by
Michael F. Uren, BVSc., MVSc.
A Thesis presented for the degree of Doctor of Philosophy
of the Australian National University.
1987
Department of Experimental Pathology John Curtin School of Medical Research
The work described in this thesis was undertaken in 1985 - 1987 within the Virology Laboratory of the Department of Experimental Pathology, John Curtin School of Medical Research, Australian National University. This thesis does not contain material which has been submitted for the award of any other degree or diploma in any University and, to the best of my knowledge and belief, does not contain material previously published or written by another person except where due reference is given in the text.
ABSTRACT
Murine T cell clones (Thy 1, L3T4+, Lyt 2-)
spec-ific for KUN, WN and MVE were generated in vitro following in vivo priming. All clones proliferated strongly and secreted high levels of IL-2 and eighteen clones secreted IL-3 in response to homologous antigen. Both KUN and WN-specific clones showed extensive cross-reactivity to KUN and WN antigen, but recognised MVE to a lesser extent. In contrast, MVE-specific clones cross-reacted strongly with both KUN and WN. These results show that antigen-specific, cells are generated during
flavivirus infection and are cross-reactive for viruses of the same subgroup.
In order to investigate the helper activity of these clones, the B lymphocyte response to primary and secondary stimulation with flaviviruses was first established using polyclonal T cells populations. Flavivirus-specific
antibody-secreting cells (ASC) were assayed directly after in vivo priming, or following secondary restimulation in
.
vitro or in vivo. The formation of ASC following restimulation in vitro depended on the presence of+
was demonstrated between KUN, WN and MVE primed ASC both in vivo and in vitro. Both KUN and WN-irnrnune B eel ls responded to restimulation with KUN and WN viruses, but to a lesser extent to MVE virus. Conversely, MVE-specific ASC responded strongly to exposure to both KUN and WN viruses, a pattern which correlates with observations on L3T4+ cell specificity.
A second group of cloned cells was used to examine the ability of individual T cells to stimulate the secretion of flavivirus-specific antibody by flavivirus-irnrnune B cells. The clones were L3T4 +, restricted by the MHC and secreted high levels of IL-2. The cross-reactivity patterns were identical to those in the first group when compared by using a standard dose of virus. The ability for individual T cells to provide specific and cross-reactive help was examined using the ELISA plaque technique. T cell clones differed markedly in their ability to stimulate antibody secretion by B cells. Two clones,
stimulate
of different specificities, any increase in IgG
were unable to
secretion after
virus but to a lesser extent with MVE virus. Numbers of
MVE-immune ASC increased when MVE-specific clones were restimulated with MVE, KUN or WN virus a pattern previously observed with polyclonal and clonal L3T4+
cells. WN-specific T cell clones stimulated antibody from
L3T4 , KUN-primed cell cultures indicating that antibody production occurs in the presence of cross-reactive T
cells. The helper activities of the cloned T cells indicated that both the physical presence of T cells and factors produced by T cells were essential for the
production of antibody.
The fine specificity of antigen recognition by virus-specific T cells was analyzed using chemically synthesized linear peptides corresponding to sequences of
the envelope glyprotein of MVE virus. Cells proliferated
in reponse to the amino acid sequence 522-543 but failed
to respond to the remaining peptides. Both WN and KUN specific clones also responded al though the response was
markedly weaker than that of MVE-immune T cells. This implies that closely related viruses possess a sequence of amino acids such that possible substitutions cause some
modification in the response to heterologously primed T eel ls. Comparisons of this region with WN and KUN virus
During flavivirus infection, therefore, L3T4+ T cells which recognise the envelope glycoprotein
lymphokines and provide help for proliferate, produce
antibody production. These activities are induced not only by the immunizing virus but also 1n response to
TABLE OF CONTENTS
Page No.
Abstract (i)
Acknowledgements (viii)
Chapter 1. Review of the Literature.
1. Virus Characteristics
1.1 Classification 1.1
1.2 Virus Properties 1.2
1.3 Virus Entry Into Cells 1.3
1.4 Virus Replication 1.5
1.5 Epidemiology 1.8
1.6 Molecular Epidemiology 1.9
2 . Host Response to Flaviviruses
2.1 Clinical Syndrome 1.13
2.2 Flavivirus Pathogenesis 1.17
2.3 The Host Response 1.21
2.4 Cross-Reactivity to Flavivirus 1.30
2.5 Antibody Dependent Enhancement 1.39
TABLE OF CONTENTS
Page No.
Chapter 2. Materials and Methods.
1. Mice 2 . 1
2. Cell culture 2. 1
3. Viruses 2. 5
4. Monoclonal antibodies 2. 9
5. Lymphocyte cultures 2. 9
6. Preparation of Stimulator cells 2.11 7. Phenotypic analysis of T Lymphocyte clones 2.12
8. Proliferation assays 2.13
9. Colorimetric MTT 2.15
10. ASC assay 2.16
11. Statistical analysis 2.18
Chapter 3. Flavivirus-specific L3T4+ T cell clones - Induction, Characterisation and
Cross-reactivity. Introduction
Results Discussion
TABLE OF CONTENTS
Page No.
Chapter 4. Flavivirus-specific Antibody Secreting Cells. Introduction
Results Discussion
4. 1 4. 3 4.19
Chapter 5. Helper Activity of Flavivirus-specific T Cell Clones.
Introduction Results
Discussion
5. 1 5. 2 5.20
Chapter 6. Recognition of Peptides of the Flavivirus Envelope Glycoprotein by Virus- specific Helper Cells.
Introduction Results
Discussion
Chapter 7. General Discussion.
Bibliography
ACKNOWLEDGEMENTS
I wish to thank my supervisor,
.
Dr. J. E. Allan, for her expert guidance and unwavering enthusiasm during thepast two and a half years. I also thank Professor P. C.
Doherty for his time and skilled advice given
.
freely during our many discussions.A special word of thanks goes to Dr. R. Ceredig and Dr. H. O'Neill whose expert advice ensured the success of the T eel 1 cloning experiments. I also thank Dr. P. D.
Jones for his valuable comments on the ELISpot technique.
Dr J. Mathews kindly donated the synthetic peptides
described in chapter 6 and collaborated in the performance of those experiments.
I wish to acknowledge the staff of the small animal
unit for their constant helpfulness regardless of the request.
Finally I owe a debt of gratitude to my wife who not only provided the encouragement to continue, but whose
PUBLICATIONS AND COMMUNICATIONS
ARISING FROM THIS THESIS
Uren, M.F., Doherty, P.C. and Allan, J.E. {1987}.
Flavivirus-specific murine L3T4+ T cell clones: induction, characterisation and cross-reactivity {J.gen. Virol. 68,2655 - 2663}
Uren, M.F., Doherty, P.C. and Allan, J.E. (1987}. Enumeration
primary and
of antibody-secreting secondary infections with (submitted for publication}
Uren, M.F., Doherty, P.C. and Allan, J.E.
cells during flaviviruses
Helper activity of L3T4+ murine T cell clones (manuscript in preparation}.
L3T4+ flavivirus-specific T cell clones. M.F. Uren, P.C. Doherty and J.E. Allan. Presented at the Annual Meeting of the Australian Society for Immunology, Newcastle, Australia; December 3-5, 1986.
Regulation of the antibody response to flaviviruses. M.F. Uren, P.C. Doherty and J.E. Allan.
International Canada; August 1987.
Congress of
GENERAL INTRODUCTION
AND
P2.l6 , Section 10.1. Lines 11 - 13 should read - Vero-passaged virus,
or control antigen was incubated with spleen cell cultures for 5
days at 37°C in the presence of 5% CO 2 . Antigen concentration
was optimised for the 5 day incubation.
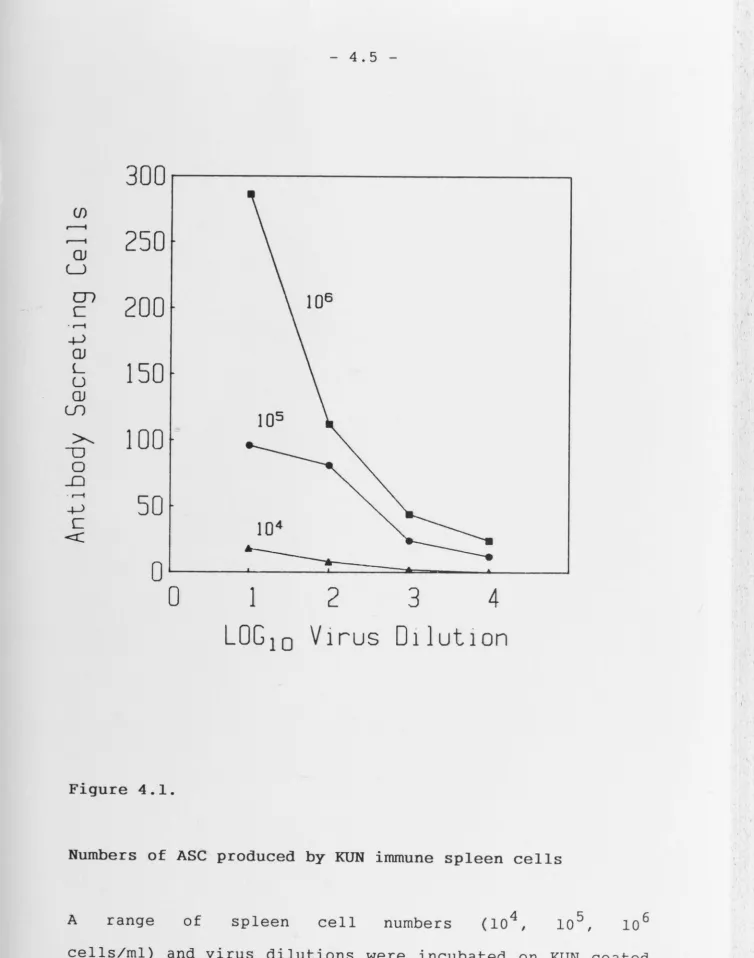
Fig. 4.1 Legend should read - A range of spleen cell numbers (104,
105, 106 cells/ml) and virus dilutions used to coat the assay
plates, were incubated for 4h.
Fig. 4.5 The following data should be added to the legend - The number
of ASC obtained from control mice immunized 20 - 30 days before,
were 96, 54 and SO for WN, KUN and MVE respectively.
Fig. 5.2 The Mab+ C column refers to the fact that the spleen cells
were T cell depleted and should have appeared next to the primed
spleen cell column.
P6.17 Line 21 should read - There are four amino acid substitutions
between MVE and WN and five between MVE and KUN, of which three
[image:15.810.14.804.25.1065.2]1.
VIRUS CHARACTERISTICS
1.1 Classification
The flaviviruses comprise a group of about 70 closely
related human and veterinary pathogens. Originally
classified as members of the Togaviridae by the
International Committee on Taxonomy of viruses (Fenner et
al., 1974) the genus has recently been reassigned the
status of Family, the Flaviviridae, based on fundamental
differences in structure, replication strategy and gene
sequence (Brown, 1986). The family Flaviviridae contains
the single genus Flavivirus. The family name is derived
from the Greek flavus meaning yellow and refers to yellow
fever, the type species.
Members of the Flaviviridae are small, enveloped
viruses of about 45nm diameter containing positive sense,
single stranded, linear RNA of molecular weight 4 x 106
(Murphy, 1980; Stollar et al., 1967; Trent et al., 1969).
Analysis of the RNA molecule has established the presence
7
of a type I Cap of the form m G( 5' )AMP Np at the 5' end
(Cleaves and Dubin, 1979) and a stable secondary structure
rather than a poly (A) tai 1 at the 3' end (Wengler and
Wengler, 1981).
is unknown.
1.2 Virus Properties
The nucleocapsid contains a single type of capsid
protein of approximately 14,000 dal tons surrounded by a
lipid bi1ayer of host origin. The latter contains an
envelope protein, E, of about 55,000 daltons which 1s
usually, but not invariably, glycosylated (Shapiro et al.,
1971, 1973; Stollar, 1969; Wright, 1982). A third, smaller
nonglycosylated membrane-like protein (M) of about 8,000
dal tons has also been identified (Wengler et. al., 1985;
Westaway et al., 1980). It has been proposed that M is
derived from a precurser glycoprotein by proteolytic
cleavage late in virus maturation leaving the
nonglycosylated M protein embedded in the virion membrane
(Castle et al., 1985; Dalgarno et al., 1986; Rice et al.,
1985). Cell-associated flavivirus particles are deficient
in this protein.
Flavivirus-infected cells contain three large
nonstructural proteins: NS!, NS3 and NS5 as well as four
lower molecular weight nonstructural proteins; ns2a, ns2b,
ns4a and ns4b ((Heinz and Kunz, 1982; Smith and Wright,
1985; Westaway, 1980). The functions of these proteins
are not known, however there is some evidence to suggest
that NS3 and NS5 are viral polymerases (Rice et al. 1985).
NSl, however, 1s unlikely to be a polymerase protein,
since it is glycosylated, fixes complement and is present
Wright, 1985). Tryptic peptide mapping has revealed
unique sequences for most of the nonstructural proteins
for Kunj in (KUN} virus {Wright et al. , 19 77; Wright and
Westaway, 1977), WN virus
.
{Castle et a 1. , 1986}, Tick-borne encephalitis virus (TBE) (Heinz and Kunz, 1982)and YF virus (Rice et al., 1985).
Recently the nucleotide sequences of yellow fever
(YF), West Nile {WN), Murray Valley encephalitis {MVE) and
dengue type 2 (DEN2) virus genomes have been investigated
(Castle et al., 1985, 1986; Dalgarno et al., 1986; Duebel
et al., 1986a; Rice et al., 1985) together with the
amino-terminal amino acid sequences of Saint
encephalitis {SLE) and DEN2 structural proteins.
Louis
These
studies revealed that the genome consists of approximately
11,000 nucleotides arranged as a single long open reading
frame. The data also show that the amino terminal of the
viral core protein (C) is followed by the amino-terminal
of the Mand E proteins.
1.3 Virus Entry Into Cells
The exact method involved in the penetration of viral
genetic material into susceptible cells has yet to be
elucidated. However, fusion with the cell membrane has
been proposed as the method of entry of a number of
enveloped viruses
.
into cells (Marsh, 1984). Geneticplasma membrane or into acidic prelysosomal endosomal compartments where replication occurs (Helenius et al., 1983). The ability to fuse with membranes probably plays a maJor role in the penetration of cells by flaviviruses (Gollins and Porterfield, 1986a}. Indeed, lysosomotropic amines can inhibit flavivirus infection of cells (Brandriss and Schlesinger, 1984; Gollins and Porterfield, 1984}, and inhibit penetration of virus cores into the cytosol from endosomal prelysosomal compartments (Gollins
and Porterfield, 1985). Examination of the entry process of WN virus by electron microscopy indicates that virus can be taken up into cells by specific receptor-mediated endocytosis (Cardosa et a 1. , 1986; Gollins and Porterfield, 1985). The finding that WN virus undergoes acid-catalysed fusion with cell membranes has been supported by experiments using NH
4Cl to raise the pH of acidic,
uncoating
intracellular compartments. This inhibited the of virus particles, thus limiting viral replication and causing a build up of virus particles in endosomal compartments (Gollins and Porterfield, 1984, 1985, 1986b). Furthermore WN is known to fuse with artificial liposomes with an optimum pH of 6. 7 and below which virus
.
compartment
may escape (Gollins
1.4. Virus Replication
1.4.1. Translation and Transcription
Radio-isotope labelling and irnmunofluorescence experiments (Boulton and Westaway, 1976; Ng et al., 1983} have revealed that the sites of translation and transcription
synthesis is endoplasmic
are distinct. associated with
reticulum which
Virus-specific both smooth
undergoes
protein and rough
extensive
proliferation to form characteristic organelles (Westaway,
1980}. Conversly, RNA replication appears to be associated with the perinuclear region (Ng et al , 1983). Both the method of initiation of transcription and
translation of the flavivirus genome, and how regulation
of the switch to preferential plus-strand RNA production
occurs, are unclear. Experiments using the incorporation
of N-formylmethionine and complimentary DNA (cDNA} cloning (Castle et al., 1985, 1986; Rice et al., 1985; Svi tkin et
al., 1984} suggest that translation in vivo is initiated with the C protein at the 5' end and proceedes sequentially
through the genome to produce one precursor polyprotein. This precursor is then rapidly cleaved to produce both
structural and nonstructural virus specified proteins.
High molecular weight proteins with properties
produce a number of large, virus-specific proteins. In support of this, Coia et al. (1988) have identified polyp rote in cleavage sites of KUN virus indicating that
NSl, NS2A and NS4B are probably cleaved in the lumen of the endoplasmic reticulum.
In flavivirus-infected cells, genomic sized RNA {44S RNA) has been the only virus specified RNA isolated (Naeve and Trent, 1978). However a double-stranded RNA of 20-22S, termed a replicative form, and a partially
ribonuclease sensitive heterogeneous 20-28S RNA, termed a
replicative intermediate, have been isolated (Stollar et al. 1967; Trent et al. 1969; Wengler et al. 1978}. Studying DEN2 virus-infected cells, Cleaves and coworkers
{1981} showed that tritiated uridine was transferred from these two forms of RNA to the 44S RNA. More recently Chu and Westaway (1985} described pulse-chase experiments in
.
which replicative form RNA functioned as a recycling
template for semi-conservative and asymmerti.c replication
in which only one strand was synthesised per cycle.
Collectively, these data indicate that viral RNA 1s transcribed into complementary minus-strand 44S RNA which remains in a 20S double-stranded replicative form and recycles as a template via
.
a replicative 20-28S intermediate form. In this fashion, progeny RNA can beframe encoding a single polypeptide. The structural proteins {C - M - E} are found within the amino terminal and the remainder of the open reading frame consists of nonstructural viral polypeptides. Mature viral proteins are then produced by post-translational cleavage of the polyprotein precursor.
1.4.2. Maturation of the Virion
The "-maturation and morphogenesis of flaviviruses are poorly understood. It has been suggested that virions mature by condensation {Murphy, 1980} and accumulate within cysternae of the endoplasmic reticulum {Leary and Blair, 1980}. Since viral proteins have been shown to be present as membrane associated molecules {Boulton and Westaway, 1976; Shapiro et al., 1973}, it has recently
been proposed that viral structural and nonstructural proteins interfere with the normal membrane traffic and that this causes the accumulation of altered membranes in
infected cells (Castle et al., 1986}. In ver tebrate cells, pathological changes are known to include
1.5. Epidemiology
With the exception of urban dengue and urban yellow fever, all of the infectious arthropod-borne flaviviruses are zoonoses (Ray, 1985). The cycle is believed to involve both arthropods and lower vertebrates with human infection a dead end (Ho, 1985). The complexity and specificity of transmission cycles between reservoir animals only partly explains the geographical distribution of these diseases. For example, dengue fever is reported in East Asia, the Pacific area and the Caribbean whereas yellow fever, which uses the same vector, has been reported only from tropical Africa and the Americas (WHO, 1985). A number of mammalian hosts which amplify flaviviruses have been described. For example, pigs are
.
known to support JE virus replication with birds, snakes and bats thought to provide incidental reservoirs (Monath, 1984). Similarly, monkeys (YF); birds and marsupials(MVE) and birds (WN) are other known amplifying hosts (Halstead, 1980; Kay et al., 1985a,b; Monath, 1984). It has been proposed that, when optimal climatic conditions prevai 1, mos qui toes become abundant leading to increased levels of transmission among the reservoir hosts. This, in turn, increases the probability of human infection
1.6 Molecular Epidemiology
1.6.1. Virus Strains
The geographic origin and genetic relatedness of different isolates of a flavivirus have been used in
.
attempts to document the evolution and epidemiology of flavivirus infections. The techniques used have varied in their ability to demonstrate homology and have included sizing of virus-specified polypeptides, peptide mapping of complete and partial proteolytic digests, protein and RNA.
sequencing, RNA-RNA hybridization and oligonucleotide
.
mapping.
Geographically defined antigenic and biological variations were first described for JE (Okuno et al., 1975; Trent, 1977); WN (Hammon and Price, 1966); YF
(Clarke, 1960); SLE (Monath et al., 1980) and DEN (Russell and Mccown, 1972). Isolates of JE, WN, YF SLE and DEN were found to vary in virulence for different species
More recently, RNA oligonucleotide mapping has been
successfully applied to the differentiation of flavivirus
isolates. Analysis of 57 strains of SLE virus from
different regions of North America has demonstrated that
SLE isolates could clearly be divided into three genotypic
sets, or topotypes (Trent et al., 1981). Geographical
strain variation has also been described for DEN (Repik et
al., 1983; Trent et al., 1983) and YF (Deubel et al.,
1986b). Different topotypes of DEN2 have also been
identified by using hybridization techniques (Kerschner et
al., 1986). In contrast, Heinz and Kunz (1981) isolated
TBE virus strains in different years from different
countries and found them to be indistinguishable. Genetic
analysis of KUN virus using HAE III and TAOl restriction
digest profiles of 15 KUN isolates from different
Australian regions spanning a ten year period have also
shown no evidence of genetic variation (Lobigs et al.,
1986).
The use of monoclonal antibody and immunofluorescence
techniques to analyse flavivirus serotype variation has
given variable results. Four YF virus-specific monoclonal
antibodies could not differentiate between strains
isolated from different regions (Monath et.al., 1984).
Moreover, Gould et al. (1985) were unable to find any
antigenic differences between 22 wild types of YF using a
panel of nine monoclonal antibodies. However the use of
distinguished between isolates of SLE (Roehrig et al.,
1983), DEN virus
.
(Henchal et a 1. , 1982) and JE virus.
(Kimura-Kuroda and Yasui, 1983). Significant differences between isolates in binding assays have been demonstrated
for TBE virus. Stephenson et al. (1984) found extensive differences between three Austrian strains of TBE, although Heinz et al. (1983) could not differentiate between these same isolates and other strains isolated in different years from different countries. A study of the
antigenic relationships between JE virus and flaviviruses using anti-JE monoclonal antibodies,
other
has
suggested that JE is closely related to MVE virus but less so to WN virus and SLE is the least closely related
(Kimura-Kuroda and Yasui, 1986).
The results from comparisons of strain variations, therefore, demonstrate remarkable differences in data
obtained from binding assays and functional assays. These
results have also been observed for influenza virus (Kendal, et al., 1981) and may reflect differences in the
HI and neutralization abilities of monoclonal antibodies
directed towards common epitopes. The mechanisms respon-Sible a re unknown, but may include slight differences in
.
the binding site on the antibody for virus or perhaps
conformational changes induced by antibodies binding to
certain epitopes on the virus which may affect the ability of virus to
.
interact with its putative receptor. Thiswhich are not involved in neutralization of infectivity
.
may still be of importance in the immune response.1.6.2. Evolution
By partial proteolysis of the nonstructural proteins NS1 and NS5, Heinz and Kunz (1982) have demonstrated marked differences between the polypeptides specified by MVE, WN and TBE. Oligonucleotide fingerprints of WN and Uganda S viruses (Wengler et al., 1978) and the four dengue prototype strains (Vezza et al., 1980) showed no detectable similarities. In a study by Westaway et al. (1977) using phosphate gel electrophoresis, considerable heterogeneity was observed among a number of viral proteins, particularly the envelope proteins of seven flaviviruses. In contrast, a study using exhaustive proteolytic digests demonstrated considerable homology for proteins of KUN, WN and MVE viruses (Wright et al., 1983).
Comparative studies between flaviviruses have also helped to define those areas in the structural and nonstructural proteins which have been conserved.
sequences for YF,
recently, partial amino acid
.
SLEMore and DEN2 viruses and complete or partial nucleotide sequences for YF, MVE, TBE, JE, WN and DEN2 viruses have been documented (Bell et al., 1985; Castle et al., 1985, 1986; Dalgarno et al., 1986; Deubel et al., 1986a; Pletnev et
1986). Glycoproteins of YF, SLE and DEN2 vi ruses were
.
shown to be 52 - 60% conserved in the regions sequenced
and 40% of the amino acids were invariant in all three
viruses. A comparison of the nucleotide sequences of the
YF, TBE, WN and MVE genomes has revealed 40 - 50% sequence
similarity over the regions
.
sequenced (Castle et a 1. , 1985, 1986; Dalgarno et al., 1986; Pletnev et al., 1986).Moreover, the DEN2 C, M, and E proteins have been shown to
be 13, 36 and 43% similar respectively to the proteins of
YF virus, and 33, 32 and 47% similar to the respective
proteins of WN virus (Deubel et al., 1986a). As a result,
it has been proposed that the amino acid sequence
similarities between MVE, YF, SLE and DEN2 is evidence
that flaviviruses have descended from a common ancestor
(Bell et al., 1985; Dalgarno et al., 1986; Deubel et al.,
1986a}.
2.
HOST RESPONSE
TO
FLAVIVIRUSES
2.1. Clinical Syndrome
By far the great majority of flavivirus infections are
asymptomatic (Westaway et a 1. , 1985). The clinical
manifestations of flavivirus infection can be divided into
two broad groups (Table 1.1} and the infection lS
characterised either by central nervous system (CNS}
by mild to severe fever with shock and haemorrhages extreme cases {Monath, 1984}.
2.1.1. The Febrile/ Haemorrhagic Syndrome
.
inThe acute febrile syndrome is typified by dengue virus infection. Mild dengue is characterised by diphasic fever, rash, malaise, myalgia, vomiting, photophobia and anorexia. Leukopenia and lymphadenopathy have also been observed {Ho, 1985}. Since 1953 dengue haemorrhagic shock/ dengue shock syndrome {DHS/DSS} has become one of the most important causes of morbidity and mortality among children in SE Asia {Halstead, 1980}. Confirmed epidemics of DHS/DSS have been reported in Burma, Cuba, Kampuchea,
Indonesia, Laos, Malaysia, Singapore, China, Thailand, India and Vietnam {WHO, 19 85} . Based on epidemiological observations it has been proposed that sequential infection with a heterologous dengue serotype may lead to manifestations of DHS/DSS {Halstead, 1980; Halstead et al., 1970}. A prospective study in Rayong, Thailand conducted by Sangkawibha and coworkers {1984 } revealed that children with pre-epidemic antibodies to DEN!
contributed a disproportionately high number of shock cases. Additionally the risk factors for DHS/DSS were
shown to result from predominantly secondary infections with DEN2 which followed primary infections with DEN!, DEN2, or DEN3 { in descending order of magnitude}. This
Virus
St. Louis
encephalitis
Yellow Fever
Dengue
Vectors
Culex pipiens
C. p.
quinque-fasciatus
C. tarsalis
C. nigripalpus
Aedes aegypti
Ae. africanus
Haemagogus spp.
Ae. simpsoni
Ae.
furcifer-taylori
Ae. luteocephalus
Ae. vittatus
Ae. aegypti
Ae. albopictus
Ae. polynesiensis
Ae. scutellaris
Ae. niveus
Ae. furcifer-taylori
Ae. luteosephalus
Geographic
Distribution
North America
South America
Caribbean
Africa
South America
all tropical
zones
Disease
Expression
encephalitis
haemorrhagic
fever
febrile
illness and
haemorrhagic
fever
Occurrence
epidemic
and sporadic
epidemic
and sporadic
Japanese
encephalitis
Murray Valley encephalitis
Tick-Borne encephalitis
References:-C. "vishnui"
C. tritaeniorhyncus C. "vishnui"
C. qelidus
C. fuscocephalus C. annulus
C. annulirostris
C. annulirostris Ae. normanensis C. bitaeniorhyncus C. pipiens
Ixodes ricinus I. persulcatus Dermacentor spp. Haemaphysalis spp.
India, East and South East Asia
Australia
PNG
Europe
encephalitis
encephalitis
encephalitis
Lopes et al, 1978; Karabatsos, 1978; Monath, 1985; Work, 1963.
- 1.15
-epidemic
and sporadic
epidemic
epidemic
low morbidity, no mortality outbreak of DEN! was followed by an outbreak of DHS/DSS caused by DEN2 in which there were 158 deaths, mostly of children under 15 {Kouri et al., 1982; Mas-Lago, 1979}. Strain variation in dengue viruses, such as those already described for DEN2 (Section 1.6.1} may also contribute to the ability to cause DSS/DHS.
DHS/DSS is characterised by a sudden onset of fever which persists for 2 to 7 days accompanied by
thrombocy-topeni a •r and haemoconcentration, hypovolaemia and
hypofibrinogenaemia (Halstead, 1980}. Later, complement 1s activated and the degree of complement depletion and hypofibrinogenemia is reported to correlate with the onset of shock. It has been suggested that the pathophysiology of DHS/DSS is related to increased vascular permeability
and disseminated intravascular coagulation, further
complicated by liver and bone marrow dysfunction (Ray,
1985; WHO, 1985}. Neurological involvement is not
associated commonly with dengue infection, however CNS disorders appear to occur more frequently with DHS/DSS than with classical dengue (Monath, 1985}. Neurological signs may include encephalopathy and polyneuritis (Acevedo et al., 1982; Sumarmo et al., 1978}.
2.1.2. Viral Encephalitis
subclinical to lethal encephalitis { reviewed by Albrecht, 1968; Monath, 1985 and Nathanson and Cole, 1970). The prodromal period may include fever, headache, anorexia,
.
nausea and vomiting. These symptoms are then usually followed by the appearance of neurologic or meningeal signs which may include paralysis, paresis and convulsions. The mortality rate varies from 20% for SLE to 50% for JE {Monath, 1985). The striking characteristic of the flavivirus encephalitides is, however, the high incidence of neurologic sequelae in those cases whichrecover {Albrecht, 1968).
2.2. Pathogenesis of Flavivirus Infections
.
The flaviviruses include those viruses which are associated with fever/joint-pain/rash syndrome such as DEN or YF, or those which are known to cause encephalitis such as JE or MVE. Al though mice have proven to be a good
model in which to demonstrate flavivirus encephalitis the same has not applied to the use of mice to study the
.
fever/joint-pain/rash and DHS/DSS syndromes. Neuroadapted DEN will produce encephalitic lesions in.
suckling, weanling and adult mice {Cole and Wisseman, 1969). The outcome of infection was shown to be influenced by dose and route of inoculation, age of host and virulence of the infecting viral strain. Interferon, reticuloendothelialand Wisseman, 1969).
Investigation of the pathogenesis and pathophysiology of both DEN and YF has relied predominantly on experimental observations in monkeys. Marchette et al.
(1973} inoculated Macaca mulatta monkeys subcutaneously with DEN and, two to three days later, virus could be recovered from multiple skin sites as well as spleen, lymph nodes, thymus, lungs, intestine, adrenals, muscle, heart and circulating lymphocytes. In uncomplicated dengue fever, changes appear in the smal 1 dermal blood vessels leading to endothelial cell swelling and perivascular oedema with mononuclear cell infiltration
(Sabin, 1952). More severe infection {DHS/DSS}, often complicated by shock, is characterised by widespread effusions into serous cavities, haemorrhage and perivascular oedema. Hepatic lesions similar to those found in other haemorrhagic fevers consist of central or paracentral focal necrosis,
.
sinusoidal acidophilic (Councilman} bodies, hypertrophy of Kupffer cells and portal mononuclear cell infiltration (Fresh et al., 1969}.Experimental evidence strongly indicates that DHS/DSS
.
1S an immunopathologic disease (Halstead, 1982).
Importantly both dengue immune monkeys and man show significantly higher v1rem1as following heterologous DEN
.
.
(Halstead, 1980; Marchette et a 1. , 19 73} .
infection
donors or from normal donors exhibit enhanced growth of virus in the presence of subneutralising concentrations of dengue antibodies. This phenomenon, termed antibody mediated immune enhancement (ADE), is discussed in greater
detail below (Section 2.5).
Yellow fever is known to produce both neurotropic and viscerotropic infections in vertebrate species (Strode, 1951). A number of monkey species are highly susceptible to lethal infection with the viscerotropic form of the
.
virus. In particular, much of the data on the pathogenesis of YF has been obtained by experimental infection of the rhesus monkey. Al though the disease is characterised by hepatic pathology, monkeys can develop mild encephalitis after intracerebral inoculation of viscerotropic YF virus
.
(Nathanson et a 1. , 1966). The hepatic parenchyma is the target organ, and the disease is characterised by coagulative necrosis of hepatocytes with the formation of Councilman bodies and intranuclear eosinophilic granular inclusions (Bearcroft, 1957). Lymphocytic necrosis in the germinal centres of theYF following the depletion of vitamin K-dependent
coagulation factors as a result of liver necrosis (Dennis
et al., 1969}.
Flaviviruses which exhibit a predilection for the CNS
have been reviewed extensively by Albrecht (1968},
Nathanson and Cole {1970}, Johnson (1982} and Monath
(1985}. The exact manner in which flaviviruses gain
.
access to the CNS is not known although it has been
proposed . that neuroinvasion may occur either by virus
crossing the blood brain barrier via the haematogenous
route (Johnson, 1982} or by infection of the olfactory
neurones and transport a long neurona 1 axons to the
olfactory lobe of the brain (Monath et al., 1983}. In
mice, the mid-brain appears most susceptible {Nir et al.,
1965}. In monkeys, the lenticulo-striate complex,
thalamus and substantia nigra are most severely affected
(Nathanson et al., 1966} and in man the substantia nigra,
thalamus and hypothalamus appear most frequently involved
(Reyes et al., 1981}. Virus reaching the CNS may cause
meningeal inflammation, or encephalitis with meningeal and
perivascular mononuclear cell infiltrates, degeneration of
neurones with neuronophagia, and glial nodule formation
2.3. The Host Response
The present understanding non-specific defence mechanisms
of the and
role of early
later, also the
specific humeral and cellular immune responses following primary flavivirus infections relies
experimental observations in
.
laboratorylargely animals.
on In
these, the immune response to flaviviruses has been shown to play a major role in recovery from severe infection while the importance of the early responses is not yet clear.
2.3.1. Non-specific Responses
1. Interferon {IFN)
Both WN and DENl viruses have been shown to produce
.
significant amounts of type 1 IFN in suckling mice brains (Cole and Wisseman, 1969; Finter, 1964). A study by Tongaongar and Gosh (1979), examined the ability of thirty-seven flaviviruses belonging to different antigenic groups to induce IFN. They found that the vi ruses could be divided into high, moderate and low inducers of gammaI FN in Vi t r O •
between titres
However, there seems to be no correlation of virus
.
and the amount of gamma IFNThe role of IFN 1n flavivirus infections needs to be examined more closely. It is evident from other studies that gamma IFN plays a central role in the immune process (Vilcek et al., 1985). Although originally described for its antiviral effect, gamma IFN 1S
.
now known to have marked immuno-modulatory activity. More specifically, gamma IFN is capable of inducing major histocompatibility(MHC) class I and class II (Ia) antigens on normal cells including endothelial (Wong and Schrader, 1985) and glial cells (Fontana et al., 1984). The induction of MHC
particularly on brain cells, may play a antigens,
significant part 1n
.
immunoprotection and theimmunopathology of flavivirus infection. Moreover, the increased expression of class 1 MHC antigens on the surface of virus-infected cells would encourage lysis by sensitized cytotoxic T cells (CTL) (Buckowski and Welsh, 1985).
Finally, it has been suggested that gamma IFN has a synergistic role in IL-2 driven proliferation of cytotoxic T lymphocytes (Farrar et al., 1981), and natural killer (NK) cells (Herberman, 1984). If this is the case, the release of gamma IFN may play a central role in the protective response to flaviviruses. Recent evidence 1n
.
support of this suggests that gamma IFN induction fromlymphocytes by DEN virus-infected monocytes 1S an important defence mechanism in primary dengue infections
2. Natural Killer {NK) Cells
NK cells have also been implicated in the response to
flavivirus infection. Cytotoxici ty arising within a few
days of KUN virus infection of Balb/c mice has been shown
to be due to NK cells (Macfarlan and White, 1980).
Recent findings by Kurane et al. ( 1986) have demonstrated
the ability of human NK cells from non-immune donors to
lyse DEN-infected cells by either antibody-dependent
cell-mediated cytotoxicity (ADCC) or by spontaneous lysis
in the absence of ADCC.
3. Macrophages
The importance of macrophages as a first line of
defence against flavivirus infection is also poorly
understood. Using silica to selectively destroy
macro-phages, Zisman et al. ( 1971) clearly demonstrated higher
titres of YF PFU/ml) in the brains of silica-treated mice as compared to titres of 106 PFU/ml
in brains of control mice. Similarly, Kulkarni and
Goverdhan (1986) showed an increased viremia and higher
mortalities in
.
JE infected mice.
treated with silica. Conversely, Chaturvedi et al. ( 1978) could demonstrate no2.3.2. Immune Response
Early studies of the importance of the immune system
during infection by flaviviruses utilised immunosupressive
techniques ( reviewed by Nathanson and Cole, 1970).
studies showed that significant depression of
These
.
immune
reactivity, including reduction in antibody levels, was
associated with enhanced morbidity, prolonged viremia,
elevated virus levels and higher mortality rates.
Cyclophosphamide treatment has been shown to convert a
subclinical infection of mice infected with a number of
flaviviruses into clinical disease (Cole and Nathanson,
1968; Nathanson and Cole, 1970, 1971) with suppression of
antibody synthesis and a reduced cellular inflammatory
response in the CNS. In another study, administration of
cyclophosphamide to mice after intracerebral injection of
Langat or WN viruses gave different outcomes. There was a
reduction in the inflammatory response to Langat
whereas
infected
less
mice
severe CNS
(Camenga
lesions were observed
and Nathanson, 1975).
.
virus
.
in WN
The
differences in host response to these two related viruses
indicate that the immune response to flaviviruses can be
both protective and immunopathological.
X-irradiation, anti-lymphocyte serum and adoptive
transfer of immune spleen cells {Bhatt and Jacoby, 1976;
Chaturvedi et al., 1978; Jacoby et al., 1980; Webb et al.,
analyse the immune
.
response. The outcome of the experiments depended largely on the virus and the host system, and immunosuppression was shown to be capable ofboth ameliorating and potentiating disease.
Administration of anti-lymphocyte serum to mice infected with YF, DEN or WN virus did not potentiate infection
{Camenga et al., 1974; Chaturvedi et al., 1978; Zisman et al., 1971), however, anti-lymphocyte serum induced acute disease in genetically resistant mice infected with Banzi virus {Jacoby et al., 1980). Similarly, the course of the infection was shown to be affected by the availability of thymus-derived T cells based on studies of athymic nude mice {Hotta et al., 1981). Results from experiments using transfer techniques have similarly been adoptive
confusing. The intravenous transfer of immune spleen cells protects mice challenged either with Banzi, Langat, WN or JE virus {Camenga and Nathanson, 1975; Camenga et
al., 1974; Jacoby et al., 1980; Mathur et al., 1983) but not when mice are infected with DEN or WN virus {Camenga and Nathanson, 1975; Chaturvedi et al., 1978). This may be due to the severity of the disease for mice, or may result from differences in the route and dose of virus. These results, therefore, remain inconclusive until techniques and methods can be standardized.
1. Antibody
flavivirus infection. Passively transferred antibodies have afforded protection in a number of experimental models using a variety of viruses.
.
Passive immunity has been demonstrated for JE (Hammon and Sather, 1973; Mathur et al., 1983), Langat virus (Camenga and Nathanson, 1975), WN (Camenga et al., 1974), YF (Zisman et al., 1971) andDEN (Chaturvedi et al., 1977, 1978). Cross-protective antibody has been observed and is discussed in more detail in Section 2.4. The overwhelming weight of evidence presented- in these experimental systems supports antibody-mediated termination of v1remia.
.
.
However, the extent to which antibody influences a primary infection in man is unclear, although it has been shown that antibodies are present in the CNS of humans following recovery from flavivirus encephalitis infection (Burke et al., 1985; Reid et al., 1971).Although it was once believed that hemagglutination inhibition antibodies (HI) played a protective role against JE infection in mice (Grossberg and Scherer, 1966) and neutralizing antibodies played a similar role in gibbons (Edelman et al., 1973), the coexistence of JE virus with HI, neutralizing and complement-fixing
infection by flaviviruses in the presence of neutralising antibody has also been observed in rhesus monkeys infected with TBE (Pogodina et al., 1981) and has been associated with chronic progressive human encephalitis resulting from Russian spring-summer
.
fever virus (Ogawa et a 1. , 1973) . Similarly WN virus has been shown to produce persistent infection and sub-acute encephalitic lesions in rhesus monkeys (Pogodina et al., 1983).In co-ncert with the production of antiviral antibody, antibody dependent enhancement of flavivirus infection has been described in vitro for several flaviviruses and in vivo for DEN (Halstead, 1979). The phenomenon of ADE is dealt with in greater detail in Section 2.5. Briefly, ADE
.
arises following the infection
monocyte-macrophage lineage in
.
of the
cells of presence
the of subneutralizing levels of antibody and this is thought to lead to an increase in viral replication ie, enhancement of infectivity.
2. The T Cell Response
(a) Cytotoxic T Lymphocytes
A number of investigators have addressed the question of immune cell reactivity during flavivirus infection. By
showed that they were cross-reactive and restricted by class 1 major histocompatibility complex (MHC) antigens {Gajdasova et al., 1981). The cytotoxic activity reached a peak on day 6 after infection and then declined rapidly. More recently, Kesson et al. ( 1987, 1988) have obtained cytotoxic T cell responses in
.
CBA/H mice following primary infection with WN virus ..
A secondarycytotoxic response was observed following secondary in vitro stimulation of primed spleen eel ls with WN virus. These cells were identified as Thyl , + and L3T4 . Cross-reactivity between KUN and WN-primed CTL has also been demonstrated (Mullbacher et al., 1986).
Cytotoxic T lymphocytes are known to lyse virus-infected cells in vitro (see Doherty, 1985, for review) and recently have been found to secrete a number of lymphokines (Kelso and Glasebrook, 1984). The extent to which CTL are involved in host recovery and the immunopathology of flavivirus infection is unknown, but requires further investigation.
(b) T Suppressor Cells
lymphocytes (Chaturvedi, 1984). cells (Chaturvedi et al., 1981) produce a soluble suppressor factor which recruits a second set of suppressor cell which in
.
turn produce a prostaglandin-like suppressor factor (SF2) (Chaturvedi and Shukla, 1981; Shukla and Chaturvedi, 1981).lymphocytes
.
suppressionwhich of the
finally humeral
SF2 then recruits Ts 3 mediate antigen-specific
response Chaturvedi, 1984). The phenotype of
(Shukla DEN2
and primed suppressors has been identified as both Lyl+ and Ly2+
(Wong et al., 1984) and, in DEN primed mice, the duration of suppressor activity of T cells has been specified as 21
days and 15 days for Tsl and Ts2 respectively (Shukla and Chaturvedi, 1985). Suppressor T cell activity has been reported to control both delayed type
hypersensitivity (DTH) and IgM B cell responses in DEN2 immune mice. A recent study of the suppressor pathway has demonstrated the ability of anti-I-J or I-A anti-sera and complement to remove the suppressor activity of Tsl' Ts2 and Ts
3 cells (Shukla and Chaturvedi, 1986).
Injection of mice with JE virus
.
also induces the generation of T suppressor cells for DTH and the IgM Bcell response (Mathur et al., 1984; Rawat et al., 1986). These cells have been partially characterised
+
Lyl (Rawat et al., 1986).
+
Although only DEN and JE viruses have been studied, it
.
is becoming increasingly clear that T cell mediated
suppression may significantly affect the immune response
to flavivirus infection, at least in mice.
.
.
The preciserole of the T suppressor pathway remains controversial. A
tenatative link between the presence of T cells and the s
formation of enhancing antibodies during dengue fever has
been suggested by Wong and coworkers (1984), however,
there is no supporting experimental data.
(c) DTH Cells
DTH responses to flaviviruses have been assayed for
DEN and WN viruses. The DTH response to DEN was measured
by footpad swelling, and was found to be transferable to
normal BALB/cJ mice by injection of immune spleen cells
(Pang et a 1. , 1982). WN, KUN, MVE and DEN vi ruses were
.
also shown to be capable of inducing a DTH response as
measured by the macrophage procoagulant assay (Allan and
Doherty, 1986; unpublished data).
2.4. Cross-Reactivity to Flaviviruses
2.4.1. Cross-protection
Animal experiments have established the occurrence of
cross-protection to heterologous flaviviruses following
flavivirus group {Sather and Hammon, 1970; Thind, 1981).
In man, infection or vaccination with YF following natural
flavivirus infection results in a broad spectrum of
antibody response to a number of flaviviruses (Hatgi et
al., 1966; Wisseman et al., 1962). Similarly, Kayser et
al.(1985) found that TBE vaccination of individuals
previously immunized with 17D YF vaccine resulted in a low
titre antibody response against the flavivirus group.
However neutralizing antibodies cross-reactive with DEN!
which arose following YF vaccination of individuals who
had previously been infected with JE did not protect
against DEN! challenge (Wisseman et al., 1966}. Although
the data are far from conclusive, the appearance of
cross-reacting antibodies does not prevent infection with
another flavivirus. It is not known to what extent they
ameliorate or potentiate subsequent flavivirus infections.
2.4.2. Cross-reactive Immunity
Serological cross-reactions among flaviviruses which
have been observed in vitro (Madrid and Porterfield, 1974;
Price et al., 1963; Westaway, 1965} and in vivo (Henderson
et al., 1970; Kayser et al., 1985) have been proposed as
the basis of cross-protection against infection (Price et
al., 1963; Tarr and Hammond, 1974; Wisseman et al.,
1962}. Cross-neutralization
assay using
Porterfield,
hyper immune
1974; Westaway,
studies performed by plaque
rabbit serum {Madrid and
arrangement of viruses of the flavivirus family into seven
sub-groups based on antigenic differences. These studies
recognised close relationships between WN, MVE, KUN and JE
with the four DEN types forming a second complex of
.
closely related viruses. Similar
with avian sera.
.
primary antibody
Boyle et
responses
al.
of
infected with MVE and KUN viruses.
results have been found
(1983) investigated the
herons experimentally
In KUN infected birds
the highest neutralizing and HI antibody titres were to
KUN, followed by WN and MVE with very low titres to JE
virus. Similar observations from JE infected birds showed
highest antibody titres to JE, followed by MVE, with low
titres to KUN and WN vi ruses. As discussed in Section
1. 6, peptide mapping and genome sequencing studies have
confirmed that these viruses are closely related.
Although previous infection or immunization with a
flavivirus has been shown to produce heterologous
cross-protection, the role of CMI is not well understood.
Evidence has been presented which suggests a minimal
effect of CMI during f lavi virus infection {Chaturvedi et
a 1. , 1978; Chaturvedi, 1981). However, others have
considered that DTH plays a more prominant role {Camenga
et al., 1974,; Hott a et al., 1981; Jacoby et al., 1980;
Pang et al., 1982). Little is known about the extent of
cross-protection afforded by T cells following infection
with heterologous flaviviruses, although investigators
flavivirus-specific cytotoxic T cells {Gajdosova et al., 1980, 1981; Mullbacher
macrophage
et a 1. , 1986} and cross-reaction using
.
the and migration inhibition assay {KelkarBanerjee, 1978). To date, there have been no published reports on the effects of cross-reactive T cells by adoptive transfer to infected mice.
2.4.3 Epitopes of Flaviviruses
Cross-=reactive antibodies appear to be directed
against group-specific flavivirus antigens, i.e. the viral glycoprotein E and its group specific epi tape {Heinz et
al., 1981; Peiris et al., 1982). The significance of antibodies directed against glycoprotein E epitopes is unknown, however, Kayser et al. {1985) found antibody
reactions in vivo against group-specific determinants
which could be responsible for the HI cross-reactivity
observed in their study.
Mapping of the epitopes of flavivirus glycoproteins
has been reviewed extensively by Heinz {1986). Monoclonal antibodies have been used to assess the sites of antigenic variations at the epi tape level, and to define critical
epitopes for the preparation of subunit vaccines. To date
the majority of monoclonal antibodies produced to
flaviviruses have been specific for the E glycoprotein, which would therefore appear to be the immunologically
monoclonal antibodies against the NS1 protein indicates
that this nonstructural protein may also induce an immune
.
response. The biological activities of haemagglutination
and neutralization are known to be associated with the E
glycoprotein {Qureshi and Trent, 1973; Eckles et al.,
1975) although epitopes have been identified which do not
appear to induce a useful antibody response {Henchal et
a 1. , 1985). Further, neutralization by antibody and
passive protection in vivo do not necessarily involve the
same epitopes, since cross-protection has been demonstrated
in the absence of cross-neutralizing antibodies (Price et
al., 1967; Tarr and Hammon, 1974). It is also clear that
neutralization of infectivity by monoclonal antibodies
does not necessarily correlate with the serological
grouping obtained by cross-neutralization with polyclonal
sera {Heinz, 1986). The results for E glycoproteins of
(1) YF, (2) DV, (3) SLE and (4)TBE will be discussed in
more detail.
(1) YF
Reactivity patterns among monoclonal antibodies
against the E glycoprotein of the l7D vaccine strain of YF
virus prepared by Schlesinger et al. {1983) initially
defined at least five distinct epitopes. This number was
extended to at least ten {Cammack and Gould, 1986). These
epitopes have been mapped to five topographically distinct
were recognised by one monoclonal each; a fourth, designated D, reacted with two monoclonals and a fifth domain {E}, comprised a major cluster of at least five overlapping antibody binding sites. Functional activities were assigned to the mapped epitopes and, although the HI
and neutralization activities were not necessarily linked, only those monoclonals with neutralization activity were able to passively protect mice against lethal infection.
The monoclonal antibodies which react with the E
glycoprotein of YF virus have also been examined for
with other
cross-reactivity
imrnunofluorescence, neutralization {Schlesinger
Paradoxically,
et a 1. ,
groupings
1983; based
Gould on
flaviviruses and
et
HI a 1. ,
by assays 1985). immunofluorescence assays did not correlate with the serological groupings defined by Madrid and Porterfield {1974}. These findings were extended by Buckley and Gould {1985) who demonstrated the cross-neutralization of a number of flaviviruses using monoclonal antibodies to YF.
(2) DEN
Monoclonal antibodies have been generated against each of the four dengue serotypes {Henchal et al., 1982}. Testing by imrnunofluorescence with DEN and other
glyco-protein. Additionally, subcomplex-specific determinants have been identified. Once again, functional analysis of these monoclonal antibodies found that results from immunofluorescence and HI assays contradicted the results obtained with neutralization tests using polyclonal sera
.
{Gentry et al., 1982). This indicates that, on the basis of single epi tapes, immunof luorescence and HI assays may be more specific than the neutralization test using monoclonal antibodies( 3) SLE
Competitive binding assays have defined antigenic domains on the E glycoprotein for
three type-, complex- and group-specific determinants on SLE virus
{Trent, 1977). Subsequent investigations using 21 SLE monoclonal antibodies prepared against the E glycoprotein
{Roehrig et al., 1983) demonstrated that, rather than separate antigenic domains, there existed a single heterogeneous domain. Based on functional and serological criteria, this single domain, which included eight different epitopes, was spatially arranged as a continuum of S1X
.
overlapping epitope sites. It was alsodemonstrated that a biologically significant epitope {E3) included all the members of the SLE, WN, JE and MVE complex as well as YF virus. One of the eight epi tapes
inoculation of the E-lc monoclonal antibody enhanced protection against SLE virus at least one thousand-fold. Antibody to this critical epitope of SLE virus, therefore,
also proved to be protective.
(4) JE
Kimura-Kuroda and Yasui (1983) have analysed the topological relationship using ten monoclonal antibodies directed against the E glycoprotein of JE. The antibodies could be divided into four types: flavivirus cross-reactive HI and non-neutralizing, subgroup-specific HI and non-neutralizing, low HI and neutralizing and non-HI and neutralizing antibody. Competitive binding assays demonstrated the existence of at least five antigenic determinants on the E glycoprotein with three of these overlapping strongly, reminiscent of the overlapping
domains described for YF and SLE determinants. Seventeen monoclonal antibodies directed against the E protein of JE virus have been characterised, and it was found that they could be divided into eight groups based on reactivity patterns in the HI test, neutralization test, ELISA and competitive binding assays (Kimura-Kuroda and Yasui, 1986).
( 5) TEE
glycoprotein {Heinz et a 1. , 1983). Eight distinct epitopes were defined, seven of which were partially linked and clustered in
.
two antigenically reactive domains. Both domains differed in their serological specificity al though each contained epi topesHI, neutralization and passive protection.
involved in
.
The differences in the competitive binding assays described above, may not be as marked as these experiments might suggest. The monoclonal antibodies used by different groups were generated in random fashion, and do not nee essarily recognise homologous epitopes on different viruses. The testing of other monoclonal antibodies may reveal additional epitopes. For example, epitopes may exist which are unlinked to the continuum described for SLE. Other epitopes may link the domains of DEN and TBE. In addition, the type of label used in binding assays may significantly alter the results. Separate domains were reported in those studies using radiolabelled antibodies {TBE, DEN and YF) and clustering of domains {SLE and JE) was characteristic of assays using peroxidase-labelled
antibodies. Peroxidase labelling may increase the total
.
size of an antibody and possibly increase the amount of overlap {Heinz, 1986).group- specific determinants using polyclonal sera. More complex relationships are apparent at the epi tope level which are specific for the test system applied thus complicating the simple explanation of type, complex and
group. Furthermore, neutralizing assays do not
nee essari ly correlate with the serogroupings obtained by cross-neutralization with polyclonal immune sera. This has also been observed for a number of other viruses including rabies (Flamand et al., 1980). The functional activity of any polyclonal sera therefore, may be a result of the interaction of antibodies to different epi topes. These antibodies may then act synergistically to achieve neutralization of virus infectivity.
2.5. Antibody Dependent Enhancement (ADE)
ADE involves the uptake of virus-antibody complexes by F receptor bearing monocytes and has been demonstrated
C
for a number of viruses including flaviviruses (Brandt et al., 1982; Halstead, 1979; Phillpotts et al., 1985). This has been shown to result in increased numbers of viral particles binding to the cell surface resulting 1n
.
a greater number of virions infecting the cell through amore efficient internalization process (Gollins and
The preparation of monoclonal antibodies and topological
flavivirus
.
mapping
structural
of the . corresponding epitopes on glycoproteins have allowed the problems of ADE to be addressed at the epitope level. Peiris and Porterfield (1982) analysed the capacity of
three monoclonal antibodies to enhance WN virus
replication in the P388Dl macrophage cell line. From these experiments they concluded that (1) the same epitope can be involved in neutralization and enhancement of
infectivity, (2) ADE can be mediated by broadly
cross-reactive, as well as type- and even subtype-specific antibodies and (3) the capacity to mediate ADE is not paralleled by other functional activities such as HI or
neutralization. Consistent with these findings,
Schlesinger and Brandriss (1983) analysed the ability of a panel of monoclonal antibodies against YF virus to cause ADE. Their results demonstrated that both type-specific and cross-reactive monoclonal antibodies, and monoclonal antibodies with different functional properties, could mediate ADE.
The ability of monoclonal antibody to mediate ADE also
appears to depend on the multiplicity of infection
(moi). Hatta et.al. (1984) demonstrated virus
type-specific ADE when testing a monoclonal antibody to DEN2 using a high moi. In contrast, at low moi, ADE was
dependent on flavivirus group-reactive rather than