JOURNAL OFVIROLOGY, Oct. 1974,p.981-996 Copyright0 1974 AmericanSocietyforMicrobiology
Vol. 14,No.4 Printed in U.S.A.
Further Studies
on
the
Replication
of Rabies and
Rabies-Like
Viruses in
Organized
Cultures of Mammalian
Neural
Tissues
S. MATSUMOTO, L. G. SCHNEIDER, A. KAWAI, AND T. YONEZAWA
Institute for Virus Research, Kyoto University, Kyoto, Japan, Department of Pathology, Kyoto Prefectural MedicalCollege, Kyoto, Japan, and Federal Research Institute for Animal Virus Diseases,
7Tabingen, BRD, WestGermany
Received forpublication 30 May 1974
Organized culturesofmammalian spinal and dorsal rootganglionswereused
foracomparativestudyoftheneurocytopathologycausedbyrabies andso-called rabies-like viruses. Electronmicroscopyandtitrationofinfectivityrevisedearlier data in whichnodifferencecouldbedemonstrated between street andfixed-virus
infection. In the present study, fixed virus produced inclusion bodies without apparentvirusassembly. Sequentialelectron microscopyrevealed that themain
sites of virus assembly were the membranes of the Golgi complex. In contrast, rabies-like virusesfreshly isolated from wild rodents produced inclusion bodies
all of whichwereassociated withvirusreplication. Electronmicroscopicevidence
has ledus toclassify these strains asstreetvirus. Nonneural cell elements from
cultivated ganglions were susceptible to fixed virus and the cultures yielded higher titers of infectivity as compared to those of rabies-like viruses. Virus
budding was shown to occur atthe cell surface as well as at intracytoplasmic membranes.
It has generally been accepted that the mor-phogenesis of rabiesvirus canbedivided intoat
least twosteps: accumulation of
nucleocapsids
(formation of the inclusionbody)
followed by envelopment of the virion(budding
via thecytoplasmic
membrane).
Thisprocessissubject
to
slight
variationdepending
upon the virus strainand thetypeofhost cellused(1,
5,8).
In apreceding
paper concerned with the in vivoneuropathology
ofrabiesinfection,
wedescribed thephenomenon
that,
inspite
ofhigh
titers of infectious virus, fixed virus particles within inclusion bodies occurred onlyrarely (8). Simi-lar evidence was obtained with 1-day-old chickenembryos
infected with theHEP-Flury
virus(A. Kondo, personal communication). Due totheobvious technical difficulties encountered inasequential studyofvirusreplicationinan in vivo system, we have adapted the technique using organized cultures of mammalian gan-glion cells (6). It was once again shown byelectron
microscopy that only a small percent-age of inclusion bodies was associated with virus assembly. Upuntilnow this in vitro system has beenunsatisfactory dueto thelowefficiencyof infection as compared withnonneural cell cul-ture systems being highly susceptible to the rabies virus. To overcome the relatively slow onset of infection in a limited percentage ofneurons,improvement has been obtained by the following: (i) choice of suitable virus strains including rabies-like viruses; and (ii) technical improvement of cultural conditions for viral inoculation. Thepresent paper compares ultra-structuralaspectsoftheneuropathologycaused byanumber of rabies and rabies-like viruses.
MATERIALS AND METHODS
Viruses. Fixed virus strains used were the
chal-lenge virus standard (CVS) strain and the plaque-cloned HEP-Flury strain both adapted to BHK-21 cells.Thefield strainsW56/71andW131/71, isolated from Microtus arvalis in southern Germany, repre-sented the so-calledrabies-like viruses (13).
Cell culture. The method used for cultivation of ganglions has been described previously (6). Dorsal andspinalrootganglions from embryos of mice of 15 to17daysinutero orofhamsters of13to 15days were
explanted on collagen-coated round coverslips and incubated with the same medium before and after inoculation. The culture medium consisted of equal volumes of balanced salt solution, Eagle minimal essential medium, calfserum, and saline extract of 9-day-old chicken embryos supplemented with glu-cose to afinalconcentration of 6mg/ml.
Virusinoculation andtitration. Explant cultures were washed twice with warm balanced salt solution (BSS) and exposed to 0.05 ml of virus suspended in Eagle minimal essential medium supplemented with 2%calfserum.Thevirus was allowed to adsorb to the 981
on November 10, 2019 by guest
http://jvi.asm.org/
MATSUMOTO ETAL.
cultures for1hat 37C. After removalofthe inoculum theculture was rinsed three times withBSS,followed by addition of culture medium and incubation at 37C.Samplesforinfectivityassayweretakenat3, 6, 12, 24, 48, and 72 h postinfection (hpi). The cell culturesupernatant fluidwasassayed for the presence of free virus. Cell-associated virus was obtained by
suspending two ganglion explants fromoneculture in 1ml of BSScontaining2% calfserumandsonicating
the suspension for1 min at0C. Cell-associated and free virus were assayed by plaque formation on chickenembryofibroblasts(HEP-Flury virus)andby
intracerebral inoculation ofsucklingmice(field virus strains).
Lightmicroscopy.Unfixed cultures were observed under the light microscope at a magnification of
x400. The direct immunofluorescent technique was used foridentificationof virusantigen usinginfected coverslip cultures. Anti-nucleoprotein (RNP) sera were prepared by immunization ofrabbits with
cell-derived, purified RNP (14) andlabeledwith fluores-ceinisothiocyanate (12).
Electron microscopy. Infected cultures were fixed in situ for 1 h at 4C with 1% isotonic buffered
glutaraldehyde andpostfixed in1%OSO4inMillonig
phosphate buffer for 1 h (7). This was followed by dehydrationin a gradedseriesofalcohol, by immer-sion in acetoneandbyembeddinginVestopalW(Fa. Schchhard, Munich, West Germany). Polymerized
specimens were placed at -20C overnight, after which coverslips became ready to remove. Serial sections were cut fromthe bottom collagensheet ina parallel direction tothe plane ofcellgrowthusinga
Reichert or Porter-Blum ultramicrotome equipped
with a diamond knife. Sections were doublystained withuranylacetateand leadcitrate, and were photo-graphed using a Siemens 101 or Hitachi HU 11D electron
microscope.
RESULTS
Cultural characteristics and kinetics of virus growth. Ganglions usually began to
flat-tenabout 24 h afterexplantation. The first
sign
of cellular proliferation was the
characteristic
zigzag
evolvementofveryfine neurofibrilsafter 24h,
followedby
rapid fibroblast outgrowth atabout 48 h. During the early stage of
cultiva-tion,
individual neurons wereindistinguishable
among thetightly packed
cellsexceptfor afew neurons flattened amongtheoutgrowing fibro-blasts. Nonneural cells and glialelements,
mainly
Schwann and satellite cells, werefully
developed
after 9-14 days, and the majority of neuronscould beidentifiedbytheir characteris-tic cell body, clear nucleus and nucleolus, and cellprocesses.Myelin
sheeths appeared firstin week 2 or 3.Figure 1showstypicalnon-infected neurons maintainedin vitro for 20days.About 10% ofthe neuronswere lost withinthe first 10days
of cultivation. The observed sequential patternofcellgrowth dependedonthe extent of connectivetissue growth withintheculture.The efficiency of the rabies virus infection
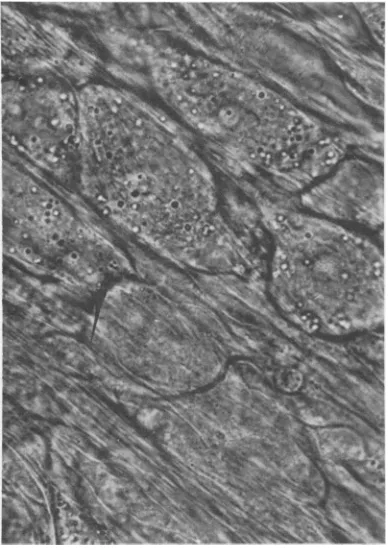
FIG. 1. Apartofunfixedcultivated dorsalroot
ganglion
ofmouseembryo. Noninfectedneuronsmaintained invitrofor20days. x700.982 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:2.502.66.452.387.654.2]RABIES AND RABIES-LIKE VIRUSES
wasfollowedby the appearanceofacytopathic effect (CPE) and by
demonstration
of virus antigen in infected neurons. The typical cyto-pathic changes, the sequence of which has already been described (6), is shown in Fig. 2. To establish the optimal time for infection of cultivated ganglions, cultures were exposed to HEP-Flury virus at different days after ex-plantation. Thedegree ofCPE and theamountof virus antigenwasestimated. Small ganglions from a 15-day-old mouseembryo wereselected tominimizethe growth of nonneuralsupportive cells. The results (Table 1) indicate that the
efficiency of infectiondependsontheageofthe explant. Therefore, only ganglions cultivated for 2 to 3 days have been used throughout the subsequent experiments.
The growth cycleof afixed (HEP-Flury) and afield strain (W56)ofrabies virus in cultivated ganglion cells is shown in Fig. 3 and 4. It was first shown that a HEP-Flury virus suspension of 107-7 PFU/mlwastitrated to 107- 5mean lethal dose
(LD50)/ml
using suckling mice. Exponen-tial virus growth started at 24 hpi. Cell-associated andfreevirus ofthe HEP strain(Fig. 3) reached a maximum of 106-2 and 105-3 PFU perculture at 72hpi. Virusyields from the field strainW56 (Fig.4) wereconsiderably lower and dropped quicklyafter 72 hpi.Morphological
findings.
Light microscopy of ganglion explants infected with different rabies virus strains showed a similar develop-mentof the characteristicneuronal CPE. Cyto-plasmicgranulation appearedat48hpiin many neurons located at the periphery of explants. The subsequent degenerative changes took place asdescribed before (6).By electron microscopy thinsections of gan-glion explants infected withfourrabiesstrains, namely W56, W131, CVS, and HEP-Flurywere compared. Specimens harvested atthe time of maximal CPE (72 hpi) exhibited a varying degree of degeneration within most neurons (Fig. 5-7). The sequence ofcytopathic changes can be summarized asfollows. Numerous
elec-FIG. 2. Neurons 3days after infectionwith rabies-like virus strain W56. Cytoplasmic granulation is seeninmostneurons. x 700.
TABLE 1. Influenceofthe ageofcultivatedganglions
on theefficiency ofinfectionwith theHEP-Flury
strainof rabies virus
Daysbetweenex- FAstainingof plantationand virus CPEofneuronsa virusantigen"
inoculation
1
+++
++
5 ++ ++
10
4-14 - _
20 _
aCytopathiceffect.
bFluorescentantibodystaining.
0L) L-75 u LiL a. S
cell associated virus
virus
id$
0 12 24 48 72
Hours post infection
FIG. 3. Growth curves offixed rabies virus (HEP-Flury) in cultivated ganglions. (0) free virus; (-) cell associated virus.
983
VOL.14,1974
id6S
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.502.58.252.222.497.2] [image:3.502.261.452.414.648.2]MATSUMOTOETAL.
I
cell, ossocioted virus
10
0 12 24 48 72
Hours post infection
FIG. 4. Growthcurvesofrabies-like virus (W56) in cultivated ganglions. (0) free virus; (0) cell-associated virus.
tron-densegranules andlucentvacuolesof vari-ous size replaced the ground substance of the
cytoplasm.Therough-surfacedendoplasmic
re-ticulum, the number of free ribosomes, and Golgi complexes decreased, and the formation
ofballooned mitochondriabecameobvious.
Nu-cleardegeneration wasfrequentlyseen in
heav-ily damagedneurons. Finally, theneurons were
shrunken andbecameonlyidentifiablebytheir
complete covering with thin layers ofsatellite
cells (Fig. 7). A small percentage of heavily necrotic neurons contained small-sized
inclu-sion bodies but no virion. Evidence of virus
replication associated with the inclusion body
wasfoundonlyinneuronsshowing slight
degen-erative changes (Fig. 5 and 6).
Neurons ofcultivatedganglionsinfected with
the rodent-origin field virus strains W56 and
W131 constantly showed large numbers of
vir-ions adjacent to the inclusion body (Fig. 5, 6, 8). Athighermagnification (Fig. 8), buddingof
virions from intracytoplasmic membranes into
the lumen ofvacuoles isclearlyseen. Infection
ofglial cellswasnot observed.
Explantsinfected with the CVSstrain
exam-ined onday3or4postinfection containedlarge
inclusion bodies within slightly damaged or
apparently intactneurons (Fig. 9). The major-ity of inclusion bodies did not contain virions
and only occasionally was virus replication associated with inclusion bodies of the peri-karyon and nerve fiber (Fig. 10). Virions were frequentlyseenextracellularlybetween the neu-ron and itssatellitecells(Fig. 11). Figure 11 also shows virus assembly within a satellite cell. However, such neuroglial infection was not commonly encountered.
The ultrastructural features of HEP-Flury virusinfectionin cultivated ganglionsobserved from 2 to 4 dayspostinfection appeared similar tothoseseen with the CVS strain. Virus assem-bly, however, occurred in asomewhatdifferent modus. Figure 12 illustrates a representative picture oftheearly changes at 24 hpi. Two small inclusion bodiesarelocated at theperipheryof the perikaryon wheremanylucentvacuoles are randomly dispersed. Few virions are seen adja-cent to the inclusion bodies. At 24 to 48 hpi, numeroussmall-sized lucent vacuolesappeared in close relation with the Golgi complex. Virus budding occurred mainly into these vacuoles whichwere notassociatedwithinclusionbodies (Fig. 13 and 14). This modus of replication progressively involved the majority of neurons by 3days afterinoculation.
Fluorescent antibody staining showed that nonneural cells, mostly fibroblasts which filled the outgrowth zone, contained massive aggre-gates ofviral antigenin the case ofHEP-Flury virusinfection. Electron microscopical observa-tions revealed that the occurrence of inclusion bodies was consistent with that of the viral antigen. Sections of nonneural cells (Fig. 14 and 15) were
prepared
fromperipheralportions ofanexplant at72hpi. Virusbuddingfrom the intracytoplasmic and cellular membraneaswell as free virions within extracellular spaces were commonly observed.DISCUSSION
The resultspresented
partially
confirmedour previous data (6) and show more elaborately thatorganized
cultures of mammalian neural tissue are a useful tool for the study ofrabies virus and host cell interaction. The mainte-nance of cell topography in vivo is the main advantage of the organ-type culture but it presents difficulties in the analysis of virus infection compared with conventional mono-layer cell culture systems. One disadvantageis that thick fibroblastic layers coveringthe origi-nal explant block virus attachment to the neurons. However, thiscanbeovercome bythe use of youngexplants
(Table 1)
which arereadily infected andhave
proved
tobeusefulfor sequential analysis of rabies virusreplication
within the neuron.
984 J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.502.56.249.56.337.2]4.~~~~~~~~~~~~~~~~--4..
-*--~eI
J..J. .
.;-,
4>.. '"
?I4IN
,'V
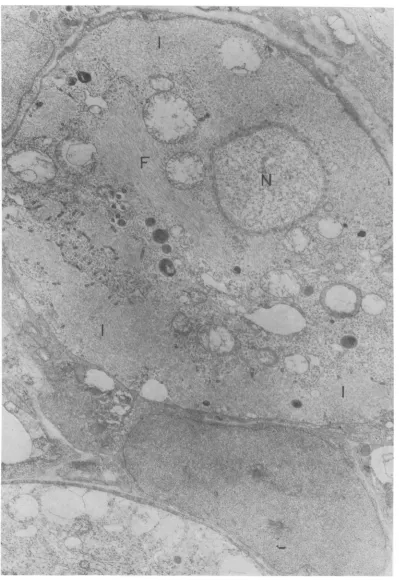
FIG. 5. Rabies virus ( W56) infected neurons.Peripheral cytoplasm of the lower neuron is almost completely replaced by inclusion bodies (I). Virions are located aroundinclusion bodies. Mitochondria are swollen, lucent vesicles of varying size appearsporadically. Part of a heavily degenerated neuron is seen in lower portion. (F) microfilaments. x 15,000.
98,
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.502.50.449.46.628.2])A- 4 4.
I
UQ'
'N
0.r
./
I
S.
4.9:
.
*_AL
Jr-C'"
[image:6.502.61.452.59.637.2]!.
FIG. 6. Rabiesvirus(W56)-infectedneurons. Numerousvirions in association with inclusion bodies(I)are seen throughout thecytoplasm ofaslightly degenerated cell(left). (M) mitochondria. Virusassemblyis not seen in a markedlydamaged neuron (right). Signs ofvirus infection are not evident within satellite cells. x18,000.
986
7
..; ;..
.?N. ., I -71, ;h
,. .*"t , ....,.
I .ffgE;l-j
.. WI
A*;
r .-w
.4.
W..,
_, h.
on November 10, 2019 by guest
http://jvi.asm.org/
RABIES AND RABIES-LIKE VIRUSES
I.'0
,i
r^.
;I:r-II-.. 117,Pk ;..-1
zt "..
to...,:.,
j.1* ,.
,..or
I -k
-k".44
Al
- .;t*
5'
~/ '.;J t
10,
IhI
N1
/
t *
A, pp.-)- 4 _
FIG. 7. Fixed rabies virus (CVS) infected neurons. Cytoplasmic groundsubstance disppears in the lower neuron.Nosign of virusinfectionisdiscernibleinthisheavily damagedcell. Small inclusion bodies(I)are seen within theupper neuron. x17,000.
V,.
f
VOL.14,1974 987
>t, .L.
l
jk.., &I
.A&
--i.71 !.
fijrk,
.0 \,
I ,
06-r-
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.502.53.448.75.610.2]'9
Mm01; .-,- f
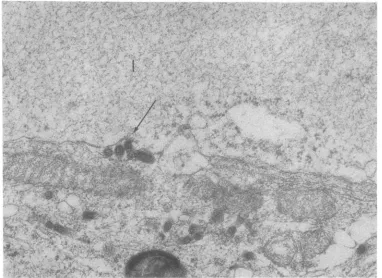
FIG. 8. Periphery of rabies virus (W56) infected neuron. Virions during the process of budding from membranes surrounding inclusion body (I). Electron dense granules somewhat larger than ribosomes are accumulatedat thesiteofvirusassembly. CS; cell surface. M; mitochondria. x45,000.
988
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.502.62.452.60.637.2]44
[image:9.502.51.438.37.480.2]-. \4.,
FIG. 9. Fixed rabies virus ((
lucentvesicles appearsincrease
'Se~~~~~~~~~~~~~~~~~~~~S
fpfniV,it
*3L4M;k;-X
Y~~
"N
-N"Z
ik P
-n
U.,~ 2 *4
~~~~~A~~~~~~~¾4e~~~~~~~~~6
FIG. 10. Fixed rabies virus (CVS) infected neurofiber cut longitudinally. Virions are located near an
inclusion body.Densegranules, as seen in Fig. 8, arefoundnearvirions. x23,000.
989
of
on November 10, 2019 by guest
http://jvi.asm.org/
[image:9.502.50.439.506.659.2]MATSUMOTO ET AL. J. VIROL.
4.i'
FIG. 11. Fixed virus (CVS) infection.Arrowindicates extracellular virions. Upper halfof figureisoccupied byaninclusion body(). Virions in lowerhalf of figurearelocated withinasatellite cell. x33,000.
Similar degenerative changes withinneurons
were observed after infection with different
virus strains. Ultrastructural findings of virus morphogenesis, however, differed markedly. After fixed-virus infection with the CVS and HEP-Flury strains, a largepercentage of inclu-sionbodieswasnotassociated with virus
assem-bly. This evidence, being inconsistent with the actual yield of infectious progeny virus, has repeatedly been obtained by in vivo and in vitro experimentswithfixedrabiesvirus(6, 8).Inthe
presentstudy this observationwas soprominent
thatchronological electron microscopical
stud-ies of the HEP-Flury virus replication within cultured neurons were undertaken. The results
indicated an unusual mode of rabies virus
morphogenesis. Itwasnotedthat, in additionto
the occurrence offew inclusion bodies
associ-atedwithvirions(Fig.12and16), the main sites ofvirusassemblywerethevesicular membranes
of the Golgi complex. Infected neurons were
easily recognized by an increasing number of
small vesicles throughout the perikaryon.
Vir-ions were individually formed and packed
within these structures. An increase of Golgi complex-associated small vesicles has also been
observed in mouse brain neurons infected with
fixedvirus, but virusbudding from these
mem-braneswasnotobserved (8). Ourrecentfindings lead us to speculate that virus assembly may
also occur at the Golgi vesicles during the in
vivoinfection but this is notdemonstrable due
totherapiddegenerative alterations of infected
neurons, possibly a consequence of
autodiges-tiveprocessesinducedbythe increased number
of lysosomes. The involvement of the Golgi complexinthereplicationofenveloped viruses, especially arboviruses, hasbeenreportedbefore
(2, 11). Arboviruses budding from the Golgi vesicles accumulated in membranous
enclo-sures, whereas HEP-Flury virus particles were
mostly contained individually.
Strikingdifferenceswereobserved after
infec-tion of cultivated ganglions with rodent-origin field viruses. Numerous virions which budded
fromintracytoplasmic membraneswerelocated
within inclusion bodies. The accumulation of
virions within brain neurons in vivo has been
emphasized as the main characteristic of the streetrabiesvirus infection (3, 5, 8, 9, 10).The
similarity of virus morphogenesis within the
neuron in vitro and in vivo suggests that the
rabies-likeviruses examined heremaybe
classi-fied asstreet viruses. Inanearlierstudy(6) we
990
.#.
/
* . .. -; 4
*.v*>;. :~~4
,;;.g, r; 'j'tS
E
I
:'' ,j
on November 10, 2019 by guest
http://jvi.asm.org/
[image:10.502.67.449.75.353.2]M
mSf
is
\-7
..---
.-_. *X\>4
I
/
FIG. 12. Fixed virus(HEP-Flury) infectedcellat24
hpi.
Lucentvesiclesoccurattheperiphery of
a neuron. Some mitochondria (M) are ballooned but ground substanceof
thecytoplasm
remains intact. Two small inclusion bodiesshowperipheralvirusbudding (arrows).x20,OOO.
991
iv
on November 10, 2019 by guest
http://jvi.asm.org/
[image:11.502.53.447.52.628.2]MATSUMOTO ET AL.
FIG. 13. Fixed virus (HEP-Flury) infectedneuron at 48hpi. Numeroussmallvesiclesaredistributed in the
cytoplasm. Virusbuddingfrom vesicular membranesisindicatedbyarrows.Inclusionbodiesarenotpresentin thisfigure. x35,000.
992 J.VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:12.502.67.442.71.640.2]'#~~~~~~~~:- 6 f a
IV
I
~~~~~~~~~~~AZ
Ik~~~I
'"J~~~~~~~~~~ J
FIG. 14. Fixed virus(HEPFlury)-infected fibroblastat72hpi.Virusassemblyisindependent frominclusio
body (I). Cytoplasmic membranes from which virus budding occurs are not clearly demonstrated at this
magnification.
x24,500.
99:3
on November 10, 2019 by guest
http://jvi.asm.org/
[image:13.502.58.450.56.630.2]MATSUMOTO ET AL. J. VIROL.
' *
[image:14.502.57.450.190.470.2]ad..
FIG. 15. Extracellular accumulationof virions(HEP-Flury). Arrow showsaintracellularvirionadjacentto theinclusionbody (I)withinafibroblast. x31,000.
994
on November 10, 2019 by guest
http://jvi.asm.org/
RABIES ANDRABIES-LIKE VIRUSES
FIG. 16. Fixed virus (HEP-Flury)-infected neuron at 24 hpi,showing development of small vesiclesfrom Golgicomplex (G). Virions are found within vesicles (arrows) and in a smallinclusion body(I). x76,000.
have shownthatasmall percentageofinclusion bodies was
regularly
associated with virus as-sembly, regardless of whether the infecting virus was of street or fixed-virus origin. The reason for these different findings has not yet been determined. Variation of virus during numerous passages in micemay beonereason, technical aspects or inadequate virus attach-ment tothetarget cellsmaybe another.Growth curves of fixed
(HEP-Flury
strain) and field (W56 strain)virusesdiffered quantita-tively and qualitaquantita-tively. The HEP-Flury virus continuedtomultiplyexponentially until72hpi(Fig. 3 and 4). Its
peak
titers were several-thousandfold higher than those of the W56 virus. The latter virusreached itspeak
titer at 48 hpi and then decreasedprogressively.
The different behaviour indicates that the HEP-Fluryvirus replicates notonly within the neu-ronbut alsowithinothersupportive cellsof the organized neural culture. Electron microscopy confirmed theproductive
infection of fibro-blasts and satellite cells (Fig. 11, 14, and 15).ACKNOWLEDGMENTS
Wewishtothank J. H. Cox for his helpinrevising the
995
VOL. 14,1974
on November 10, 2019 by guest
http://jvi.asm.org/
[image:15.502.55.446.77.496.2]MATSUMOTO ET AL.
manuscript. S. M. ismostgrateful for the facilities offered by M.MussgayatTulbingen.
This investigation was supported by the World Health Organization, the Japanese MinistryofEducation, and the Public Health Service grant NB-03173 from the National Institute ofNeurological Diseases.
LITERATURE CITED
1. Iwasaki, Y., T. J. Wiktor, and H. Koprowski. 1973. Early
events of rabies virus replication in tissue cultures.
Lab.Invest. 28:142-148.
2. Lyons, M. J., and J. Heyduk. 1973. Aspects of the developmental morphology of California encephalitis virus in cultured vertebrate and arthropod cells and in
mousebrain.Virology 54:37-52.
3. Matsumoto, S. 1963. Electron microscope studies of rabies virus inmousebrain. J.Cell Biol. 19:565-591.
4. Matsumoto, S. 1970. Rabies virus. Advan. Virus Res. 16:257-301.
5. Matsumoto. S., and A. Kawai.1969.Comparative studies
ondevelopment of rabies virusindifferent hostcells. Virology 39:449-459.
6. Matsumoto, S., and T. Yonezawa. 1971. Replication of rabies virus in organizedculture of mammalian neural tissues. Infect.Immunity 3:606-616.
7. Millonig, G. 1961. Advantages ofphosphate buffer for OSO4 solution in fixation. J. Appl. Physics 32:1637. 8. Miyamoto, K., and S. Matsumoto. 1967. Comparative studies betweenpathogenesisofstreetand fixed rabies
infection. J.Exp. Med. 125:447-456.
9. Murphy, F. A., S. P. Bauer, A. K. Harrison, and W. C. Winn. 1973.Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site tothe central nervous system. Lab. Invest. 28:361-376.
10. Murphy, F. A., A. K.Harrison, W. C. Winn, and S. P. Bauer. 1973. Comparative pathogenesis of rabies and rabies-like viruses. Infection of the central nervous
system and centrifugal spread of virus toperipheral
tissues. Lab. Invest. 29:1-16.
11. Murphy, F. A., A. K. Harrison, and S. G. Whitfield. 1973. Bunyaviridae; morphologic and morphogenetic simi-larities ofBunyamwera serologic subgroup viruses and several other arthropod-borne viruses. Intervirology 1:297-316.
12. Schneider, L. G. 1973. A rapid method for fluorescein labelling of rabies antibodies, p. 336-338. In M.M.
Kaplan and H. Koprowski (ed.), Laboratory tech-niques in rabies, 3rd ed. World HealthOrganization, Geneva.
13. Schneider, L. G., and U. Schoop. 1972. Pathogenesis of rabies and rabies-like viruses. Ann. Inst. Pasteur 123:469-476.
14. Schneider, L. G., B. Dietzschold, R. E. Dierks, W. Matthaus, P. J. Enzmann, and K. Strohmaier. 1973. Rabiesgroup-specificribonucleoprotein antigen anda
testsystemforgrouping and typing of rhabdoviruses. J.
Virol. 11:748-755.