genesis of rabies virus. Morphogenesis of the investigated strain of fixed rabies virus parallels that ofcertain myxoviruses. The assembly of the viral components is similar to that of some paramyxoviruses, suchas parainfluenza type 2 or SV 5, although the nucleoprotein component is narrower (6, 12). Theintracytoplasmic
ground substance consists of strands about 15 nm indiameter,
asmeasured ontheir sections. Synthesis ofthenucleoprotein substance occursinthe cyto-plasm, with subsequent virus assembly on mem-branes. Apossible
mechanism of incorporation of theribonucleoprotein
into viral particles dur-ing their maturation onmembraneswasobserved onlyinfrequently
when labeled strands could be seen to be associated with thebudding
particle. In mostinstances,
the nucleoprotein fibrils wereonly found near the budding site or they were absent in the plane of the section.
Intracytoplasmicinclusionsconsisting ofhelices have been described for another member of the rabies group of
viruses,
namely thevirus ofviral hemorrhagicsepticemia
of the rainbow trout. Similar to theparamyxoviruses,
these helical strands havea diameterof 18 nminsections(34).
Theabilitytogrowrabiesvirus intissuecultures
to relatively high titers and then to purify it made itpossible to investigate the structure ofthe ribonucleoprotein in negative contrast.
Thefollowing evidence indicates that thehelices in Fig. 9 represent the viral nucleoprotein: (i) the bandobtainedaftercentrifugationina sucrose density gradient of deoxycholate-disrupted
puri-fied virus contained essentially all of the RNA present in the original viruspreparation, and (ii) this material reacted strongly in complement fixation tests withspecific antiserum but did not yieldanyhemagglutinin. Theincorporationofthe matrix strands into capsids during the morpho-genesis of the rabies virus and their diameter in sections make it obvious that they are identical with the nucleocapsid isolated from virions, as seenin negative contrast.
The data obtained are consistent with the data of Pinteric and Fenje (28), who foundhelical structures 15 to 16 nm in diameter after dis-ruptionofpartially purified virus; however, these structures were not shown to contain the viral RNA.
Similarly,
vesicular stomatitis virus, another member of thisgroup,wasfoundtorelease helices with a diameter of 15 nm after disruption (7, 20, 31).The morphology of the nucleocapsid of the investigated rabies strain exhibited a close rela-tionship with the nucleocapsids of some myxo-virusesofsubgroup II,such as SV (5),Newcastle diseas2 virus (16), and HVJ (11). Biophysical data have emphasized this similarity (Sokol et al., in preparationz). It would be prema-ture, nevertheless, to classify rabies virus in the myxovirus group. The striking morphology of the virion sets it apart from myxoviruses.
VOL.2, 1968 1197
on November 11, 2019 by guest
http://jvi.asm.org/
[image:7.489.80.403.64.245.2]in the matrices without obvious involvement of pre-existing cell membranes. This was not ob-served in the present experiment in which a
different strain of fixed rabies virus was used.
Differences in the pathogenesis of rabies virus strains have been described. Miyamoto and Matsumoto (26) found that, whereasstreet virus infections resulted in the production of large Negri bodies in mouse brain, fixed virus strains induced only small amounts of matrices in
neurons but exhibited marked cytopathogenicity. These differences in pathogenesis may also
ex-tend to morphogenesis.
No qualitative differences in antigenicity
be-tween fixed strains was evident in the present
study. Although immune serum was prepared
against PM virus and the experimental agent used was the CVSstrain, both thenucleoprotein
strandsand the viruscoatwereeffectivelylabeled,
and the nucleocapsid obtained from HEP-Flury strains reacted strongly with anti-PM serum in
the complement fixation test. The antigenic composition and thediscrepancy in development of theseviruses require further investigation.
ACKNOWLEDGMENTS
Thisinvestigation was supported by PublicHealth Service grants AI-04911, Al-02954, and AI-02405 from the National Institutes ofAllergy andInfectious Diseases, and by a grant from the World Health Organization. K. H.wastherecipient of Public Health
Service award K3-HD-22708. LITERATURE CITED
1. Andrew, G. A., K. C.Hsu,andB.C. Seegal. 1966.
Immunoferritin technique for the identification
ofantigens by electron microscopyp. 527-570.
InD. M.Weir (ed.), Handbook of experimental
immunology. F.A.DavisCo.,Philadelphia,Pa.
2. Atanasiu, P., P. Lepine, and P. Dragonas. 1963.
Etude cinetique du virus rabiqueenculturede
tissusaI'aidedes anticorps fluorescents et des
study of moderate and virulent virus-cell in-teractions of the parainfluenza virus SV5. Virology 30:411-426.
7. David-West, T. S., and N. A. Labzoffsky. 1968. Electromicroscopic studieson thedevelopment of vesicular stomatitis virus. Arch. Ges. Virus-forsch. 23:105-125.
8. Davies, M. D., M. E. Englert, G. R. Sharpless, and V. J. Cabasso. 1963. The electron mi-croscopy of rabies virus in cultures of chicken embryo tissues. Virology 21:642-651. 9. Diamond, L. 1967. Two spontaneously
trans-formed cell lines derived from thesamehamster embryo culture. Intern. J.Cancer. 2:143-152. 10. Goldwasser, R. A., and R. E. Kissling. 1958.
Fluorescent antibody staining of street and fixed rabies virus antigens. Proc. Soc. Exptl. Biol. Med. 98:219-223.
11. Hosaka, Y., H. Kitaro, and S. Ikeguchi. 1966. Studies on the pleomorphism of HVJ virions. Virology 29:205-221.
12. Howe, C., C. Morgan, C. de VauxSt.Cyr, K. C. Hsu,andH. M.Rose. 1967. Morphogenesisof type 2 parainfluenza virus examined by
light
and electronmicroscopy.J.Virol. 1:215-237. 13. Hummeler, K., H.Koprowski, andT. J. Wiktor.
1967. Structureanddevelopmentof rabies virus in tissue culture. J. Virol. 1:152-170. 14. Johnson, R. T., and E. H. Mercer. 1964. The
de-velopment of fixedrabies virus inmousebrain. AustralianJ.Exptl.Biol. Med. Sci. 42:449-456. 15. Kaplan, M. M., T. J. Wiktor, R. F. Maes, J. B. Campbell, and H. Koprowski. 1967. Effecton
polyionsontheinfectivityofrabies virus in tis-sue culture: construction of a single-cycle growth curve. J. Virol. 1:145-151.
16. Kingsbury, D. W., and R. W. Darlington. 1968. Isolation and properties of Newcastle disease virusnucleocapsid. J. Virol. 2:248-255. 17. Kissling, R. E., R. Q.Robinson, F. A. Murphy,
and S. G. Whitfield. 1968. Agent of disease contracted from green monkeys. Science 160: 888-890.
18. Kitajima, E. W., and A. S. Costa. 1966. Morphol-ogy and developmental stages of Gomphrena virus. Virology 29:523-539.
19. Lowry, 0. H., N. J. Rosebrough, A. L. Farr,
on November 11, 2019 by guest
NUCLEOPROTEIN COMPONENT OF RABIES VIRUS
and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275.
20. McCombs,R. M., M.Benyesh-Melnick, andJ. P. Brunschwig. 1966. Biophysical studies of vesicu-larstomatitis virus. J.Bacteriol. 91:803-812. 21. MacLeod, R., L. M. Black, and F. H. Moyer.
1966. The fine structure and intracellular localization of potato yellow dwarf virus. Virology 29:540-552.
22. Macpherson, I., and M. Stoker. 1962. Polyoma transformation of hamster cell clones-an in-vestigation of genetic factors affecting cell com-petence. Virology 16:147-151.
23. Matsumoto, S. 1962. Electronmicroscopy ofnerve cells infected with streetrabies virus. Virology 17:198-202.
24. Matsumoto,S. 1963. Electron microscopestudies of rabies virus inmouse brain.J.Cell Biol.19: 565-591.
25. Matsumoto, S., and K. Miyamoto. 1966. Elec-tron-microscopic studies on rabies virus multi-plication and thenatureof the Negri body. In Symp. Ser. Immunobiol. Standard. Karger, Basel1:45-54.
26. Miyamoto, K., and S. Matsumoto. 1967.
Com-parative studies betweenpathogenesis ofstreet
and fixed rabies infection. J. Exptl. Med. 125: 447-456.
27. Murphy, F. A., P. H.Coleman, and S. G.
Whit-field. 1966. Electron microscopic observations of Flanders virus. Virology 30:314-317. 28. Pinteric, L., and P. Fenje. 1966. Electron
micro-scopic observations of the rabies virus. hI Symp. Ser. Immunobiol. Standard. Karger, Basel 1:9-25.
29. Roots, E., andI.M. Schultze. 1963. Neuere elek-tronenmikroskopische Befunde an Gehirnen nach Infektion mit Tollwutvirus. Zentr. Bak-teriol. Parasitenk. Abt.IOrig. 188:159-173. 30. Siegert, R., H.-L. Shu, W. Slenczka, D. Peters,
and G. MUller. 1967. Zur Atiologie einer un-bekannten, von Affen ausgegangenen men-schlichen Infektionskrankheit. Deut. Med. Wochschr. 92:2341-2343.
31. Simpson, R. W.,and R. E. Hauser. 1966. Struc-turalcomponents of vesicular stomatitis virus. Virology 29:654-667.
32. Sokol,F., E. Kuwert, T. J.Wiktor,K.Hummeler, and H. Koprowski. 1968. Purification of rabies virus grown in tissue culture. J. Virol. 2:836-849.
33. Zlotnik, I., D. 1. H. Simpson, and D. M. R. Howard. 1968. Structure of thevervet monkey disease agent. Lancet 2:26-28.
34. Zwillenberg,L. O., M. H. Jensen, and H. H. L. Zwillenberg. 1965. Electron microscopyofthe virus of viral hemorrhagic septicemia of rain-bow trout (Egtved virus). Arch. Ges. Virus-forsch. 17:1-19.
VOL. 2, 1968