JOURNAL OF VIROLOGY, Aug. 1979,p.341-349 Vol.31,No. 2 0022-538X/79/08-0341/09$02.00/0
Simultaneous
Purification of Murine
Mammary
Tumor
Virus
Structural Proteins: Analysis of Antigenic Reactivities of
Native gp34
by Radioimmunocompetition Assays
STUART L.MARCUS,* REBECCA KOPELMAN, ANDNURUL H. SARKAR
Laboratoryof Molecular Virology, MemorialSloan-KetteringCancerCenter,NewYork, New York 10021 Received forpublication7March 1979
Allthe structural proteins (gp47, gp34, p27, p23, p16, and p12) of the murine
mammary tumor virus (MuMTV) were simultaneously purified utilizing
alkyl-agarose chromatography as the initial fractionation step. Least-hydrophobic
MuMTVpolypeptides (p23, p16) and theslightly hydrophobicp27wereseparated
from moderately hydrophobic proteinsgp47 and p12 bypassage through
octyl-imino(C8)-agarose; thegp47and p12 could be removed from the matrix by elution
withethylene glycol, whereas themosthydrophobic MuMTV protein,gp34, was
eluted using nonionic detergent together withethyleneglycol. Subsequent
puri-ficationstepsinvolvedion-exchangeorgelfiltrationchromatography. The
result-ing proteinpreparations appeared near-homogeneous on analysis by
polyacryl-amide gelelectrophoresisin the presenceofsodium dodecylsulfate. Recoveries
of MuMTVproteins, basedontheirapproximate individual contributiontototal
virus protein, ranged from about 20% forgp47 togreaterthan100% for the minor
structural component p23, the major phosphoprotein of MuMTV. Antiserum
againstpurifiedC3H MuMTVgp34,together withpurified, radioiodinatedgp34,
wasusedtodevelop aradioimmunoassay which showed thatfrom13 to 14% of
total MuMTV protein by weight is gp34. Using this assay system, the
group-specific antigenic reactivity of gp34 was also demonstrated. When solubilized
preparations of O3H,RIII, andGR MuMTV's wereusedascompetingantigens
ingp34radioimmunoassays withanti-C3H MuMTVserum,bothgroup-and
type-specificdifferences inantigenic reactivitywerefound.
The type B murine mammary tumor virus
(MuMTV)containsapproximatelysix structural
polypeptides,includingtwoglycoproteins(gp34
andgp47) and thenonglycosylatedproteinsp27,
p23, p16, and p12 (5, 13, 21-23, 26, 30). The
glycoproteins gp47and gp34appear to serve as
the extramembrane projections and the
intra-membrane component, respectively, based on
structural dissection in the virions (21, 30) as
well as studies on the individual properties of
theglycoproteins (12,22). Thenonglycosylated proteins have previously been assigned to the
viralcorelocation (12, 22), butrecentevidence
(4) suggests that p12 may functionas a
mem-brane-associatedor matrixprotein. MuMTV is
unique among murine oncornaviruses in
con-tainingtwoglycoproteins andtwo
phosphopro-teins. The major phosphoprotein of MuMTV
was recently shown to bethe minor structural
polypeptide p23,and, in addition, a
subpopula-tionofthemajorcoreprotein (p27) appearedto
bephosphorylated (23).
Purification of MuMTV structural
polypep-tideshas been accomplished usingavariety of
classical biochemicalprocedures(1, 6, 15-17, 21,
25,27,29).However, thetechniqueofgel
filtra-tion in thepresenceofguanidinehydrochloride,
which has proven effective for the purification
oftypeC viralproteins (16),appears to
irrevers-iblydenature and inactivate the antigenicityof
someMuMTVproteins (16, 17).Nosingle
non-denaturing procedurehasyetbeendescribed by
whichall of the structural proteinsof MuMTV
may be purified simultaneously. Additionally,
classicalpurification procedurespose aproblem
for purification of the extremely hydrophobic
membrane-associated peptide, gp34,which has
onlybeen purifiedin thepresence of the
dena-turing detergent, sodiumdodecylsulfate (SDS)
(6).
Immunological characterization of MuMTV
polypeptideshas beenreported forgp47(25, 26),
p27 (27), and p16 (1). The major glycoprotein,
gp47, was the first soluble MuMTV antigen
shown to containgroup specificity (17, 21),and
subsequent studies have shown that antisera
prepared againstgp47, p27,andp14do not
cross-react with any other than their homologous
341
on November 10, 2019 by guest
http://jvi.asm.org/
342 MARCUS, KOPELMAN, AND SARKAR
MuMTV protein and that they all contain
group-specific antigenic determinants (1, 15,24,
25, 27). Biological distinctions in the
tumori-genicity and hormonedependenceofMuMTV's
fromdifferentmousestrains(14) have prompted
asearch for the presence oftype-specific
anti-genic determinantsonMuMTV structural
com-ponents. Several laboratories (1, 10, 15) have
reported that the use ofradioimmunoassay
pro-cedures served to identify only group-specific
reactivities on the variousantigens tested.
Re-cent reports have indicated the presence of both
group- and type-specificantigenicdeterminants
ongp47 (24, 25) andp27(27). Immunochemical
characterization of other MuMTV proteins, in-cluding gp34, has not been reported.
We have recently reported the use of
alkyl-agarose derivatives in determiningthe relative
hydrophobicity(potential forhydrophobic
inter-action) of both type B and type C murine
on-cornavirus structural proteins (12) and reverse transcriptases (11). In this paper, we report the
development of a protocol for thepurificationof
all six MuMTV structuralproteins fromasingle
batchof C3H mouse tissue culture cell-derived
virus usinghydrophobic chromatography as the
initial step in the purification, and without the
use of protein-denaturing reagents. Purified
gp34 was radioiodinated and used in the
devel-opment of a radioimmunocompetition assay.
Competition studies carried out using homolo-gous virus and solubilized preparations of RIII mouse-derived and GR mouse cell-derived
viri-ons revealed the presence of both group- and
type-specificantigenic determinants on the gp34
polypeptide.
(A preliminary reportof this work has been
published [S.L. Marcus and N.H.Sarkar, Abstr.
Annu. Meet. Am.Soc. Microbiol. 1979, S(H)21,
p.
297].)
MATERIALS AND METHODS
Viruses. The MuMTV that was used for all protein purification wasfrom the Mm5mt mousemammary tumorvirus-producingcellline (7), and was obtained as double isopycnically banded, concentrated prepa-rations from the Virus Cancer Program. The GR mouseMuMTV strain was purified fromsupernatant medium of the GR-3A cellline grown as previously described(19). The RIII MuMTV was obtained from RIIImousemilkandpurified aspreviouslydescribed (21).Avianmyeloblastosis virus, Rauscher murine leu-kemiavirus, and Mason-Pfizer monkey virus wereall obtainedasdoubleisopycnically banded and concen-trated preparations through the Virus Cancer Pro-gram.
Reagents.Alkyl-agarosematriceswerepurchased from MilesLaboratories, Inc. Nonionic detergent P-40 (NP-40) was from Particle Data Co. 125I forprotein iodinationwaspurchased fromNewEngland Nuclear
Corp. Sephadex G-75 was obtained from Pharmacia, andphosphocellulose came fromWhatman, Inc.
Antisera. Anti-C3H MuMTV serum prepared against whole, solubilized virions waskindly provided by R. Cardiff (serum no. 1)andJ. Schlom (serum no. 2). Anti-RIII MuMTV serum was prepared against whole, detergent-solubilized virus as previously de-scribed. All of the anti-MuMTV seraused in this study wereshown toprecipitate both of the MuMTV gly-coproteins. Antiserum prepared against C3H-derived MuMTV gp34purified using sodium dodecyl sulfate (SDS) was obtained through thecourtesy of R. Car-diff, andwasalsopreparedin our ownlaboratorywith the participation ofJ. Racevskis.
Radioiodination of protein. MuMTV gp34 or solubilized virus (2to 4jgofprotein),purified using the protocol described herein, was radioiodinated us-ing Iodogen (1,3,4,6-tetrachloro-3a,6a-diphenylgly-couril)obtainedfrom PierceChemicals,aspreviously described (13).
HydrophobicchromatographyofMuMTV. Vi-rusdisruption, in buffer containing 1%(vol/vol) NP-40,0.5%(wt/vol) sodiumdeoxycholate,10mM dithi-othreitol, 0.4 MKCI,10% (vol/vol)glycerol, and 0.05 M Tris-hydrochloride (pH 7.8), was carried out as previouslydescribed (12). Columns of2- to3-ml bed volume containing octylimino (C8)-agarose and de-cylimino(C,o)-agarosewerepreparedandequilibrated with wash buffercontaining 0.05 M Tris-hydrochloride (pH 7.9),1mMdithiothreitol, 10%(vol/vol) glycerol, and1MKCIat4°C.Theprotein concentration of the finaldisrupted virion solution was kept tobelow 10 mg/ml, and to avoidoverloading no more than5mg ofprotein was applied toany one column of alkyl-agarose matrix. After collection of column flow-through fractions, proteins were eluted with wash buffercontaining8.5 Methylene glycol,and themost tightlybound MuMTV structuralproteinswerefinally eluted from the columns with wash buffercontaining both 8.5 Methylene glycol and 1% (vol/vol) NP-40. Detailedhydrophobicchromatographyproceduresfor thefractionation of oncornaviralproteinsaregivenin references12and13.
Ionexchangeandgelfiltration chromatogra-phy. A column ofphosphocellulose (0.5 by8cm)was preparedandequilibratedwithbuffercontaining0.05 M Tris-hydrochloride (pH 7.8), 1 mMdithiothreitol, 10%(vol/vol)glycerol,and0.05MNaCl. The flowrate of thecolumnwasmaintainedat12ml/hwith theuse of a Pharmacia MP-3 peristaltic pump. Molecular sievechromatographywascarriedoutusingSephadex G-75,whichwasswelledtofinal volume inwaterand equilibrated with buffercontaining50mM Tris-hydro-chloride(pH 7.8), 1mMdithiothreitol,0.1%(vol/vol) NP-40, 10% (vol/vol) glycerol, and0.1 MNaCl. De-scendingchromatographywasperformedwitha pres-sure head of25 cm. The dimensions of the column usedwere 1.6by92 cm.
SDS-PAGE. Polyacrylamide gel electrophoresis (PAGE) wascarriedout inthe presence of SDS and the Laemmnli buffer system in 20-cm-long, 5to 17% (wt/vol) polyacrylamideexponential gradientslabsas previouslydescribed(18)orincylindricalgelsas pre-viously described(22).Ifnecessary,proteinswere
con-centratedbefore SDS-PAGEbyeitherlyophilization J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
MuMTV PROTEIN PURIFICATION: gp34 RIA 343
orprecipitation withtrichloroacetic acid,aspreviously described (12).
Radioimmunoassays. Normalrabbit serum and goatanti-rabbit serum werepurchasedfrom Poconos Rabbit Farms. After radioiodination of MuMTV gp34, free iodine was removedbychromatography on Seph-adexG-25,and the materialelutingatthe voidvolume was usedimmediately for immunoassay or stored at 00C.Titration of theimmunoprecipitation of'"I-gp34 was carried out in a volume of 500 yt using serial dilutionsof antiserum in a buffercontaining1%(vol/ vol)normalrabbit serum, phosphate-bufferedsaline, 0.1% (vol/vol) TritonX-100, and a fixed amount of labeledprotein (12,000 to 20,000 cpm).Incubation was carried out for2h at370Candovernightat40C.The next morning, 50
pl
of goat anti-rabbit serum was added to each reaction,mixed,and incubated for 1 h at370Cand then3 h at 40C.Precipitated protein was pelUeted by centrifugation for 5 min in a Beckman microfuge, and theresultingpelietwaswashed once withphosphate-buffered saline solution. "Ilabel in theprecipitatewascounteddirectlyin a gamma spec-trometer. The concentration of the various antisera necessary toprecipitate50%ofmaximallyprecipitable label was determined in this manner and, unless oth-erwisestated,wasroutinely used for radioimmunoas-say. In competition radioimmunoassays, serialdilu-tions of unlabeled antigens, as detergent-disrupted virus orunlabeledC3HMuMTV gp34, were used as described above, both with a fixed concentration of antiserum.Virionsweredetergent disrupted, in a total volume of 100 to 200,uland at a proteinconcentration of1 to 1.8 mg/ml, by the addition of NP40 and sodium deoxycholate to final concentrations of 0.5% (vol/vol) and 0.1%(wt/vol),and the mixture was incubated for 30min at370C.Proteinconcentration wasdetermined usingthe Bio-Rad protein assay (3).
RESULTS
Purification of MuMTV structural
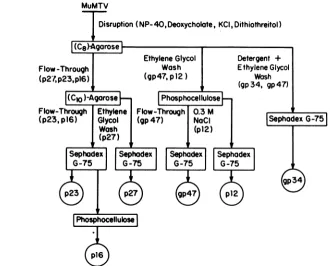
pro-teins. A flow chart of the protocols used to
obtain the simultaneous purification of all six
MuMTVstructuralproteinsfromC3H MuMTV
is shown in Fig. 1. We have previously used
(C1O)-agarose for the purification of the
least-hydrophobic proteins p23 and p16 from
MuMTV, in aprocedurewhichcanalso be used
toisolateR-MuLVppl2and plO(13).Toextend
the purification procedure using hydrophobic
chromatographytoall of theMuMTV proteins,
(C8)-agarose
wasusedasthe initial column stepbecause (i) under theconditions used,p27, p23,
MuMTV
FIG. 1. Flow chart showing the protocolsfor the simultaneous purification of MuMTV proteinsfrom a single preparation ofpurified virions. For details ofpurification refer to the text. Column matrices used in purificationareindicated with rectangles. Thebuffer constituents used for column elution or development are showntotheleft side of each appropriate arrow, and the protein(s) which represents the major constituents of each column fraction is showninparentheses. Circled polypeptide designations represent the final product of the purification.
VOL. 31,1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.504.73.403.337.603.2]344 MARCUS, KOPELMAN, AND SARKAR
and p16are all obtained in the initial column
wash (flow-through) fractions (12), and (ii)
in-complete recovery of gp34wasobserved in
stud-ies with (Clo)-agarose (12). The peak
flow-throughfractions from the (C8)-agarose column
were immediately pooled and passed over a
(C1o)-agarosecolumn, which retainedonlyp27.
The p23 and p16 in the (Clo)-agarose column
wash fractions were initially separated by gel
filtration, andcomplete purification of p16 was
achieved bychromatography on
phosphocellu-lose carried out inbuffercontaining0.3MKCl.
The basic p16protein bindstophosphocellulose
at 0.3 MKCl and thencanbeeluted in a single
stepwith1MKCI.Themajorinternal MuMTV
protein, p27, is eluted from (C1o)-agarose with
ethylene glycol-containing buffer and purified
byafinal gelfiltration step. It isimportant to
note, forallof the proceduresinvolving
hydro-phobicchromatography,that thistechnique
uti-lizes 1 M salt in all washbufferstominimize the
possibility of ionic interactions. Before storage
orsubsequentchromatographyonion-exchange
resins or Sephadex, all fractions were dialyzed
againstlow-ionic-strengthbuffer andlyophilized
aspreviouslydescribed for murine oncornaviral phosphoproteins (13).
Of the MuMTVproteinsthatremained bound
tothe (C8)-agarose column,those thatare
mod-erately hydrophobic (gp47, p12) were eluted
with ethylene glycol-containing elution buffer
(12). After dialysis to remove ethylene glycol
andsalt (13), theethyleneglycol eluatefraction
wasconcentratedbylyophilization andapplied to a phosphocellulose column equilibrated as
described in Materials and Methods. Themajor
MuMTVglycoprotein,gp47,wasobtained in the
phosphocellulose column wash fractions, and
thep12 fraction was eluted with buffer
contain-ing 0.3 M NaCl. Final purificationofboth
poly-peptides was achieved by chromatography throughSephadexG-75 as described in Materi-als and Methods.
The strongly hydrophobic MuMTV gp34
re-mainedbound to (C8)-agarose during elution of
moderately hydrophobic proteins with 8.5 M
ethylene glycol (12). The gp34 was removed
from the column by application ofbuffer
con-tainingbothethyleneglycol and detergent (see
MaterialsandMethods).Aquantityofgp47was
also removed with the gp34, butcould be
sub-sequently separated (after dialysis and
lyophili-zation) by gel filtration in the presenceof
non-ionic detergent. Under these conditions the
stronglyhydrophobic gp34 wasobtainedinthe
voidvolume of the Sephadex G-75 column (data
notshown). The individual preparations of
pu-rifiedMuMTV structural proteins obtained as
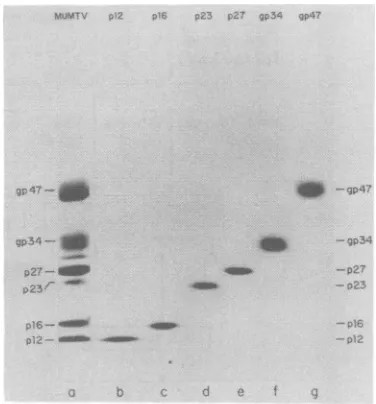
outlinedinFig.1weresubjectedtoSDS-PAGE
J. VIROL.
analysis asdescribed in Materials and
Methods,
and theCoomassie blue-stained gel is sho*n in
Fig.2.From the appearance of thegel the
struc-tural proteinpreparations were nearly
homoge-neous, indicating that hydrophobic
chromatog-raphy is efficacious for the purification of all
MuMTV structural proteins, and that the
ex-tremehydrophobicity of gp34, which creates a
problemwhen other fractionation methods are
attempted, can actually be exploited as an aid to
itspurification.
Recovery of purified MuMTV structural
proteins. To determine theefficiency of
purifi-cation and the recoveries of the purified
poly-peptidesshown inFig. 2, the percent
contribu-tion of each protein to the total amount of
protein present in the virus should be known. The percentage of amino acid label or the
inten-sity of Coomassie blue stainingassociated with
proteinbandsseparatedbySDS-PAGEanalysis
hasbeen previously reported as useful in
esti-matingthe fraction of virion protein represented
by eachpolypeptide for MuMTV (5, 22, 26, 30).
For the major internal core protein, p27,
esti-matesobtained in this manner werefound to be
ingood agreement withvalues obtained by the
more reliable method ofcompetition
radioim-munoassay (13). For the other five MuMTV
structural proteins, however,
radioimmunoas-sayseither
v7,;
havenotM
beendevelopedorwere notr 3. >!'
;4jW-El -lp4:'
Elk gpv34-40
p2-r "'
-3p3, -p2'f7
53)I,
FIG. 2. Purified MuMTV structural protein prep-arations.SDS-PAGE in the slab gelwascarriedout asdescribed in thetext. (a) 60pgof C3HMuMTV protein usedasmarkerfor the other lanes; (b)8pgof MuMTVp12; (c) 6ptgof MuMTV p16; (d) 10pgof MuMTVp23; (e) 9pg ofMuMTVp27;
(t)
12pgof MuMTV gp 34; (g) 13pgof MuMTV gp47. Proteins werevisualizedbystainingwithCoomassie blue._.
gF
4'7 _on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.504.261.449.388.590.2]MuMTV PROTEIN PURIFICATION: gp34 RIA 345
used to calculate the percentage of individual
protein in isolated virions. We have therefore
used the values obtained from SDS-PAGE,
which admittedly may be subject to variations
due to the methods used, to approximate the
percentrecovery of virionpolypeptides purified
using hydrophobic chromatography (Table 1).
The bestrecoveries were observed for the
low-molecular-weightMuMTVproteins, with
recov-ery of p23equalingorexceeding 100% insome
preparations, as previously reported (13). Due
tothe addition ofagelfiltration steptoremove
acontaminating18,000-dalton proteinof
uncer-tain origin, recovery of p27 wasless with this
procedure than in an earlier report (13),
al-though recoveries in excess of50% were rou-tinely observed. Recovery of the moderately
(p12 and gp47)andstrongly(gp34)hydrophobic
MuMTVproteinsrangedfrom20 to50%(Table
1). This lower recovery relative to the other
MuMTVstructuralproteinsmayreflect the fact
that theseproteins bindtoandmustbe eluted
from alkyl-agarose columns, with subsequent
ion-exchange and/or gelfiltration
chromatogra-phy, and the useof severalsteps decreases
re-coveryof the viralproteins.
Development of radio'mmunoassay for
MuMTVgp34. We havepreviouslyshown (13) thatpurification ofp27using alkyl-agarose
de-TABLE 1. Recovery ofMuMTVproteinspurified using hydrophobic chromatography
Protein in final Individual Approx % Poly- prepn polypeptide/ recovery of peptide p totalprotein individual
Mga centb inv c(%) proteind
gp47 0.7 7 26-31 22-27
gp34 0.6 6 16-21 29-37
p27 0.9 9 16-18 50-56
p23 0.24 2.4 1-3 >100
p16 0.7 7 7-9 80-90
p12 0.3 3 6-8 37-50
a
Purified
MuMTV preparations (10 mgof protein)wereused inarepresentative purification. The final purified productsobtainedwereanalogoustothe in-dividualpolypeptide preparationsshown inFig.2.
bVariations of10to20%wereobserved indifferent experiments.
cValues for the approximate percentage of total virionproteinrepresented byeachpolypeptide were obtainedfrom the percentage of totalradioactivity in eachprotein peak after SDS-PAGE using virions la-beled in vivo with amino acids (30) and from the relativeintensity of Coomassie blue stain associated witheach protein band insimilarly analyzed virions (22).
dExpressedasthe ratio of percentinputproteinto thepercentcontributionof the individualpolypeptide tototal viralprotein.
rivatives yielded preparations which did not
have alteredantigenic properties, as determined
by their ability tocompete with p27 inwhole,
detergent-disrupted, radioiodinated MuMTV preparations. We therefore attempted to
de-velop a radioimmunocompetition assay that
would allow the precise quantitation of gp34
within MuMTVvirions.
MuMTV gp34 purified asdescribed above was
radioiodinated in anIodogen-mediated reaction
as described in Materials and Methods. After
removal of the majority of free iodine by gel
filtration, the radioiodinated protein was
ana-lyzed by SDS-PAGE in cylindrical gels. The
1"I-labeled gp34 migrated as a single peak, consist-entwiththepurityof the gp34 preparation (Fig.
2),and indicating that little or no breakdown of
the protein had occurred during the iodination
process (data notshown).
Radioimmunoprecip-itation of
'"I-gp34
wascarried out asdescribedinMaterials and Methods, and all anti-MuMTV
andanti-gp34 seraused in thisstudy produced
a sigmoid radioimmunoprecipitation titration
curve similar or identical to those previously
described (24, 25, 27). From 60 to 90% of the
labeled gp34 could be precipitated with
appro-priate antisera. In the absence ofspecific
anti-serum, however, <2% of inputradioactivity was
found in the pellet afterincubation with goat
anti-rabbit serum (data not shown).
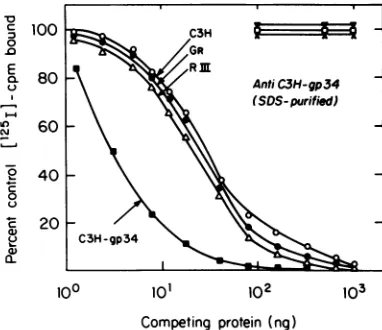
Radioim-munocompetition assaysfor gp34using
antise-rum to SDS-purified C3H gp34 and purified,
unlabeled C3H MuMTV gp34 or
detergent-dis-rupted virions of C3H MuMTV, RIII, MuMTV,
and GR MuMTV as competing antigens are
shown inFig. 3.Thisstudy indicated that gp34
comprises approximately 13 to 14% of the total
MuMTV protein, a value in good agreement
with the more conservative approximations
reached by the analysis of stained gels after
SDS-PAGE (22,30). The anti-C3H gp34 serum
used in thisstudydidnot reactwithsolubilized
preparationsof avianmyeloblastosis virus,
Ma-son-Pfizer monkey virus, or Rauscher murine
leukemia virus, even athigh virus protein
con-centrations. This result indicates the presence of
stronggroup-specific antigenic determinantson
gp34.
Analysisoftype-specific antigenic
deter-minantson gp34. Previousreports have
dem-onstrated that, when usingantiserum directed
against the purifiedMuMTVgp47 (24, 25) and
p27 (27) in radioimmunocompetition assays,
type-specific aswell asgroup-specific antigenic
determinantsmaybefoundonthesestructural
proteins. The factthat, in radioimmunoassays
utilizing antisera against SDS-purified C3H
MuMTV gp34, all three strains of MuMTV tested showed identical immunocompetition VOL. 31,1979
on November 10, 2019 by guest
http://jvi.asm.org/
346 MARCUS, KOPELMAN, AND SARKAR curves
with C absenc
the po
presen
altered
10( 8(
6(
4( 2(
,aswellasabilitytocompletely compete that,when antiserawasraised againstit, a
pop-'3H gp34 (Fig. 3) suggested a possible ulation ofantibodies against type-specific
deter-eoftype-specific determinants.However, minants may have been absent.
Radioimmu->ssibility existed that purification in the noassays utilizing antisera against whole
IceofthedenaturingagentSDSmayhave MuMTV andpurifiediodinatedgp47have been
I the conformation of the protein such shown effective for the identification of
type-specific reactivity (24, 25). Therefore, several
preparations qf anti-C3H MuMTVserumwere
.___. __._ obtained(see Materials andMethods) and used
:)
C3H§
- in theradioimmunoassay
analysis.
Becausepre-GR vious studies indicated that low dilutions of
an-RZ
t\iXntiC3H-9p34
tiserumprovided
increasedsensitivity
totype-(SDS-purified) specific differences (24), we used two dilutions
D of
antiserum,
one of which was sufficient toprecipitate 50% of the maximally precipitable
quantity of
1251-gp34
whereas the otherwas10-O _ to 15-fold less diluted. Anti-C3H MuMTV serum
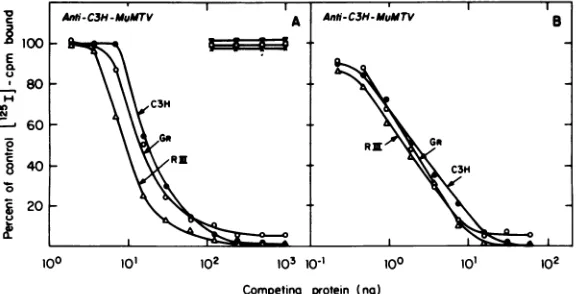
no. 2 used in this study (Fig. 4) showed strong
O _ / \ \<^S _ group
specificity
ongp34
atlowserumdilutionC3H-gp34 (Fig. 4A). With this specific anti-C3H MuMTV
serum at this concentration, differences were
0oo
lo] 102 103 observed both inslopes
oftheimmunoprecipi-10o
102lOl° tation competition curves among MuMTV ofCompeting protein (ng) C3H, GR, and RIII mouse origins and in the
3. Competition radioimmunoassay for C3H extentoffinal
competition
withlabeledantigen
'Vgp 34 using antiserum prepared against athigh concentrations
of competing antigens.trifiedC3Hgp34 and1251-gp34purified as de- Although the homologousC3Hvirus was ableto in Fig. 1. Antiserum was used at a final completely compete with labeled antigen for
a of1:15,000, which was sufficient to precipi- antibody, detergent-disrupted GR and RIII
%ofmaximally precipitable label. Detergent- MuMTV only competed toamaximumof 60%.
ted, solubilized avian myeloblastosis virus When the same anti-C3H MuMTV antiserum zuscher murine leukemia virus(V), andMa- was used at a final dilution of 1:180,000 (the ,zermonkey virus (O) were used as competing concentration capable of precipitating 50% of Istodetectgroup-specificantigenic reactivity. maximally precipitable labeled antigen), the
ized C3H(0),GR (0), and RIII (A) MuMTV of peting antigen ),
the
Rso used ascompetingantigens in addition to slopes ofcompetingantigens from all threetypes iC3Hgp34
(U)
toquantitate the percentcon- of MuMTVappeared quite similar.However, it )nof thatpolypeptide to totalMuMTVprotein. was evident that only the homologous C3H mmunocompetition assays and virus solubili- MuMTVprovidedsufficient antigenic determi-were carried out as described inthe text. nants to allow complete competition with the5 Anti C3H-MuMTV
E 100
0~~~~
" 80 \
S 60 -G
X5 40
-1 20 -R
II IC3H-gp34
101 102 103 100 101 102 103
Cornpeting protein (ng)
FIG. 4. Radioimmunocompetition assayfor MuMTVgp34using anti-C3H MuMTVserumno. 2 (see the
text) at (A) 1:10,000and (B) 1:180,000final dilution (that dilution ofserumallowing50%precipitationof labeledantigen). Competing antigenswere asdescribedin thelegendtoFig. 3.
c
C
0
QE
-N
C
0
2
c--)
c
a.
FIG. MuMT SDS-pu scribed dilutioA tate50f: disrupto (x), Ra son-Pfi antigen Solubil wereal
purifiec
tributio Radioir zationi
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.504.61.252.154.319.2] [image:6.504.116.405.478.633.2]MuMTV PROTEIN PURIFICATION: gp34 RIA 347 labeledgp34.The GR and RIII MuMTV's
com-petedto85and90%,respectively(Fig. 4B),even
at 10-fold higher protein concentrations than
those showninthefigure. Therefore,
type-spe-cific antigenic determinants onthe gp34
mole-cule were observed using anti-C3H MuMTV
serum no. 2,and the presence ofsuch
determi-nantscould be amplified through theuseof low
dilutions of antiserum.
Using rabbit serum no. 1 to C3H MuMTV
(see Materials and Methods) at low (1:10,000)
and high (1:100,000) dilution, the slopesofthe
immunocompetition curves for C3H, RIII, and
GRvirusesappearedidentical(Fig. 5).The
fail-ureofGRMuMTVtocompetecompletelywith
labeled antigen was the only apparent signof
type-specific antigenic differences, although
group-specificreactivitywasstill apparent(Fig.
5A). Two other anti-C3H MuMTV sera
pre-pared and used in this study at high and low
dilution revealed notype-specific
immunoreac-tivity ongp34for the viruses examined.
There-fore, our studies using various rabbit antisera
prepared against C3H MuMTV show that the
abilitytoidentify type-specific antigenic
deter-minantsonMuMTVgp34 is dependent on the
specific antiserum used.
DISCUSSION
Hydrophobic chromatography has only
re-cently been appliedtothe study ofoncornavirus
proteins (11-13). We have shown that these
mat-ricescanbe usedtodetermine thepotential for
hydrophobic interaction (relative hydrophobic-ity) of structural proteins, and have arbitrarily
defined fourcategories ofincreasingrelative
hy-drophobicity based on these procedures (12).
The fractionation of viral structural proteins
observed onthese matricesnaturallysuggested
X Arin-C3H-MuMTV E
80
- 60
GM
0
20-theiruse asreagentsinprotein purification. The
lack of binding of both murinetype C andtype
B phosphoproteins to (Clo)-agarose has been
reported as a rapid and effective method for
theirpurification (13).Modificationof this
pro-tocol, as described above (Fig. 1), has allowed
the simultaneous purificationofallthe MuMTV
proteins, whichappearnear-homogeneousupon
SDS-PAGEanalysis,asdetermined by
Coomas-sieblue staining (Fig. 2).Traces of
contaminat-inglow-molecular-weight proteins ofuncertain
originare,however, sometimesseenin p23
prep-arations (13). The fact thatgp34 aggregates in
aqueous solution prompted other investigators
to resort totheuseof SDS in its purification(6).
Methods suchaslectin-agarosechromatography
(20, 29)aswe7lasion-exchange chromatography
(6),which haveprovensuccessfulin the
purifi-cation ofgp47, are notuseful forgp34isolation.
Indeed, gp47andgp34 copurify througha
vari-etyoffractionationsteps(29) and doso even to
a degree during hydrophobic chromatography
(12). Therefore, in addition toprovidinga
pro-cedurefor the simultaneous isolation of all six
MuMTV structural proteins in good yields
(Ta-ble 1), the protocol shown in Fig. 1 represents
the first method for purifyinggp34 without the
useofdenaturingreagents, through the
exploi-tation of its extremely hydrophobic nature. It
should be mentioned, however, that the
MuMTV DNApolymerase is not a likely
can-didate for future purification on alkyl-agarose
matrices, since,incontrast tostructuralproteins,
it bindstoboth(C8)-and
(Clo)-agarose
with suchavidity that neither ethylene glycol-containing
buffernorthesamebuffer alsocontaining
deter-gentsisabletoelute active enzyme(11).
By preparing aradioimmunocompetition as-saywithpurifiedgp34,wewere ableto
quanti-o0 o10' 102 103 lo-, 100 lol 102
Competing protein (ng)
FIG. 5. Radwimmunocompetition assayforC3HMuMTVgp34usinganti-C3H MuMTVserum no.1 (see
thetext)atdilutionsof (A) 1:10,000and(B) 1:100,000 (thatdilutionofantiserumallowing50%precipitation
oflabeledantigen).Symbols for competing antigensareidenticaltothosedefinedin thelegendtoFig.3. VOL. 31,1979
on November 10, 2019 by guest
http://jvi.asm.org/
[image:7.504.112.405.486.633.2]348 MARCUS, KOPELMAN, AND SARKAR
tatethe amount ofgp34present inmaturevirus.
We estimated that gp34 accounts for
approxi-mately 13to14% of the totalproteininpurified
virions. The absence of deoxycholate, or an
in-crease in virusproteinconcentration in the
sol-ubilization reaction mixture above 1.5 mg/ml,
resulted inunderestimationof the percentage of
gp34 in thevirion (datanotshown). This result
may bedue to the tenacious association of gp34
with the virusmembrane, toitslipophilic
prop-erties, to its tendency to aggregate in aqueous
solution (whichperhapsresults in the masking
of certainantigenicsites),or toall of these. The
factthatlipophilic proteins bindlargequantities
of detergents (9) suggests that theweightratio
ofprotein to detergent may be acriticalfactor
inthe solubilization of certain virus proteins.
Although several reports using differing
im-munological techniques have shown the pres-ence of group-specific antigenic determinants
associated with MuMTV (1, 15, 16, 21),
identi-fication oftype-specificdeterminantsonpurified
MuMTV proteins have onlybeen reported for
gp47(24, 25) and p27 (27).Blair (2) was the first
toreport thepossible presence oftype-specific
differences amongMuMTV's,usingwhole virus
preparations in immunodiffusion analysis. We
have been able to demonstrate the apparent
existence of both group- andtype-specific
anti-genicreactivitiesonthe gp34polypeptide (Fig.
3 to5) usingdetergent-solubilized preparations
of thehomologous (C3H)MuMTVand RIII and
GRmousecell-derivedMuMTV'sascompeting antigens. Ofthree different anti-C3H MuMTV
seratested having similaror identical
radioim-munoprecipitationtiters forgp34,onlyone
(se-rum no. 2, Fig. 4) showeddistinct type-specific
differences in gp34antigenicreactivity;theRIII
and GR viruseswereunabletocompletely
com-pete withC3H gp34 for the available antibody
population. Teramoto and Schlom (28) have
obtained similar results with antiserum no. 2.
Type-specific antigenic determinants on
MuMTV gp34 also appear to bemost
demon-strable at low antiserum dilution (Fig.4A).
Al-though only one of theseratestedshowed clear
type-specific differences,all anti-C3HMuMTV
seraused in this study demonstrated
group-spe-cificreactivities on MuMTV gp34. The finding
oftype-specific antigenic reactivities on a
puri-fied viralproteinwith oneantiserummay
there-forenotnecessarily mean that identical results
willbeachievedwith other antiviralsera, even
ifthey show comparable titers in
radioimmu-noprecipitationassays.
Trypticpeptidefingerprinting analysisof
ra-dioiodinated viral proteins has recently been
used to analyze possible strain-specific
differ-ences in the gp47, gp34, and p27components of
RIII,C3H,andGRMuMTV (8). Differences in
the peptide maps of gp47 and p27 were found
consistent with previous descriptions of type
specificity (24, 25, 27). However, significant
dif-ferences in the gp34 peptide maps from all three
virus strains were notdetected (8). These results
are in contrast to the demonstration of
type-specific determinants by at least one
anti-MuMTV serum in our studies (Fig. 4). It is
possible that this antiserum contains a unique population of antibodies to specific and perhaps
minorchanges inglycosylationofthe C3H gp34
as compared with other gp34molecules of the
RIII and GR MuMTV strains. These changes
would not be detectable in peptide mapping. Alternatively, type-specific differences may
re-side inpeptideslacking tyrosine residues, which
would be undetected by the procedure used for
the analysis of the peptide maps of MuMTV
proteins (8). We are currently preparing antisera
against gp34 purified by the above procedure
(Fig. 1 and 2) to determine whether antisera
prepared against the nativeformof gp34 can be
used to detecttype-specific reactivities on this
protein.
ACKNOWLEDGMENTS
We are grateful to R. Cardiffand J. Schlom for their generousgiftsof MuMTVantiseraandtoC. Sherr forhelpful discussionsregarding radioinununoassay protocol.Wethank J.GruberforprovidingtheC3H MuMTV and nonmammary oncornavirusesused in thisstudy.We also thank J. Racevskis and E. Whittington for anti-C3H gp34 serum and for SDS-PAGEanalysis.
This workwassupported,inpart,byPublic HealthService grants CA-17129 and CA-08748 from the National Institutes of Health.
LITERATURE CITED
1. Arthur, L.O., C. W.Long, G. H.Smith, and D. L, Fine. 1978.Immunologicalcharacterization of the low-molecular weight DNA binding protein of mouse mam-mary tumor virus. Int. J. Cancer 22:433-440. 2. Blair,P. B. 1970.Immunologyofthe mouse mammary
tumorvirus: comparisonof theantigenicity of mam-mary tumor virusobtained from several strains of mice. Cancer Res.30:625-631.
3. Bradford,M. M.1976.Arapid andsensitive methodfor
the quantitation of microgram quantities ofprotein utilizing theprinciple of protein-dye binding. Anal. Bio-chem. 72:248-256.
4. Cardiff,R.D., M. J.Puentes, L.J.T.Young, G. H. Smith,Y. A.Teramoto, B. W.Altruck,and T. S. Pratt.1978.Serologicaland biochemical characteriza-tion of the mousemammarytumor viruswith localiza-tion ofplO.Virology 85:157-167.
5. Dickson,C., andJ. J.Skehel. 1974.Thepolypeptide composition ofmousemammarytumorvirus.Virology 58:387-395.
6.Dion,A.S., C.J.Williams,andA. A.Pomenti.1977.
The major structural proteins of murine mammary
tumorvirus:techniques for isolation. Anal. Biochem. 82:18-28.
7. Fine,D.L.,J.K.Plowman,J. P.Kelley,L.0. Arthur,
on November 10, 2019 by guest
http://jvi.asm.org/
MuMTV PROTEIN PURIFICATION: gp34 RIA 349
and E. A. Hillman. 1974. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tu-morcell cultures. J. Natl.CancerInst.62:1881-1886. 8. Gautach, J. W., R. Lerner, D. Howard, Y. A. Tera-moto, and J. Schlom. 1978. Strain-specific markers for the majorstructural proteinsof highly oncogenic murine mammary tumor viruses by tryptic peptide analyses. J. Virol. 27:688-699.
9. Helenius, A., and K. Simons. 1972. The binding of detergents to lipophilic and hydrophilic proteins. J. Biol.Chem.247:3656-3661.
10. Kimball, P. C., R. Michalides, D. Colcher, and J. Schlom. 1976. Characterization of mousemammary tumorvirusesfor primary tumorcell cultures. II. Bio-chemical andbiophysicalstudies. J. Natl.CancerInst. 66:119-124.
11.Marcus,S.L., andS. W. Smith. 1978. The interaction of retroviral DNA polymeraseswithalkyl-agarose mat-rices.Biochem. Biophys.Res. Commun. 89:220-228. 12.Marcus, S. L, S. W. Smith, J. Racevskis, and N.
Sarkar. 1978. Therelativehydrophobicity of oncorna-viralstructural proteins. Virology 86:398-412. 13.Marcus, S.L, S.W.Smith,J.Racevskis, and N. H.
Sarkar. Purification ofoncornaviralphosphoproteins usingalkyl-agarose matrices. J. Biol. Chem., in press. 14.Nandi,S., and C.M.McGrath.1973.Mammary
neopla-sia in mice. Adv. Cancer Res. 17:353-414.
15. Noon,M.C.,R.G.Wolford, andW. P. Parks. 1975. Expression of mouse mammary tumor viral polypep-tides inmilksand tissues. J. Immunol.115:653-658. 16. Nowinski,R.C.,N. H.Sarkar, andE. Fleissner. 1973.
Isolation of subviral constituents and antigens from oncornaviruses, p. 237-285. In H. Busch(ed.),Methods in cancer research, vol. 8. Academic Press Inc., New York.
17. Nowinski,R.C.,N. H.Sarkar, L.J.Old,D.H.Moore, D. J.Scheer,and J.Hilgers.1971.Characteristics of thestructuralcomponentsof the mouse mammary tu-morvirus.Virology46:21-38.
18. Racevskis, J.,andN. H. Sarkar. 1978.Synthesisand processing of precursorpolypeptidestomurine mam-mary tumor virus structuralproteins. J. Virol. 25:374-383.
19.Ringold,G.,E. Y.Lasfargues,M. J.Bishop,and H. E. Varmus.1975.Production of mousemammary
tu-morvirus bycultured cellsinthe absence andpresence of hormones:assaybymolecularhybridization. Virology 65:135-147.
20. Ritzi, E., A.Baldi, and S.Spiegelman. 1976.The pu-rification of a gsantigenof the murine mammarytumor virus and its quantitation byradioimmunoassay. Virol-ogy75:188-197.
21. Sarkar,N. H., and A. S. Dion. 1975. Polypeptides of the mouse mammary tumor virus. I. Characterization of twogroup-specificantigens.Virology 64:471491. 22. Sarkar,N.H.,N. E.Teraschi,A.A.Pomenti, andA.
S. Dion. 1976. Polypeptides of the mousemammary tumor virus.II.Identification of two major glycopro-teins with theviral structure.Virology 69:677-690. 23. Sarkar, N.H.,E.S.Whittington,J.Racevskis,and
S. L. Marcus. 1978. Phosphoproteins of the murine mammary tumor virus.Virology91:407-422. 24. Teramoto, Y.A.,D.Kufe,and J. Schlom. 1977.
Type-specific antigenicdeterminants on the majorexternal glycoprotein of high-and low-oncogenic murine mam-mary tumor viruses.J.Virol. 24:525-533.
25. Teramoto,Y.A.,D.Kufe,and J.Schlom.1977. Mul-tiple antigenicdeterminants on the major surface gly-coprotein of murine mammary tumor viruses. Proc. Natl. Acad.Sci. U.S.A.74:3564-3568.
26. Teramoto, Y.A.,M. J.Puentes,LJ. Y. Young, and R. D.Cardiff.1974.Structure of the mouse mammary tumorvirus: polypeptidesandglycoproteins. J. Virol. 13:411-418.
27. Teramoto, Y.A., and J. Schlom. 1978. Radioimmu-noassays demonstrating type-specific and group-spe-cificantigenic reactivitiesfor themajor internal struc-tural proteinof murine mammarytumorviruses. Cancer Res. 38:1990-1995.
28. Teramoto, Y. A., and J. Schlom. 1979. Radioimmu-noassaysfor the36,000-dalton glycoprotein of murine mammary tumor virusesdemonstrate type, group, and interspeciesdeterminants.J.Virol. 31:334-340. 29. Westenbrink,F., W. Koornstra, and P. Bentvelzen.
1977.The majorpolypeptideof the murinemamnmary
tumorvirusisolated by plant-lectin affinity chromatog-raphy. Eur.J. Biochem. 76:85-90.
30. Yagi, M. J., and R. W. Compans. 1977. Structural
componentsof mouse mammary tumor viruses. I. Pol-ypeptidesof thevirion.Virology 76:751-766. VOL. 31,1979
on November 10, 2019 by guest
http://jvi.asm.org/