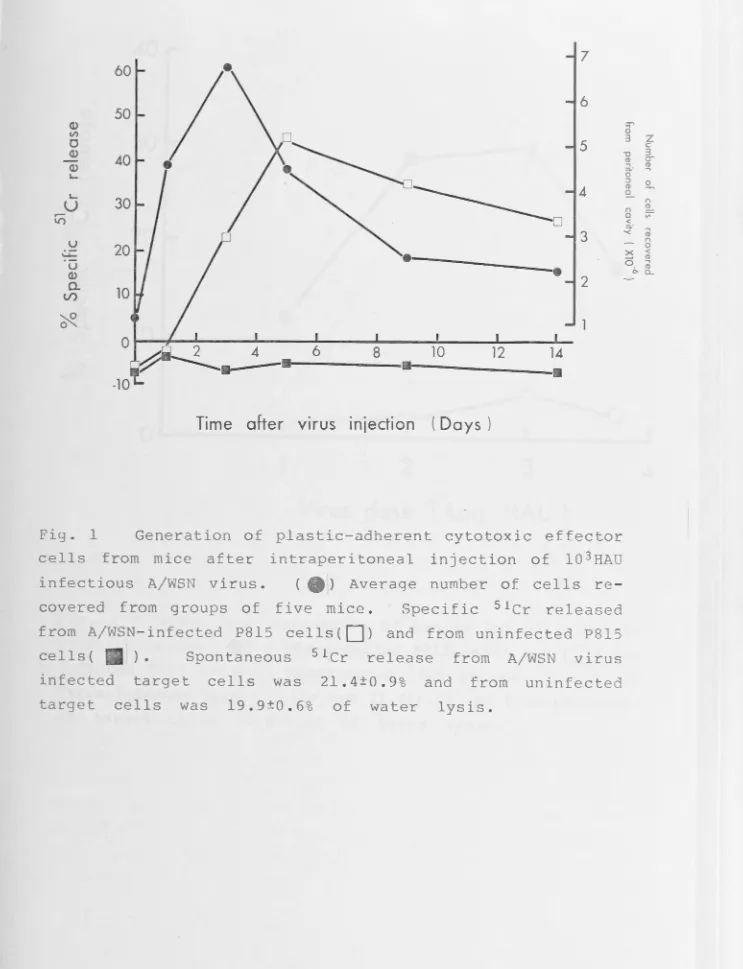
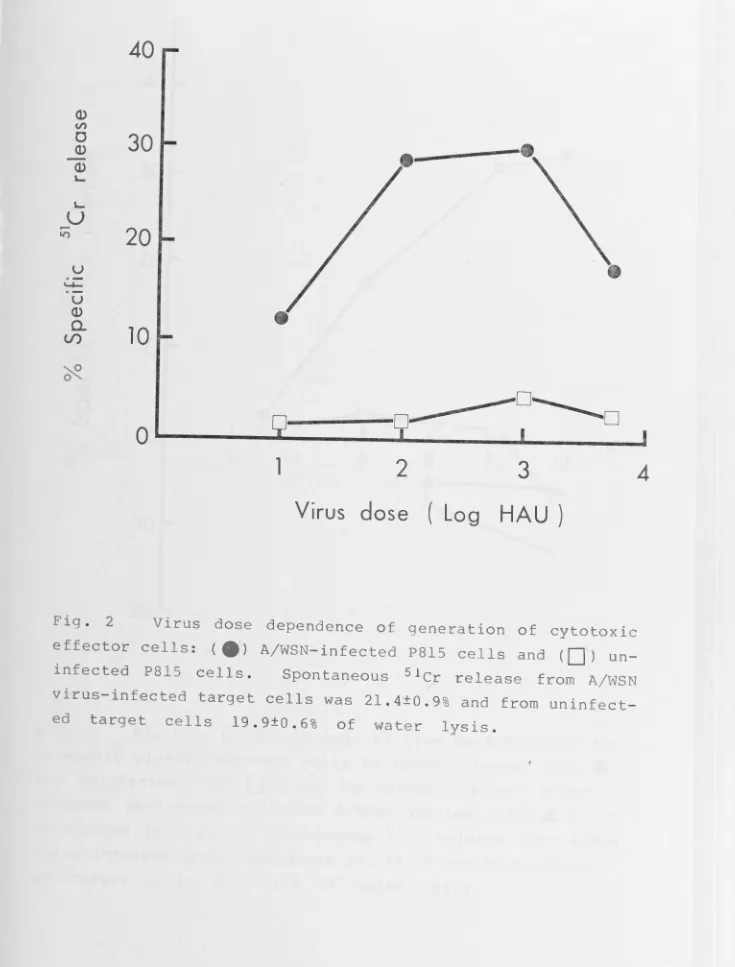
INFLUENZA VIRUS INFECTION
by
MAK NAI KI
A thesis submitted for the degree of Doctor of Philosophy (Ph.D)
in the
Australian National University
CONTENTS
Page.
Staternerit ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• (i) Acl<rlowledgernerits ••••••• · ••••••••••••••••••••••••••••••••••••••••••••• (ii) Al:)breviations •••••••••••••••••••••••••••••• -••••••••••••••••••••••••• (iii) Abstracts ••••••••••••••••••••••••••••••••••••••••••• ~ •••••••••••••••. (v)
Chapter
1 -
General Introduction: A Literature Review •••••••••••••••1.
Chapter 2 - lliterials arid 1-1ethods. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 70.
Chapter 3 - The Generation of Cytotoxic llicrophages in Mice during Infection with Influenza A or Sendai viruses. • • • • • • • • • • •
Chapter 4 - Acquisition of Anti-influenza Virus Activity by
llicrophages. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • •
Chapter
5 -
Protection of Mice against Influenza Virus Infection: Enhancement of Non-specific Cellular Responses byCorynebacterilll11
parvum.
• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • / 'Chapter 6 - HlilTIOral arid Cellular Responses of Mice to Infection
95.
110.
124.
with a Cold-adapted Influenza A Virus Variarit ••••••••••• 137.
Chapter 7 - The Sensitization of Mice with a Wild-type arid Cold-adapted Variant of Influenza A Virus: Secondary
Cytotoxic
T
Cell Responses ••••••••••••••••••••••••••••••150.
Chapter
8 -
Concluding Discussion.• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 164.
Publications •••••••••••••••••••••••••••••••••••••••••••••••••••••••• 179.
B·b1· h
1. 1.ograp y •••.••••••••••••••••••••••••••••••••••••••••••••••••••••
180.
STATEMENT
Part of the work presented in this thesis was done in collaboration with Drs. Y.E. Zhang and C. Sweet while they were Visiting Fellows in the Microbiology Department, John Curtin School of Medical Research, and with Dr.G.A.Tannock (University of Newcastle). The experiments described in
.
Table 11 and Fig.4 of Chapter 3, and Table 2, Fig. 3, of Chapter 7 were done joinly with Drs. K.N. Leung, and C. Sweet, respectively. The experiment described in Fig. 6 of Chapter 5 was performed by Miss E.Schiltknecht. The titrations of serum HI antibody and infectious virus.
tissue culture were done by Dr. G.A. Tannock. All other experiments described in this thesis were my own original work and were carried out by myself under the supervision of P fessor G.L. Ada.
July, 1983.
N.K. MAK
Department of Microbiology John Curtin School of Medical
Research.
(ii)
ACKNOWLEDGEMENTS
I am most grateful to my supervisor Professor G.L.
Ada, a remarkable scientist, for his valuable advice and guidance for the past three years during which I carried out the work presented in this thesis.
I would also 1 ike to thank Drs. R. V. Blanden, K. N. Leung, G.A.Tannock, Y.H.Zhang, and C.Sweet with whom I had stimulating discussion and/or enjoyable collaboration.
My thanks also go to Professor P. Wildy and Dr. G.R.
Shellam for their supply of antiserum and interferon standards. My friends and colleagues in the John Curtin
School of Medical Research have been most helpful and I would like to express my appreciation to them.
ABBREVIATIONS
ADCC . . . Antibody-dependent cell-mediated cytotoxicity
B cell . . . Bursa of Fabricius (or mammalian equivalent) derived lymphocyte
BCG . . . Bacilli Calmette Guerin
C' . . • . . . • . . . Complement
ca-variant .. Cold-adapted variant C.parvum . . . . Corynebacterium parvum CMI . . . • Cell mediated immunity CMV . . • . . . Cytomegalovirus
Con A . . . Concanavalin A CPE . . . • . Cytopathic effect
CRBC . . . • . Chicken red blood cell(s)
DTH ...•••. Delayed-type hypersensitivity
EBV •••... Epstein Barr virus
EID 50 • •••••• 50% Median egg infectious dose E / T . . . . • . Effector to target cell ratio
F- .••..•... Fusion negative
FBS ..•..••.. Foetal bovine serum
H-2 . . . • H-2 region of MHC in mice HA . . . • . Haemagglutinin
HAU . . . • . . Haemagglutination unit(s) HI . • . . . Haemagglutination inhibition
HI-FBS . . . . • . Heat-inactivated (56°C,30 min) foetal bovine serum hr . . . Hour(s)
RSV .•....•.• Herpes simplex virus
IA . . . • . . . . IA sub-region of H-2 complex IFN . . . • . . . Interferon
lg . . . • Immunoglobulin IL . . . Interleukin
ISC . . . Infected spleen cells
1251-UdR . . . . 1251-5-iodo-2 '-deoxyuridine i.n . . . Intranasal
i.p . . . Interperitoneal i.v . . . Intravenous
LAF . . . Lymphocyte activating factor
-LPS . . . ... Bacterial lipopolysaccharides LT . . . Lymphotoxin
Lyt . . . Lymphocyte differentiation antigen
M¢ . . . Macrophage(s)
MAF(MCF) . . .. Macrophage activating factor (macrophage cytotoxic factor)
MAS . . . Macrophage activating supernatant MCMV .. . . Murine cytomegalovirus
MDCK . . . .. Madin- Darby canine kidney cells 2 -ME . . . 2 -mercaptoethanol
MEM . . . Eagle's minimum essential medium MHC . . . Major histocompatibility complex MHV . . . Mouse hepatitis virus
MPO . . . Myeloperoxidase
NA . . . • . Neuraminidase
NDV . . . Newcastle disease virus NK cells . . . . Natural killer cells
+; +
nu nu •.... NSC • . . . • .
PBL • . . . . • . . . PBS . . . • • ..
Athymic nude mice
Normal spleen cell (s)
Peripheral blood leukocytes Phosphate buffered saline PEC . . . • . Peritoneal exudate cells
PMN . . . Polymorphonuclear cells
PSN . . . • . . . . • Penicillin, streptomycin, and neomycin
S.D . . . • . . . Standard deviation S.E . . . Standard error
SFV . . . . • . . . . Sernliki Forest virus SRBC . . . Sheep red blood cells
T cell . . . Thymus derived lymphocyte TCGF ... . . T cell growth factor
TCID 50 •••••• 50% tissue culture infectious dose
/
(iv)
TG-M¢ . . . Thioglycollate induced peritoneal macrophage(s) Thy 1 . . . Theta antigen
ts .. . . temperature sensitive Tc . . . Cytotoxic T cells
Td . . . .. T cells mediating DTH reaction Th . . . T helper cell(s)
Ts . . . .. . . T suppressor cell(s)
UV ... . .. . . Ultra-violet
ABSTRACT
Non-specific host factors represent the main line of defence during the first few days of infection in a naive host. Specific host responses which are acquired during vaccination or infection are the main defence mechanisms against reinfection with the same or antigenically closely related organisms. The work reported in this thesis studied some aspects of non-specific cellular defence components and the acquisition of protective immunity to influenza virus infection by using a highly attenulated strain of influenza virus which has been proposed as a master strain for influenza virus vaccine production.
Cytotoxic macrophage-like cells could be recovered from the peritoneal cavity of mice after injection of infectious but not of non-infectious influenza A virus or of infectious or non-infectious Sendai virus. The effector / cells were cross-reactive in that cells activated by an influenza A strain virus or Sendai virus lysed target cells infected with the same or other A strain viruses or with Sendai virus. The effector cells were plastic adherent and their activity was greatly diminished by exposure to agents cytotoxic for macro-phages, e.g.silica. Effector cells with similar cytotoxic activity could also be recovered from the peritoneal cavity of athymic nude mice or from the lungs of mice 15 days after intranasal inoculation of infectious influenza virus.
(vi)
induced peritoneal macrophages (TG-M¢), on exposure to a virus infected spleen cell culture supernatant (MAS), were found to be cytotoxic for influenza virus or Sendai virus infected P815 cells. Both infectious or non-infectious Sendai or influenza virus were effective at inducing MAS. TG-M¢ show spontaneous cytotoxic activity ·toward influenza virus infected but not uninfected L929 cells. The sharing of the H-2 region between effector cells and target cells was not required for target cell killing. Cell fractionation
experiments showed that the cytotoxic activity of TG-M¢ from ~~ high density fraction was higher than TG-M¢ from low density fraction. Furthermore, the effector cells were phagocytic and ra-. These observations suggested that macrophage populations
e
are heterogenous.
"
Macrophages, on exposure to MAS, were not only cytotoxic for influenza virus infected cells, but also becqme resistant to subsequent infection with influenza/
virus. The mediator(s) which activated macrophages in this way was most likely type 1 IFN.
The role of early non-specific defence mechanisms ~n ' influenza virus infection was further demonstrated by using an immunomodulator, C. parvum. Administration of C. parvum intranasally (i.n.) to mice 3 days before i.n. inoculation of an otherwise lethal dose of a mouse adapted influenza A virus protected all or most of the mice in a group from death. The lungs of C. parvum-treated mice contained significantly less infectious virus than did control mice. The protection
on
lung interferon levels (or enhanced ability of lung cells to produce IFN), (2) lung NK cell activity, and (3) lung macrophage content. Normal resident lung macrophages were susceptible to infection by influenza virus, whereas macrophages recovered from the lungs of C. parvum treated mice resisted infection. The level of serum anti-HA
antibody, cytotoxic T cell activity, and DTH reaction remained unchanged by C. parvum treatment. Thus, the protective effect was attributed to an enhanced non-specific defence mechanisms.
The ability of an infectious attenuated cold-adapted influenza A virus (A/Ann Arobr/6/60-ca) and its parental virus A/Ann Arbor/6/60 to induce primary and secondary immune responses was compared. The ca-variant virus was attenuated
and did not cause significant lung damage when inoculated intranasnally. At the dose of 105 TCID
50 , the replication of
,-variant was highly restricted in mouse lungs, but the ca-variant could induce a significant increase in primary cell mediated immune responses (Tc, NK cell, macrophage, and DTH reaction) and serum HI antibody.
The ca-variant virus was also able to sensitize mice for secondary immune responses. Challenge of vaccinated mice with a serologically distincte:d mouse adapted influenza virus
(viii)
spleen. Vaccination of mice intranasally with the ca-variant
virus resulted in a more than 20 fold increase in memory Tc cell frequency in their lungs. Although the ca-variants are
defective (temperature sensitive, restricted replication at above 37-38°C), the ·minimurn replication of the virus in the mouse lungs was able to provide sufficient antigenic
General Introduction:
Preface • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 1. General introduction of body defence mechanisms against viral
infection ... • ... • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • •
1.1 Non-specific defence mechanisms .••••••••••••••••••••••••.•. 1. 1 • 1 Natural killer cells.
1.1.2 Macrophages. 1.1.3 Interferon.
1.1.4 Tne complement system.
Page. 1
3
4
1 .2 Specific defence mechanisms • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 14 1 .2 • 1 Hurroral antibody responses.
1.2.2 Cell mediated inrnune responses.
1.3 Evaluation of the relative importance of specific mechanisms versus non-specific mechanisms in viral infections ••••••••• 19
2 • Macrophages in irrrnuni ty • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 2 4
2. 1 Macrophage origin and heterogeneity •••••••••••••••••••••••• 24 2.2 General characteristics of macrophages ••••••••••••••••••••• 26 2.3 Macrophages in natural irrrnunity ••••••••••••••••••••••••••••• 26 2 .4 t1a.crophage activation • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 2 7
2.4.1 Methods for the detection of activated rnacrophages. 2.4.2 Generation of activated macrophages.
2 .4.3 :Mechanisms of macrophage activation.
2.4.3a Activation against microorganisms. 2.4.3b Activation against abnorTIBl cells.
2.5 Secretion of soluble products by macrophage •••••••••••••••• 34 2.5.1 Products involved in host defence mechanisms.
2.7
M3.crophages as effector cells in host defence mechanisms ••••••••40
2.7.1
M3.crophages as effector cells against microbial infection.2.7.2
Cytocidal mechanisms against microorgansims.2.7.3
M3.crophages as effector cells against neoplastic cells.2.7.4
Cytotoxic mechanisms against neoplastic ce1ls.2.8 The importance of m1crophages during viral infection •••••••••••• 46
3. Acquisition of i.m:mmity against viral infection •••••••••••••••••• 50 3.
1
Non-specifically acquired inmunity against viral infections..51
3.2
Specifically acquired irrmunity against viral infections •••••51
3.2.1
Passively acquired irrrnunity.3.2.2
Actively acquired irrrnunity.4.
Attenuation of influenza virus • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 594.1
Criteria of an ideal live influenza virus vaccine ••••••••••• 60 4 .2 Host range mutants • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 614.3
Temperature sensitive (ts) mutants •••••••••••••••••••• ,... • • • • • 62 4. 4 Cold-adapted ( ca) mutants • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 644.4.1
The ca donor virus.4.4.2 Production of ca reassortants.
Preface
Although certain virus diseases such as measles are being controlled in certain parts of the world and smallpox has been eradicated from the world, influenza still remains one of the major infectious diseases in man. The work presented in this thesis on the one hand reports the role of various defence mechanisms and particularly the responses of macrophages to influenza virus infect-ion; and on the other hand, reports on the acquisition of immunity to influenza virus by using a live-attenuated cold-adapted influenza virus. Hence, the introductory chapter is a literature review which provides general information about the role of various immune components in response to a viral infection and the methods of acquisition of immunity to viral infection particularly to influenza virus infection.
The contents of this chapter are divided into 4 main sections. The first section discusses host defence mechanisms, including both non-specific (natural killer cells, interferon, and complement) and specific arms
( humeral antibody responses and T cell mediated
react-
rht.-ions) of ,.. immune system.
Part of the work presented in this thesis was to study the responses of macrophages to influenza virus infection, including mechanisms of macrophage activation. The second section summarizes different functions of macrophages in both the induction and effector phases of
immune responses with particular emphasis on the mode of
the effector phase in viral infection.
Since the final goal of investigating various
aspects of defence mechanisms is to seek an effective
means of immunizing a host so that viral infection can be
controlled. The third and fourth sections discuss the
means of acquisition of immunity to viral infection, and
the current status of work with live-attenuated
cold-adapted influenza virus which is suggested to be a
candidate for future vaccine.
In view of the rapid growth of knowledge in these
areas, some of the information may not be covered and
only those topics that are relevant to the work done in
3
1. General introduction of body defence mechanisms against
viral infection
In order for a virus to produce an infection, i t must
penetrate the physical barrier of the body surface. This
physical barrier may be intact
which protects the epithelial
skin, or the mucous membrane
digestive tracts. Microbial
cells of
and other
respiratory and
foreign particles
trapped within the adhesive mucus may be removed by coughing,
sneezing, and defecation. Mucus and other body secretory
fluids may exert antiviral activity (Wasserman,1968).
Acid-labile viruses such as influenza virus and rhinoviruses cannot
infect the small intestine as do other acid stable
enteroviruses or reoviruses (Fenner et al., 1974). Most
enveloped viruses are not pathogenic by the enteric route
because their lipoprotein surface membranes are susceptible to
hydrochloric acid and to enzymatic lysis. ,
The first step in the initiation of infection is the
attachment of virus particles to a cell surface. Many viruses
have a surface protein which allows attachment to specific
cell membrane receptors. The haemagglutinin of influenza
virus attaches specifically to N-acetylneuraminic acid
residues on the target cell membrane, and removal of these
receptors prevents viral attachment (Howe & Lee, 1972; Choppin & Tamm, 1964). Similarly, other enveloped viruses have
g lycoprote ins and phosphol ipids on their surface which form
projections and in many cases they are necessary for
the presence critical for
of a specific receptor at the the successful outcome of a
cell surface is viral infection. Once infection is established, two main defensive operations come into action. One is nonspecific and the other is specific defence mechanisms and they will be discussed in the following sections.
1.1 1 . 1 .1
Non- specific defence mechanisms Natural killer cells
Lymphoid cells from humans or experimental animals which have not previously been sensitized to a particular antigen may be cytotoxic in vitro against a variety of target cells including lymphoid and non-lymphoid tumors. This phenomenon is called natural cell-mediated cytotoxicity
( NCMC) . Two main subgroups of NCMC effector cells have been defined: Natural killer (NK) cells directed against lymphoma targets (Kiessling & Wigzell, 1979; Kumar et al., 1979) and natural cytotoxic (NC) cells directed against solid non-lymphoid tumors (Stutman et al.,1980).
The reported presence of low concentrations of 0-antigen has led Herberman and others to propose that NK cells may be prethymic T cells (Herberman et al., 1978). In general
5
The role of NK cells in the resistance of the host to
viral infections is still not completely understood. It has
been proposed that interferon(IFN) could limit viral infection
indirectly by activating NK cells which would kill
virus-infected cells and also protect other unvirus-infected cells from
nonspecific lysis (Trinchieri et al., 1981). NK cells with
enhanced cytotoxic activity have been recovered from animals
during infection with virus (reviewed by Welsh, 1981). In
vitro studies showed that a number of virus infected cells
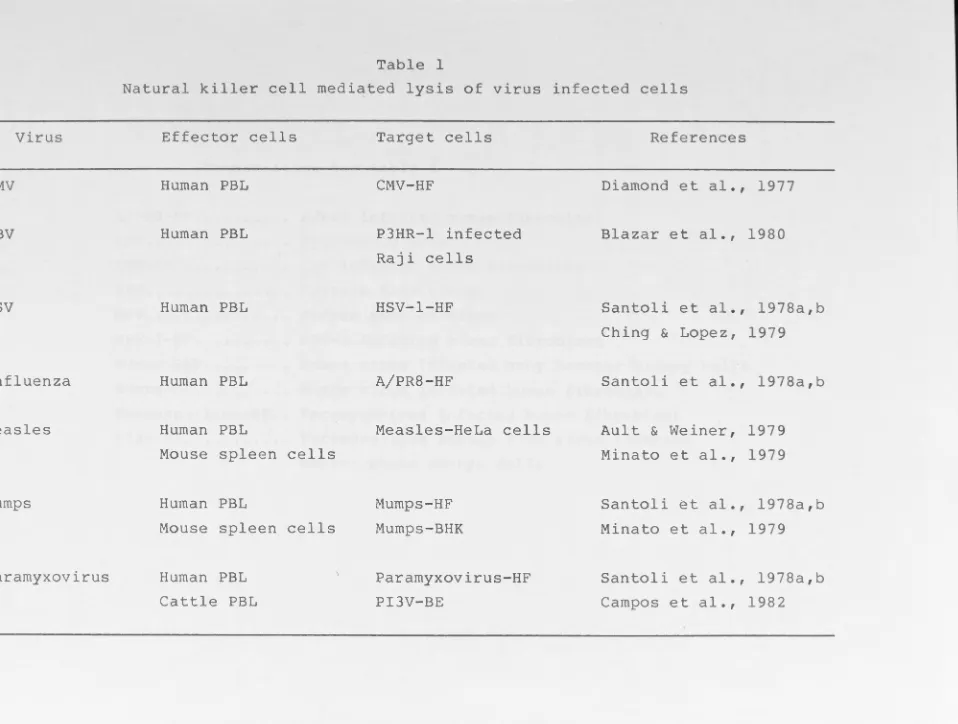
could be lysed by NK cells (Table 1), and IFN has been
implicated as the factor responsible for the augmentation of
the cytotoxic activitv of NK J. cells.
Recently, Fitzgerald et al (1982) suggested that
although IFN can augment NK cell-mediated cytotoxicity against
HSV-1-infected fibroblasts, the preferential in vitro lysis of
the virus-infected target cells cannot be attributed solely to
augmentation by the endogenously produced IF and may also be
virus dependent. In addition to the cytotoxic properties of
NK cells, NK cells may play an important role in virus
infection by secreting IFN, which can directly inhibit virus
replication (Welsh, 1981).
Recently, an in vivo function for NK cells in viral
infection has been proposed following experiments using MC"1V
and beige mutant mice (Shellam et al.,1981). In mice, a point
mutation, called beige (bg) on linkage group 14 in C57BL/6J
mice, leads to a marked impairment in NK cell activity (Roder
& Duwe, 1979). This impairment in NK cell function 1s
Virus
CMV
EBV
HSV
Influenza
Measles
Mumps
Paramyxovirus
Effector cells
Human PBL
Human PBL
Human PBL
Human PBL
Human PBL
Mouse spleen cells
Human PBL
Mouse spleen cells
Human PBL
Cattle PBL
Target cells
CMV-HF
P3HR-l infected
Raji cells
HSV-1-HF
A/PR8-HF
Measles-HeLa cells
Mumps-HF
Mumps-BHK
Paramyxovirus-HF
PI3V-BE
References
Diamond et al., 1977
Blazar et al., 1980
Santoli et al., 1978a,b
Ching & Lopez, 1979
Santoli et al., 1978a,b
Ault & Weiner, 1979
Minato et al., 1979
Santoli et al., 1978a,b
Minato et al ., 1979
Santoli et al., 1978a,b
[image:19.1133.167.1125.16.740.2]Abbreviation for table 1
A/PR8-HF . . • . . . • . A/PR8 infected human fibroblast CMV . . • . . • • . ..•.•.• Cytomegalovirus
CMV-HF . . . CMV infected human fibroblast EBV . . . • . . . • Epstein Barr virus
HSV . . . • . Herpes simplex virus
HSV-1-HF • . . . HSV-1 infected human fibroblast
Mumps-BHK . . . • . Mumps virus infected baby hamster kidney cells Mumps-HF • . . . • . • . . . Mumps virus infected human fibroblast
Paramyxovirus-HF .• Paramyxovirus infected human fibroblast PI3V-BE . . . • . . . . Paramyxovirus strain PI3V virus infected
marrow (Roder,1979). The beige defect seems to be selective for NK cells as spleen cell mediated antibody-dependent cell-mediated cytotoxicity (ADCC) against cRBC, promonocyte mediated ADCC against tumor cells and alloimmune or lectin-generated T cells function is relatively normal (Roder et al.,1979b).
Shellam et al (1981) have compared the susceptibility of homozygous bg/bg and heterozygous bg/+ C57BL/6J mice to
infection with MCMV. Higher NK cell activity was detected in
bg/+ mice than in bg/bg mice despite higher titres of type 1
IFN in the latter after infection with a sublethal dose of MCMV. bg/bg mice developed 30 to 40 fold higher virus titres
by day 3 in the liver, spleen, and kidney than bg/+ mice. Thus, the normal host IFN may be important during MCMV
infection because of its stimulatory effect on NK cells and
its antiviral activity on target cells.
Another approach (Biron & Welsh, 1982) has been used to evaluate the role of NK cells in controlling viral pathogene-sis. If the non-specific cytotoxic effect of NK cells is
important in controlling viral infection, lysis of virus
infected cells (which occurs in vitro) would occur in the
early stages of infection. These authors examined the
clearance rate of 125I-UdR labelled virus-infected L929 cells
. .
in mice and found that L929 cells infected with LCMV, HSV,
CMV, or Sindbis virus were cleared about twice as fast as ~
Although the detailhmechanisms have not ye t uninfected cells.
7
attempts to demonstrate the importance of NK cells during
viral infection have been less successful.
1 .1. 2 Macrophages
Metchnikoff, the great Russian zoologist working at the
Pasteur Institute in Paris in 1892, was the first person to
recognize that macrophages might be important in the defence
of the organism against a variety of extraneous materials.
Macrophages are widely distributed throughout the body being
in bone marrow, lymphoid organs, peritoneal cavity, lung,
liver and brain. These cells are able to remove microorganisms
().,,,.<d,
by phagocytosis, t-e initiate immune reactions by presenting
antigen to lymphocytes. Macrophage function will be discussed
in detail in section 2.
1.1.3 Interferon
Interferons (IFN) first discovered by Isaacs and
~
Lindenmann (1957) are a group of heterogenpus glycoproteins of
cellular origin. They are antiviral agents which are capable
of initiating a non-specific intracellular inhibition of virus
replication.
type II IFN.
IFN is conventionally classified as type I or
Type I IFN is induced following infection of
1 e uk o c y t e s ( INF-a ) or f i bro b 1 as t s ( I F N - B ) w i th v i r us whereas
type II IFN ( IFN-y ) is produced from antigen stimulated
lymphocytes or from mitogen activated lymphocytes. Over the
capable of stimulating IFN production both 1n vivo and 1n vitro, such as Brucella abortus (Youngner & Stinebring, 1964), Toxoplasma gondii (Rytel & Jones, 1966), E. coli ( Ho, 1964),
poly I
.
.
poly C (Field et al., 1967),
pyran copolymer(Merigan, 1967) and tilorone (Mayer & Krueger, 1970). Recent advances 1n protein purification techniques and monoclonal antibody technology has enable research workers to investigate
the biological effects of IFN (Knight, 1980) in more detail.
It is clear that IFN can modulate both immune and non-immune
responses in ways that are distinct from the traditional
antiviral activity of these substances.
Nonimmune effect of IFN
IFN containing preparations can inhibit normal or malignant cell proliferation (reviewed by Brouty-Boye,1980). The anti-cell growth property of IFN has been further
support-ed by using purifisupport-ed mouse and human IFN (reviewed by Knight,
1980). In addition, IFN derived from human leukocytes, human
fibroblasts, and mouse fibroblasts was found to inhibit the motility of bovine capillary endothelial cells and skin
fibroblasts (Brouty-Boye & Zetter,1980).
Effect of IFN on immune function
IFN is involved 1n the modulation of antibody
responses, cell-mediated immune responses, macrophage function, and expression of membrane components. Braun & Levy
(1972) showed that administration of large amounts of type I
9
suppression of plaque-forming cells (PFC) responses whereas
administration of lower doses of IFN resulted in an
enhancement of the PFC response to SRBC. Similarly, type II
IFN also showed a time-dependent and dose-dependent effect on
the antibody response to SRBC. However, type II IFN was more
potent in its immunosuppressive and immunoenhancing activity
than was type I IFN when the IFN were compared on the basis of
antiviral titre (reviewed by Sonnenfeld, 1980).
The effects of IFN on cell-mediated immunity have been
mostly studied using type I IFN. Type I IFN, when injected
into sensitized animals one day before or on the day of
challenge, inhibits the development of DTH to SRBC (DeMaeyer
et al. ,1975). Type I IFN also inhibits graft-versus-host
reactions (Hirsch et al. ,1974) and blastogenesis of rabit
lymph node cells in response to dinitrophenylated bovine
y-globulin (Thorbecke et al.,1974). Recently, it was found that
IFN can either suppress (Gresser et al.,1973) or enhance
cellular DNA synthesis. Matheson et al (1981) showed that
type I IFN inhibited blast transformation to T-cell dependent
mitogens PHA, ConA and PWM, but B-cell mitogenesis induced by
Staphylococcus Cowan I was enhanced by type I IFN, indicating
that IFN may be a regulator of immunocompetent cell activity
(Kadish et al.,1980).
IFN may enhance cytotoxic T cell function. Sethi and
Brandis (1978) demonstrated that specific murine T cell
cytotoxici ty for RSV-infected cells was enhanced if the T
cells were preincubated with type I IFN. Administration of
of HSV-1 prolonged the survival of RSV-infected mice and such
mice had a higher Tc cell activity. Thus, both in vitro and
in vivo studies suggested that IFN may be important in the development and activation of Tc cells.
The effect of IFN on other cell types such as NK cells, and macrophages has also been studied. Herberman et al(l979b) showed that type I IFN could enhance NK cell activity and enhance ADCC mediated by peripheral blood lymphocytes
(reviewed by Welsh, 1981).
Several types of macrophage activity have been shown to be affected by IFN. Treatment of mice with IFN inducers such as Newcastle disease virus resulted in increased phagocytosis of IgG-coated sheep red blood cells by macrophages {Hamburg et
al., 1978). Schultz et al (1978) showed that purified IFN could accelerate the spreading of macrophages on plastic. Macrophages recovered from virus-infected animals manifested enhanced virostatic or virucidal effects in vitro (Morahan et
al., 1977; Schultz et al., 1974). A direct role for IFN in the modulation of macrophage antiviral activity was suggested by the observation that type II IFN was able to protect mouse peritoneal macrophages against the cytopathic effects of
influenza and mouse hepatitis viruses (Virelizer et al.,1977). Furthermore, alveolar macrophages from influenza virus infected mice do not support the replication of influenza virus. Exposure of normal alveolar macrophages to type I IFN
or bronchoalveolar washings from influenza virus infected mice
11
infection. The protective activity of bronchoalveolar washings
was abolished by anti-IFN serum (Rodgers & Mims,1982).
Stebbing et al (1978) suggested that the development of
resistance to viral infection may be attributed to the
activated macrophages with enhanced virus trapping ability.
Recently, Stanwick et al (1980, 1982) showed that IFN-a could
enhance the cytotoxic effect of adherent monocytes-macrophages
from periheral blood leukocytes to HSV infected human
fibroblasts but not to uninfected cells. r u.,,,,,,:l
'-1
Ghalm&r et al(l982)
suggested that early production of type I IFN is a significant
factor in the resistance against mouse cytornegalovirus in some
strains of mice. Furthfrmore, the role of IFN in macrophage
activation was supported by the observation that many agents
which induce IFN production and release in vivo also induce
the production of activated macrophages (Schultz, 1980).
Modulation of cell surface antigen expression
Both in vitro and in vivo studies showed that IFN are
able to modulate the expression of cell surface antigen
expression . Lindahl et al (1973) showed that IFN enhanced the
expression of cell surface antigen on murine Leukemia Ll210
cells. Further studies demonstrated that type I IFN ( from
mouse C243 cells) was able to enhance the expression of H-2
antige~s on Ll210 cells (Gresser et al .,1979). Both in vitro
and in vivo studies showed that IFN-S decreased the number of
cells expressing Lyt-1 antigen, whereas the number of cells
expressing Lyt-2 antigens was increased (Kumagai et al.,1982).
correlated with the observation that IFN enhanced Tc cell development but suppressed DTH reactions, as effector Tc cells were Lyt 2+ and the majority of effector T cells responsible for DTH reactions was Lyt 1+ (Kumagai et al.,1982). Wong et al (1982) have since shown that IFN-y induced the expression of Ia antigen on cultured progenitor mast cells. In summary,
IFN are important antiviral and immunoregulatory substances.
1.1.4 The complement system
The complement system is composed of a series of serum proteins that are important in a variety of specific and non-specific immune defence mechanisms. The complement system can be activated via two mechanisms: the classical and the altern-ative pathways (Fothergill & Anderson, 1978). Both systems are important as a host defence mechanism against viral infection. The complement system may interact with free virus in the presence or absence of specific antibody, or interact
with viral antigens expressed on the surface of virus infected
cells.
A. Complement-antibody-virus interaction
13
Complement may interact with antibody to enhance
antibody mediated neutralization under circumstance where
virolysis does not occur, for example , in herpesvirus (Snyder
et al., 1981), vaccinia virus and vesicular stoma ti tis virus
(Leddy et al., 1977). The complement components of Cl-C3 of
the classical pathway are responsible for the enhanced
neutralization o f these viruses by antibody.
B. Complement and virus interaction
The complement system may be activated by virus in
the absence of specific antibody. In vitro studies have
shown that antibody-independent activation of the classical
pathway may result in lysis of type C virus particles (Cooper
et al., 1976; Barthalomew et al., 1978), or neutralization of
VSV (Mills & Cooper, 1978) and Sindbis virus (Hirsch et al.,
1980b).
C. Complement-antibody-virus infected cell interaction
Anti-viral antibody coated virus-infected cells can
also be lysed by complement before release of viral particles
(reviewed by Oldstone et al., 1980). Rouse (1977) showed that
neutrophil mediated ADCC could be enhanced in the presence of
a low concentration of complement, indicating that complement
could cooperate with such cytotoxic effector cells in
eliminating antibody coated virus-infected cells.
In vitro studies showed that mice depleted of
complement by treatment with Cobra venom factor were more
susceptible to severe infection by Sinbis virus (Hirsch et
al. , 1978), influenza virus (Hicks et al., 1978) or rabies
play a protective role by limiting viremia, . . its action also
contributes to the immuno-pathological pattern: e.g. LCMV
(Oldstone & Dixon, 1971) and Sindbis virus infection (Hirsch et al.,1978). This may be particularly important if it occurs
in vital organs. In summary, complement may be involved at
several stages during infection of a host by viruses.
1 . 2 Specific mechanisms
1 . 2. 1 Humoral antibody response
Most if not all viral infections induce a humoral
anti-body response. Studies with many viruses show a similar
pattern of antibody response. Infection of rabbits (Svehag &
Mandel , 1964) or humans (Ogra et al., 1968) with poliovirus
results in production of neutralizing IgM antibody which
reaches maximal titres in 3 to 4 weeks but is not detected at
3 months. IgG titres rise in parallel with IgM but may
increase for several months and persist at low levels for
years. Secretory IgA responses occur at local sites of
infection of the mucosal surface.
Effect of antibody on free virus and on virus-infected
cells
Serum antibody, produced in response to a viral
infect-ion, may be of great importance in preventing the spread of
Danie 1 s , 197 5 ) . In some cases,
body is enhanced by complement.
virions, and complement alone
15
virus neutralization by
anti-Complement can lyse enveloped
may lyse or neutralize some
viruses in the absence of antibody (see section 1.1.4).
In the absence of an effector system, such as K cells
and complement, antibody binding to the surface of
virus-infected cells can affect virus production and release.
Hence, the release of influenza virus was inhibited by
antibody to haemagglutinin and, less effectively, by antibody
to neuramin idase ( Dowdle et al., 19 7 4) . Joseph and Old stone
(1975) found that culture of measles virus-infected HeLa cells
in the presence of anti-measles antibody rendered the cells
resistant to lysis by complement. They called this
"antibody-induced viral antigenic modulation". Furthermore, modulated
measles vir s-infected cells were also resistant to
antibody-dependent cell-mediated cytotoxicity (Oldstone & Tishon,1978).
Shedding of viral antigen-antibody complex was a maJor
mechanism of modulation.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
A variety of target cells (e.g. erythrocytes, tumor
cells, or virus-infected cells) when sensitized with specific
antibody can be lysed in vitro by certain effector cells.
This phenomenon is called ADCC. ADCC has been described in
certain viral system~} such as varicella-zoster virus (Kamiya
et al., 1982), HSV (Kohl et al., 1979), influenza virus
1977), measles virus (Kreth & Meulen, 1977) and respiratory
syncytial virus ( Kaul et al., 1982). Two types of effector
cells have been defined. The first type is phagocyt ic and
adheres readily to surfaces, and with many properties similar
to monocyte-rnacrophages. The second one is a non-adherent
lymphocyte-like cell. The effector cells which mediate ADCC
are Fe receptor-bearing cells. Usually, the amount of cell
bound specific antiviral antibody required for ADCC is at
least 10- to 100-fold less than that required for
antibody-dependent com pl emen t mediated cytotoxic i ty (reviewed by
Sissons & Oldstone, 1980)
In summary, antibody may play an important role in vivo
( further discussion in section 1. 3). The spread of v 1rus
particles may be limited by the mechanisms mentioned above.
However , the phenomena of antibody-induced viral antigenic modulation may be important in the establishment and maintance
of persistant infection 1n certain viral infection (reviewed
by Sissons & Oldstone, 1980).
1 . 2 . 2 Cell mediated immune responses
Cell mediated immune responses (CMI)
specific and non-specific
usually regarded as the
cellular components.
predominant effector
involve both
T cells
cells of
are
CMI
responses. However, other cell types such as macrophages ,
NK cells and K cells may also play a role. Memory, specificity
funct-0 ~ soecici T cell mediated 1m uni~
10. S - ,_ ... The ec1 an1s s b
,,;_ ic'
2:~r
responses co tro i~al 1 feet ions are ( 1) direct;cilli a _J o:: 1~ s-infected cells, ( 2) release of ly. _ '.:10' 1 es
recr i t monocytes into the sites of infection and
a i ~a~e monocyte- 1 acrophages, and ( 3) p~oduction of ot er
:: actors s ..ic as 1,1 une interferon .
Ge:1eratio o:: v1r s specific c totoxic T cells (Tc) las
been de, o st~ated 1n a large number of viral infections
Zi Ker agel ad Do.erty , 1979; & refer to Table 2
)
.
Spec.fie Tc ce_ls lyse i~ s infected histocompatible (K , Dreg:.on o:: e H- 2 gene complex) target cells but not
uninfect-ed ce ls .
not er mec' an1s" by w .ich v1~us infected cells could
ne l.L sea as D,__,C (see section 1 . 2 .1). Recently, 1n vitro
st dies s~ o<,.,ed that monocyte- . acrophages could kill certain
.,,. S 1 cecte cells bv a non- DCC. ecna ism (refer to Table
6) . Letvin et al (1982) sho ed t at mac~op'.ages from unor1 ed
ice e~e canable of lysing reovirus type- 1 fected cells 1n
t e absence o:: specific antibody , a d this stri 1ng target
ce:::.l specificity 1as a p~operty of the a- 1 protein ( t. e vira_
.. ae. - agg ti i ) . I . some case s , I F _
.e . ed · a tors ·' ich en 1
a need macrophage
as fond to be one of
ediated cytotoxicity
aga1ns 11rus infected cells (Stanwic~ et al.,1982). Ho,ever ,
.. e exact ec1
a is, lS n . o vn .
' .. o : <2r
,..,::r
mec' anis=n for the control of viral infect-0. S lS he orod c tio o:: 11
ly, oho.,<ines11
hich are soluble
,.e iacors pro ced by sensitized
o:1s ( · mo:1 e et al ., 1969) .
ce ls during immune
Virus
Banzi virus Bebaru virus Ectromelia
HSV
Influenza LCMV
MCMV
VEEV
Group
Togavirus(Flavivirus) Togavirus(Alphavirus) Poxvirus
Herpesvirus
References
Jacoby-et al., 1980·
Hapel, 1975 Blanden, 1971 Cl,
Rager-Zisman & Allison 1976
Orthomyxovirus Yap & Ada, 1978b
Arenavirus Johnson & Cole, 1975 Herpesvirus Starr & Allison, 1977 Togavirus(Alphavirus) Rabinowitz & Adler,
1973
HSV: Herpes simplex virus.
LCMV: Lymphocytic choriomeningitis virus. MCMV: Murine cytomegalovirus.
18
been studied extensively , such as macrophage activation factor
(MAF), macrophage inhibitory factor (MIF), lymphotoxin (LT),
immune interferon (IFN-y), chemotactic factors (Cohen et al.,
1979), interleukin-2 (IL-2) and interleukin-3 (IL-3).
IL-3 which can be induced by viral antigen activated
lymphocytes (Ihle et al., 1981a) may promote an early step in
the differentiation of the T cell lineage (Ihle et al.,198lb).
IL-2 may activate T cell growth (Watson and Mochizuki, 1980).
Lymphotoxin, which is secreted by activated lymphocytes
( Ev ans , l 9 8 2 ) , i s a d ire c t acting c y tot ox i c lymph o k in e w i th
cytostatic and/or cytolytic activities depending on the kind
of target cells being affected (Evans & Heinbaugh, 1981;
Rosenau,1981). However, the role of LT in viral infections is
still unknown.
Chemotactic factors may function by recruiting
monocyte-macrophages to the site of infection. These cells could be
retained within infected tissue by MIF and be activated by MAF
and/or IFN. It has been demonstrated in various systems that
infection of a variety of cells by viruses such as mumps, NDV
(Ward et al., 1972; Flanagan et al., 1973) and simian virus
(SV40) in vitro (Yoshida et al.,1975), or infection of the
animal with mumps virus (Yoshida et al., 1974) can lead to the
production of MIF. In vitro studies showed that macrophages
could be activated by MAF to become cytotoxic for tumor cells
or cells with abnormal growing properties, or could be
activated by interferon to kill virus infected cells (Stanwick
et al., 1982). In the case of reov1rus virus infection,
reov1rus infection (Letvin et al., 1981). These investigators
further showed that macrophages from these athymic mice have
higher levels of cytolytic activity against reov1rus type-1
infected cells than do macrophages from nu/+ and their normal
littermates.
Blanden (1974) showed that T cell m~diated recruitment
of blood rnonocytes to the foci of infection is clearly
import-ant 1n the recovery of mice from ectromelia infection.
Although the role of in vivo activated cytotoxic macrophages
in viral infection is s t i l l unknown, in vitro studies
suggest-ed that macrophages could be cytotoxic for a number of virus
infected cells but not uninfected cells via non-ADCC mechanism
(see Table 6).
1.3 Evaluation of the relative importance of specific
mechanisms versus non-specific mechanisms in viral
infections.
The host defence involves the complex interaction of
both specific and non-specific components of cellular and
humoral factors. The relative importance depends on the model
virus used for studies and the modes of replication and spread
of virus. Generally speaking, antibody is effective in
controlling systemic viral infections when the virus spreads
by extracellular routes during which viraem1a occurs. . . CMI
seems to play an important role 1n the recovery f rorn those
viral infection in which virus spreads from cell to cell and
[image:35.745.15.726.63.1109.2]20
modifications of the surface of infected cells (Burns & Allison, 1977 Blanden et al., 1977).
Direct evidence showing that specific mechanisms (Tor B cells) may play an important role in recovery from viral
infection has been derived from studies using adoptive transf-er of immune T cells or passive transfer of immune sera or monoclonal antibody. The protective effect of T cells has
been illustrated with LCMV, Venezuelan equine encephalo-myelitis virus, influenza virus, HSV and ectromelia virus
(Table 2). On the other hand, passively transferred virus-specific antibodies also confer protection against certain viral infections, such as yellow fever virus (Zisman et al.,
1971), influenza virus (Virelizier, 1975; Yap & Ada, 1979),
and MCMV (Araullo-Cruz et al., 1978). This evidence suggested that both humeral antibody and specific CMI responses are important.
However, a viral infection elicits both specific immune responses and non-specific anti-viral reactions. These included the elevation of body temperature, the synthesis of type I IFN from virus infected cells or type II IFN from sensitized lymphocytes, and the activation of macrophages and NK cells.
Although there 1s plenty of evidence showing that specific immune responses may play a decisive role in recovery
tke_
from viral infection, other non-specific components of ~i mmune system may also play a role in controlling viral infection. Mice, depleted of complement components, developed severe
Sindbis virus (Hirsch et al., 1978) or influenza virus (Hicks et al., 1978). Hicks et al ( 1978) further demonstrated that the time of appearance and level of serum neutralizing antibodies and virus specific cytotoxic T cells were equivalent in both decomplemented and non-decomplemented influenza virus infected mice. Thus complement may play a potentiating role in the final elimination of infectious virus, perhaps by chemotaxis of mononuclear cells to the site of viral infection, neutralization of virus particles, or by complement-mediated lysis of infected cells coated with antiviral antibody (Verbonitz et al., 1978).
Although the protective effect of T cells against HSV infection had been reported (Rager-Zisman & Allison, 1976), adoptive transfer of macrophages from 8 weeki\ old mice to 3 weeks old mice also conferred on the latter a defence capacity against HSV-2 hepatitis almost as .effective as that seen in adult mice (Mogensen, 1978). Stohlman et al (1980) showed that young susceptible mice can be protected from encephalitis induced by intracranial inoculation with the JHM strain of mouse hepatitis virus after adoptive transfer of T- cell depleted adherent spleen cells from adult resistant animals.
22
viruses to high titre 1.n visceral organs, and early death.
Similar results were obtained 1.n m1.ce infected with mouse
hepatitis v1.rus (MHV-3) (Virelizier & Gresser, 1978). In
influenza v1.rus infection, the inborn resistance of A2G m1.ce
to influenza v1.rus infection is due to high sensitivity of
their cells, including macrophages, to IFN action (Haller,
1981). The above work suggested that IFN is important in the
restriction of viral infection .
The concept that NK cells may contribute to resistance
to viral infection was initially proposed by Welsh ( 197 8) •
Al though cytotoxic T cells protected m1.ce from lethal MCMV
infection , substantial evidence suggested that NK cells may
also play a role in the host defense mechanism against MCMV
infection ( Quinnan & Manischewi tz, 1979; Bancroft et al.,
1981 ; Chalmer et al. , 1982, Shellam et al.,1981).
Apart from the non-specific cytotoxic activity , NK
cells may mediate ADCC against specific target cells. Many
investigators have suggested that NK cells and K-cells
(mediators of ADCC) may represent identical, overlapping, or
similar populations of effector cells (Ojo & Wigzell, 1978;
Herberman et al.,1979a,b; Timonen, 1979). On the other hand,
NK cells, on exposure to v 1.rus or tumor cells, may produce
high levels of IFN (Trinchier et al., 1978). Welsh ( 1981)
suggested that these cells may also play a role in resistance
to v1.rus infections by a mechanism other than cytotoxicity.
In summary, although conflicting results have been
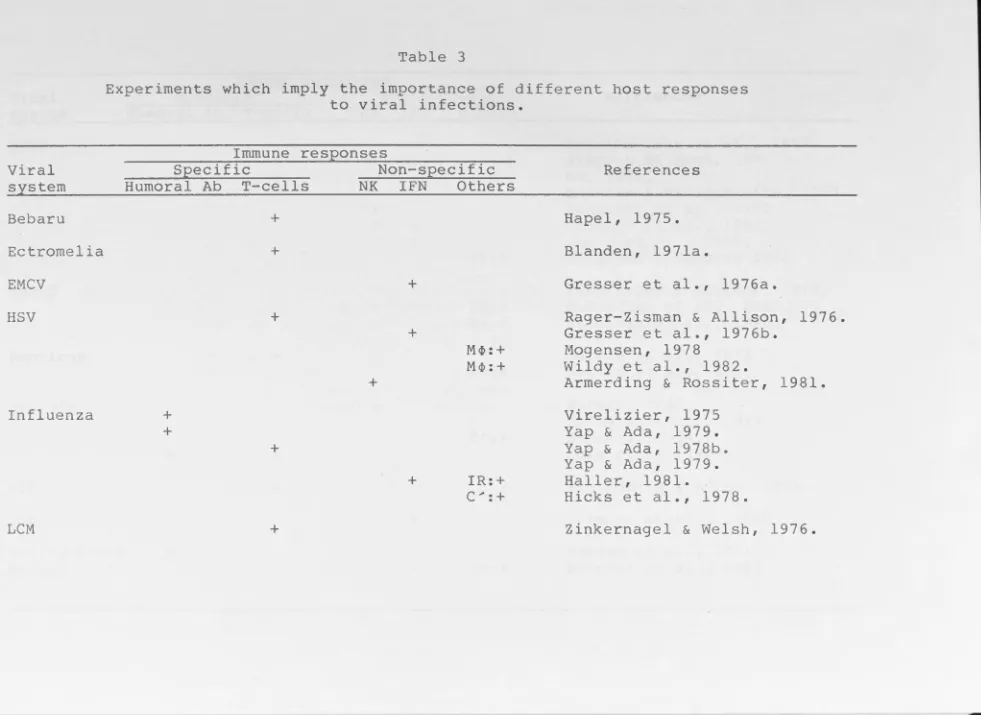
viral infections (Table 3), both specific and non-specific
components of the host immune system are involved in
controlling viral infect.ion. Skin and mucosal surfaces may
provide a physical barrier to prevent certain microorganisms
from invading the host. Non-specific immune responses may
represent in particular the early defence mechanisms which would contribute to a flexible and broad sprectrum of
protective mechanisms against infection. The central features of specific T and B cells immunities are specificity and memory, which provide specific protection against subsequent
Table 3
Experiments which imply the importance of different host responses to viral infections.
Immune res_eonses
Viral Specific Non-specific
system Humoral Ab T-cells NK IFN Others
Bebaru +
Ectromelia +
EMCV +
HSV
Influenza
LCM
+ +
+
+
+
+
+
+
M~:+
M~: +
IR:+ C ... : +
References
Hapel, 1975. Blanden, 1971a.
Gresser et al., 1976a.
Rager-Zisman & Allison, 1976. Gresser et al., 1976b.
Mogensen, 1978
Wildy et al., 1982.
Armerding & Rossiter, 1981. Virelizier, 1975
Yap & Ada, 1979. Yap & Ada, 1978b. Yap & Ada, 1979. Haller, 1981.
Hicks et al., 1978.
[image:40.1133.144.1125.15.730.2]MCMV MHV-3 Reovirus Sindbis VEE
vsv
Yellow fever virus + + + + +
-+ + + + -+ + + + -IR:+ IR:+M.4>: +
M <l> : +
C ... : +
IR:+
Araullo-Cruz et al., 1978.
Starr & Allison, 1977
Ho, 1980.
Quinnan & Manischewitz, 1979.
Bancroft et al., 1981.
Chalmer et al., 1982.
Chong et al., 1983.
Selgrade & Osborn, 1974.
Virelizier & Gresser, _1978.
Schindler et al., 1982.
Stahlman et al., 1980.
Le tv in et al . , 1981
Le tv in et al., 1982
Hirsch, 1981.
Reinarz et al., 1971.
Hirsch, 1980a.
Cole et al.,1982.
Rabinowitz & Adler, 1973.
Gresser et al., 1976b.
Zisman et al., 1971.
Abbreviations for table 3
+ •••••••• • • • • • • • •
Ab . . . • . •
C.,,,. • •• • •• • •
EMCV . . . . HSV . . . •
I FN ••••••• IR ••••••••
LCM . . • . • . . MCMV ..••.• MHV-3 •..•. M <P •• • • • • • •
N K ••••••••
VEE .•..•• •
vsv . ... .
Relatively important. Relatively unimportant.
Antibody.
Complement system.
Encephalomyocarditis virus. Herpes simplex virus.
Interferon.
Inborn resistance ( genetically determined). Lymophcytic choriomeninglitis virus.
Mouse cytomegalovirus. Mouse hepatitis virus. Macrophages .
Natural killer cells.
2 • Macrophages 1n immunity
The role of macrophages in the immune response 1s both
diverse and critical. Recent findings have demonstrated the
wide range of functions for this cell type. They are
involved in both the inductive phase and the effector arms
of the immune response to infection. The properties include
the production of lymphocyte activating factor(LAF or IL-1)
and presentation of antigens to appropriate lymphocytes,
removal of invading microorganisms by phagocytosis,
regulat-ion of other cellular functregulat-ions by productregulat-ion of different
secretory products, or to be activated to become cytotoxic
effector cells.
2 .1 Macrophage origin and heterogeneity
Macrophages are derived from bone marrow committed stern
cells. The most immature cell of the mononuclear phagocyte
system identified in vivo is the prornonocyte (van Furth et
al.,1970), and the most primitive cell identified 1n
colon-1es grown in vitro lS
.
the rnonoblas t (Goud et al., 1975).Circulating rnonocytes, which are derived from prornonocytes,
differentiate into tissue macrophages in different organs.
This pathway 1s followed both in the normal steady state
(van Furth & Chon, 1968; van Furth & Diesselhoff-den Dulk,
1970), and 1n acute and chronic inflammations (Volkman,
1966; van Furth & Chon, 1968; van Furth et al., 1973).
Generally, fully differentiated macrophages in tissue do not
25
local proliferation does occur in the tissues (North, 1969).
The differentiation pathway of macrophages is shown in
figure 1 .
Functionally distinct subpopulations of macrophages at
various stages of differentiation can be separa ted by
fractionation of murine peritoneal cells according to their
cell size (Lee, 1980), because macrophage maturation is accompanied by cell enlargement (Gordon
&
Chon, 1973).Thus, Farr am and Nelson (1980) showed that activated
macrophages of medium to high density (1 .08/1 .07) were more
effective in tumor cell killing than lighter cells
(1.07/1 . 06) . Similary, Chap es and Tompkins ( 1 981) showed
that high density peritoneal macrophages from vaccinia
virus infected hamsters were cytotoxic for HSV infected BHK
cells , whereas low density macrophages from the same source
when added to bone marrow culture caused the appearance of
NK cell activity in the culture, i.e the macrophages had a
"helper" or "stimulatory" efffect.
Lee (1980) showed that small Ia+ macrophages were
required to induce antigen specific T cell proliferation,
whereas C.parvum induced macrophages which were cytostatic
against tumor cells , were relatively large cells. The
spleen may play an important role in controlling the
differentiation of peritoneal macrophages. Thus, Yacov et
al (1981) showed that macrophages from splenectomized
animals had an impaired ability to present antigen and to
N E M A R R 0
w
B L 0 0 D T I ss
u
E Mitotic compartment ( ±1 day)Post-mi to tic { compartment
( (1 day)
Transit
compartment ( ±1 day)
Functional
compartment (1-5 weeks)
{
Monoblast Promonocyte MonocyteI
Monocytel
Exudate M~(small M<P)
i
Exudate-resident M<P
l
Resident M<P
(Large M<P)
• • • • • • • • • • • • • • • • • • • Promonocyte
(1
MacrophageFig.l Schematic representation of the origin and kinetics
of mononuclear phagocytes. The dotted line
indicat-ed that some of immature mononuclear phagocytes divided 1n the tissue. (Modified from van Furth et
26
2.2 General characteristics of macrophages
Mononuclear phagocytes can be characterized (Cooper~ al., 1982) as: 1) avidly phagocytic for particles greater than O.lµm and pinocytotic for particles less than O.lµm; 2)
-capable of firm attachment to glass; 3) possessing surface
membrane receptors for the CH 3 domain of immunoglobul ins (IgG 1 and IgG3 ) and for complement which play an important role in the attachment phase of phagocytosis; and 4) inability to synthesise immunoglobulin.
2.3
1892 .
Macrophage 1n natural immunity
The term "macrophage" was introduced by Metchnikoff in He found that these cells which resided in various tissue were important in resistance to bacterial infection. Some of the evidence showing a role for macrophages in natural immunity is:
1 . In bacterial infections, resistant mouse strains were found to have a 2- to 3-fold greater accumulation of peritoneal macrophages after infection with Listeria. The macrophages from these mouse strains were more responsive to complement-derived chemotactic factors ( Stevenson et al., 1981 ) •
1n vitro ability of the virus to grow 1n macrophages and the
pathogenicity of the disease.
3. The in vitro cytotoxic activity of macrophages 1n
athymic m1-ee is correlated with the 1n vivo resistance of
these mice to reov1rus infection (Letvin et al., 1981;1982).
4. Blanden(l97lb) showed that regression of ectromelia
virus infection in mice was associated with the invasion of
virus-infected foci by mononuclear phagocytes.
5 • The nonespecific cytotoxicity of peritoneal
~
macrophages from athymic nude mice to tumqr cells observed
in vitro (Meltzer, 1976) was correlated with the observed
I.{,
low incidence of spontaneous tumo~s in these athymic animals
(Custer et al.,1973; Rygaard & Povlsen,1974).
This evidence suggests that macrophages may be
important 1n the control of tumor and infectious disease
development. The role of macrophages 1n viral infection
will be discussed 1n section 2.8.
2. 4 Macrophage activation
Resting macrophages when exposed to a variety of
stimulatory agents undergo a number of changes. These
include the enhancement or expression of certain functional
activities which are poorly or not expressed by macrophages
in their resting state. Such changes include a change in
enzyme contents, in secretion products, phagocytotosis, and
28
of these changes occur, the macrophages are often said to
be activated. In addition, macrophage activation can be
regarded as a differentiation process in which cells of the
monocyte-macrophage system are induced to differentiate and
thus acquire new functional and biochemical characteristics.
In the following sections, the methods of detection and
the mechanisms of activation of macrophages will be
discussed with particular emphasis on the cytotoxic .effect
towards other cells .
2. 4 .1 Methods for the detection of activated macrophages
Activated macrophages can be recovered from animals
inoculated with a variety of agents (microorganisms, tumor
cells or chemical agents) or obtained by exposure of normal
macrophages to certain activating agents (e.g. lymphokines)
in vitro. The methods used to measure the state of
activation are summarized in table 4.
2 . 4 . 2 Generation of activated macrophages
Activated macrophages can be recovered from animals
infected with Listeria or Brucella (Hibbs et al. ,1972b),
Toxoplasma (Remington et al., 1972), or a virus such as
vaccinia virus (Chapes & Tompkins, 1979; Morahan et al.,
1980) . Macrophages activated by alloantigens or tumor
cells may be cytotoxic for the immunizing cells (Evans &
Alexander, 1972; Gallily, 1975; Schultz et al., 1976).