Contribute to Superinfection with Gram-Positive Respiratory
Pathogens
Jenni N. Weeks-Gorospe,aHeather R. Hurtig,bAmy R. Iverson,aMargaret J. Schuneman,bRichard J. Webby,aJonathan A. McCullers,a and Victor C. Huberb
Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA,aand Division of Basic Biomedical Sciences, University of South Dakota, Vermillion, South Dakota, USAb
A combination of viral, bacterial, and host factors contributes to the severity and overall mortality associated with influenza vi-rus-bacterium superinfections. To date, the virulence associated with the recently identified influenza virus protein PB1-F2 has been largely defined using models of primary influenza virus infection, with only limited assessment in models ofStreptococcus
pneumoniaesuperinfection. Specifically, these studies have incorporated isogenic viruses that differ in the PB1-F2 expressed, but
there is still knowledge to be gained from evaluation of natural variants derived from a nonhuman host species (swine). Using this rationale, we developed the hypothesis that naturally occurring viruses expressing variants of genes, like the PB1-F2 gene, can be associated with the severity of secondary bacterial infections. To test this hypothesis, we selected viruses expressing vari-ants in PB1-F2 and evaluated outcomes from superinfection with three distinct Gram-positive respiratory pathogens:
Strepto-coccus pneumoniae,Staphylococcus aureus, andStreptococcus pyogenes.Our results demonstrate that the amino acid residues
62L, 66S, 75R, 79R, and 82L, previously proposed as molecular signatures of PB1-F2 virulence for influenza viruses in the setting of bacterial superinfection, are broadly associated with enhanced pathogenicity in swine in a bacterium-specific manner. Fur-thermore, truncated PB1-F2 proteins can preferentially increase mortality when associated withStreptococcus pyogenes superin-fection. These findings support efforts to increase influenza virus surveillance to consider viral genotypes that could be used to predict increased severity of superinfections with specific Gram-positive respiratory pathogens.
I
nfluenza A viruses typically express 12 proteins (58), including the recently identified virulence factor PB1-F2 (24). The PB1-F2 protein contributes to viral polymerase activity (13,31,34,58), induces apoptosis by interacting with host cell mitochondria (24,25,40,61), and affects host immune responses directed toward the virus (29,54). Full-length PB1-F2 is frequently maintained at 87 to 90 amino acids (24,33), with specific amino acid residues that are associated with virulence (1), including a critical one at posi-tion 66 (14). Interestingly, the pandemic influenza virus isolates from 1918, 1957, and 1968 expressed full-length, virulent forms of PB1-F2 (32), but changes in this protein have been correlated with the overall reduction in virulence observed as sustained transmis-sion within humans was established (33). In addition to point mutations within PB1-F2, virulence can be reduced when trun-cated forms of this protein are expressed, including natural vari-ants that are 11, 25, 34, 57, and 78 amino acids in length (13,33). A significant proportion of deaths during influenza epidemics and pandemics are associated with secondary bacterial complica-tions (37). To date, the impact of PB1-F2 on virulence has largely been described within models of primary influenza virus infection (62), with little consideration for the contributions of PB1-F2 to-ward deadly secondary bacterial complications (33). Since a vari-ety of PB1-F2 genes exist in both avian and swine populations (13,
15), natural reassortment events have the potential to yield viruses with a virulent PB1-F2 expressed on a background suitable for sustained human transmission. At this time, prioritizing the po-tential risk from such reassortment events will allow us to identify specific viral proteins that are associated with increases in primary influenza virus illness (30,43) and, more appropriately, progres-sion to deadly secondary bacterial infections (35).
Historically, three Gram-positive pathogens have contributed to community-acquired secondary bacterial pneumonia: Strepto-coccus pneumoniae,Staphylococcus aureus, andStreptococcus pyo-genes(12,27,36). Reports detailing the prevalence of secondary bacterial invaders during pandemic and epidemic influenza virus infections demonstrate variation in the dominant bacterial species associated with secondary pneumonia (37), and this does not ap-pear to be directed by either the hemagglutinin (HA) or the neur-aminidase (NA) proteins expressed at the viral surface. To reca-pitulate virus-bacterium superinfections in humans, we have developed virus-bacterium superinfection models for all three of these Gram-positive pathogens (12, 27, 39), and we use these models to evaluate outcomes after these lethal, synergistic inter-actions.
We developed the hypothesis that virally expressed proteins, like PB1-F2, dictate the severity of secondary bacterial infections, which can vary based on the bacterial species to which the host is exposed. To test this hypothesis, we selected naturally occurring viruses expressing PB1-F2 genes of swine origin based on their predicted virulence. We then tested these variants within three distinct models of influenza virus-bacterium superinfection to de-termine the extent that knowledge of the PB1-F2 variant expressed
Received13 February 2012 Accepted30 May 2012
Published ahead of print6 June 2012
Address correspondence to Victor C. Huber, victor.huber@usd.edu.
Copyright © 2012, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.00369-12
on November 7, 2019 by guest
http://jvi.asm.org/
can be used to predict the severity of secondary bacterial infection. Our data demonstrate that different virus-bacterium combina-tions yield different superinfection outcomes but that in general the predicted virulence of the PB1-F2 protein expressed correlates with increases in both lung bacterial titers and overall mortality. These findings are discussed in the context of expanding influenza virus surveillance to include sequencing of internal genes, like PB1-F2, in an attempt to identify genetic signatures associated with deadly secondary bacterial infections.
MATERIALS AND METHODS
Mice.Six- to eight-week-old female BALB/c mice (Jackson Laboratory, Bar Harbor, ME, or Harlan Laboratories, Indianapolis, IN) were main-tained in a biosafety level 2 facility in the Animal Resource Center at either St. Jude Children’s Research Hospital (SJCRH) or the University of South Dakota (USD). All experimental procedures were approved by the Animal Care and Use Committee at SJCRH or USD and were done under general anesthesia with inhaled (2.5%) isoflurane (Baxter Healthcare Corpora-tion, Deerfield, IL).
Viral and bacterial strains.The St. Jude strain of mouse-adapted in-fluenza virus A/Puerto Rico/8/34 (H1N1; PR8) was generated by reverse genetics and grown in Madin-Darby canine kidney (MDCK) cells fol-lowed by 2 passages through eggs for stock. The resulting allantoic fluid was stored at⫺80°C. The hemagglutinin, neuraminidase, and PB1 genes were sequenced to ensure no inadvertent mutations were present, and the viral stock was further characterized in MDCK cells to determine the infectious dose for 50% of tissue culture wells (TCID50). Swine virus iso-lates were grown in 10- to 11-day-old embryonated chicken eggs for 3 days at 35°C. The resulting allantoic fluid for each virus was stored at⫺80°C. Further characterization for each virus included sequencing of the PB1 gene to determine the expression of the PB1-F2 protein as well as TCID50 and 50% lethal dose (LD50) in MDCK cells and BALB/c mice, respectively (Table 1). Separation of these primary swine virus isolates into three dis-tinct virulence groups was based on both published (1,33,62) and un-published (I. V. Alymova, St. Jude Children’s Research Hospital, Mem-phis, TN, personal communication) findings with influenza viruses expressing H1N1 or H3N2 surface proteins. These previous studies were designed to specifically define PB1-F2 virulence and included models of both primary influenza virus infection and influenza virus-bacterium su-perinfection using isogenic viruses that differed only in PB1-F2.
Streptococcus pneumoniaestrain A66.1 (type 3) was engineered to ex-press luciferase and resistance to kanamycin as described previously (20). S. pneumoniaewas grown in Todd-Hewitt broth (Difco Laboratories,
De-troit, MI) to an optical density at 620 nm (OD620) of approximately 0.7 and then frozen at⫺80°C and mixed 2:1 with 50% sterile glycerol, and the titer was subsequently determined on tryptic soy agar (TSA; Difco Labo-ratories, Detroit, MI) supplemented with 3% (vol/vol) sheep erythrocytes. Streptococcus pyogenesstrain MGAS 315 is a serotype M3 strain that was obtained from Michael S. Chaussee at the University of South Dakota (12).S. pyogeneswas grown in Todd-Hewitt broth supplemented with 0.2% yeast extract to the mid-exponential phase at an OD600of approxi-mately 0.47. The infection stocks of 3.1⫻108CFU/ml were supplemented with 25% glycerol and frozen at⫺80°C in single-use aliquots, and the titer was subsequently determined on TSA plates supplemented with 10% de-fibrinated sheep blood (Becton, Dickinson, Sparks, MD) after 24 h of incubation at 37°C, 5% CO2.
Staphylococcus aureusNRS-193 (USA400) is a clinical strain from a patient with necrotizing pneumonia and was obtained from the Network for Antimicrobial ResistantS. aureus. Infection stocks were grown on confluent TSA plates and scraped off in brain heart infusion supple-mented with 25% (vol/vol) glycerol and frozen at⫺80°C in single-use aliquots, and the titer was subsequently determined on TSA.
Murine superinfection model.Groups of 5 mice were inoculated in-tranasally with 0.25 murine LD50(MLD50) of the individual influenza viruses (Table 1) in a volume of 100l phosphate-buffered saline (PBS). After 5 days, mice were inoculated in a similar manner with either 100 CFU ofS. pneumoniae(0.1 MLD50), 1E108CFUS. aureus, or 1E106CFU GAS (0.1 MLD50).
Titers of virus and bacteria within lungs.Lungs were aseptically col-lected from mice at 0, 24, or 48 h after bacterial inoculation for quantita-tion of virus and bacteria present. Immediately after mice were euthanized by CO2inhalation, lungs were sufflated with PBS, and right lobes were removed and homogenized in PBS using a tissue homogenizer (Omni TH, Kennesaw, GA). Bacterial titers were determined on either TSA forS. aureusor TSA supplemented with 3% defibrinated sheep blood forS. pneumoniaeand grown overnight at 37°C. Bacterial titers within lungs from mice inoculated withS. pyogeneswere determined using TSA plates supplemented with10% defibrinated sheep blood (Becton, Dickinson, Sparks, MD), after 24 h of incubation at 37°C, 5% CO2. Alternatively, viral titers were determined using standard inoculation of MDCK cell mono-layers, as described previously (26).
Statistical analyses.Statistical analyses were done using analysis of variance (ANOVA) for comparison of titers and the log rank test on the Kaplan-Meier data for comparison of survival. Significance was defined as aPvalue of⬍0.05. SigmaStat for Windows (SysStat Software, Inc.; version 3.11) was used for all analyses.
RESULTS
PB1-F2 sequences of selected primary influenza virus isolates.
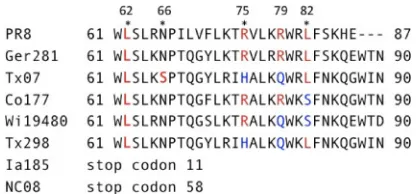
PB1-F2 contributes to the virulence of highly pathogenic influ-enza viruses within models of primary influinflu-enza virus infection, and specific amino acids within the C terminus that contribute to increased cellular infiltration and cytokine release have been iden-tified (1,14). Comparatively, the contribution of PB1-F2 toward development of deadly secondary bacterial infections has been only briefly noted by studies from our group (1, 33). With the emergence of the 2009 pandemic H1N1 influenza virus as a triple reassortant composed of human, avian, and swine influenza virus genes (9), we sought to examine the contribution of PB1-F2 within naturally occurring swine influenza virus isolates toward virulence during secondary bacterial infections. We selected vi-ruses with various combinations of amino acids associated with virulence (1) and divided them into three broad groups (Table 1). Group 1 contains the mouse-adapted PR8 virus and the primary swine influenza virus isolates GE81, TX07, and CO77, which in-dividually contain either 3 (TX07 and CO77) or 4 (PR8 and GE81) of the 5 previously characterized amino acids associated with
vir-TABLE 1.Characteristics of PB1-F2-expressing viruses used in this study
Group and virus name Abbreviation TCID50a MLD50b
Group 1: virulent PB1-F2
A/Puerto Rico/8/34-H1N1 PR8 9.2 2.5
A/swine/Germany/2/81-H1N1 GE81 9.0 5.5
A/swine/Texas/042995-27/2007-H1N2 TX07 7.1 4.3
A/swine/Colorado/1/77-H3N2 CO77 7.5 6.0
Group 2: avirulent PB1-F2
A/swine/Texas/4199-2/98-H3N2 TX98 6.67 4.8 A/swine/Wisconsin/194/80-H3N2 WI80 6.7 4.2
Group 3: truncated PB1-F2 A/swine/North Carolina/057225/
2008-H1N2
NC08 7.4 6.0
A/swine/Iowa/1/85-H1N1 IA85 5.5 3.0
a
Values are reported as log10TCID50/ml. bValues are reported as log
10TCID50/0.1 ml.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:2.585.39.288.87.245.2]ulence (Fig. 1). Group 2 viruses contain 2 of these 5 amino acids and are represented here by TX98 and WI80. Group 3 viruses (NC08 and IA85) contain a premature stop codon in the PB1-F2 open reading frame that leaves them devoid of all amino acids associated with virulence. As indicated inTable 1, all viruses grow to high titers under MDCK cell culture conditions and demon-strate lethality within the murine model of influenza virus infec-tion.
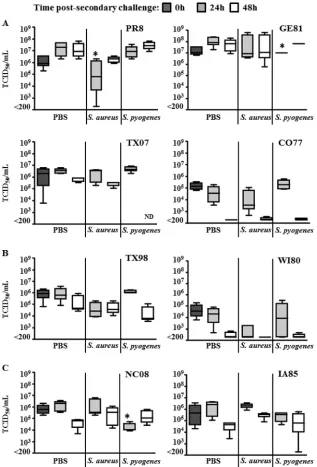
Viral titers after secondary challenge.Viral titers were quan-titated within the lungs of mice inoculated with individual swine influenza virus isolates either alone (Fig. 2, PBS columns) or after bacterial superinfection. The 0-h time point serves as an indicator of virus level at day 5 postinfection with a sublethal dose (0.25 MLD50) of each influenza virus and represents viral loads at the
point when mice were either mock challenged with PBS or chal-lenged with the bacterial species indicated. Samples from lungs taken 24 and 48 h after secondary challenge demonstrate viral titers after inoculation with PBS,S. aureus, orS. pyogenes. Since secondary bacterial infection withS. pneumoniaehas consistently been shown to enhance lung viral titers in prior studies by our group (57), this parameter was assessed only withS. aureusandS. pyogenesin the present study.
Our results demonstrate that virus clearance kinetics differed even in the absence of bacterial superinfection (PBS alone), with lung viral loads demonstrating a noticeable increase in peak viral titers at either day 6 or 7 (24 and 48 h after mock challenge with PBS) for both the PR8 and GE81 viruses. Alternatively, lung viral titers were detected at similar levels at day 6, with noticeable re-ductions in viral titer between days 6 and 7 for the TX07, TX98, NC08, and IA85 viruses. Lung viral titers demonstrated the most complete clearance profiles after day 5 for CO77 and WI80. Lung virus clearance kinetics did not correlate with PB1-F2 genotype and, with a few notable exceptions, secondary infection with ei-therS. aureusorS. pyogenesdid not appreciably alter this pattern. These exceptions include viral titers afterS. aureussuperinfection that were reduced to statistically significant levels on day 6 (PR8) compared to those of PBS-inoculated mice (P⬍0.05) and viral titers afterS. pyogenessuperinfection that were lower on day 6 than in PBS-challenged mice for two viral strains (GE81 and NC08). Mice superinfected with TX07 andS. pyogenesdid not survive to have viral titers measured at day 7. It is worth noting that despite differences in the MLD50doses (Table 1), all viruses
were detected at high levels within the lungs of mice at 5 days after influenza virus challenge (Fig. 2) and that a certain level of vari-ability within lungs at this time is expected as the host immune system works to clear these viruses. Based on these findings, we
conclude that lung viral load is only minimally impacted by bac-terial superinfection with eitherS. aureusorS. pyogenes, without a consistent pattern related to PB1-F2 genotype.
Bacterial titers after secondary challenge. Next, we deter-mined the bacterial titers within the lungs at 24 and 48 h post-inoculation. In the absence of virus (PBS as the primary inocu-lum), bacteria were detected in the lungs 24 h after challenge in mice that received eitherS. pneumoniaeorS. aureusalone and then decreased between 24 and 48 h (Fig. 3, PBS columns). Bac-terial lung infection could not be detected in mice that receivedS. pyogenesalone, corroborating previous observations of rapidS. pyogenesclearance within mice by our group (12). However, when mice were infected with group 1 influenza viruses prior to bacte-ria, lung titers ofS. pneumoniaebacteria were both noticeably (PR8 and GE81) and significantly (TX07,P⬍0.05) increased at 24 h postinoculation compared to those in PBS controls. Further-more, increases in lung bacterial titers at 48 h were statistically significant for all three of these viruses compared to those in PBS controls (P⬍0.05), with CO77 being the lone exception to this observation among group 1 viruses (Fig. 3A). Alternatively, S. pneumoniaetiters were stable or decreased after inoculation with group 2 viruses (TX98 and WI80;Fig. 3B), and a single virus within group 3 (NC08; Fig. 3C) demonstrated significant in-creases in bacterial titers compared to those in PBS controls, but theS. pneumoniaebacterial loads did not increase from 24 to 48 h postinoculation in this group. Surprisingly, mice inoculated with the IA85 virus that expressed a truncated PB1-F2 (11 amino acids) demonstrated titers ofS. pneumoniaethat noticeably increased between 24 and 48 h.
In the murine coinfection model with influenza virus and
Staphylococcus aureus, a large dose of the bacteria (108CFU) was
required to observe clinical signs of pneumonia, and as such only clearance kinetics of the bacteria were observed (27). In response to the group 1 viruses PR8, GE81, and TX07, both noticeable (PR8) and statistically significant (GE81 and TX07;P⬍ 0.05) increases in lung bacterial titers at 48 h were observed compared to those in the PBS control (Fig. 3A). Similarly, one group 2 virus (Fig. 3B) and both of the group 3 viruses (Fig. 3C) demonstrated statistically significant increases inS. aureustiters at the 48-h time point compared to those in the PBS control group. Of note, two viruses demonstrated lung bacterial titers at 48 h that were similar to those in the PBS control group (CO77, group 1; WI80, group 2). Despite these differences, titers ofS. aureusdecreased between 24 and 48 h for PBS and all viruses, regardless of the PB1-F2 variant expressed.
With regard to secondary inoculation withS. pyogenes, bacteria were no longer present within lungs of previously uninfected mice by 24 h after bacterial inoculation. However, in mice that were infected with influenza virus, bacteria were supported to various degrees with all viruses examined in this study. Similar toS. pneu-moniae, mice superinfected with the group 1 viruses (PR8, GE81, or TX07) andS. pyogenesshowed statistically significant increases in bacterial titers by 24 h after bacterial inoculation for the TX07 virus (P⬍0.05) and 48 h after bacterial inoculation for both the PR8 and GE81 viruses (P⬍0.05), compared to mice primed with PBS (Fig. 3A). When superinfections were established with the TX07 virus andS. pyogenes, all mice were dead at 48 h, with no lung samples available for measuring titers with this specific virus-bacterium combination. The CO77 virus supportedS. pyogenes FIG 1C-terminal sequences of PB1-F2 from selected swine flu isolates.
Amino acids that have been previously identified and characterized (1) are in red (virulent) and blue (avirulent).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:3.585.60.270.65.162.2]bacteria at 24 h and 48 h postinoculation compared to PBS con-trols, but these differences failed to achieve statistical significance. Within the lungs of mice challenged with the TX98 (group 2;
Fig. 3B), NC08 (group 3;Fig. 3C), and IA 85 (group 3;Fig. 3C) viruses, the titers ofS. pyogeneswere increased to statistically sig-nificant levels (P⬍0.05) compared to those in PBS controls at 24 h (TX98 and NC08) and 48 h (TX98 and IA85) after bacterial inoculation. While still supported within the lungs after inocula-tion with virus, the burden ofS. pyogenesbacteria was lowest after
infection with the WI80 virus, which contains a full-length PB1-F2 protein with amino acids associated with an avirulent phenotype. This reduced bacterial burden after WI80 was similar to the observation made withS. pneumoniaeand supports recent evidence that PB1-F2 proteins can express anti-inflammatory amino acids at position 82 that may contribute to antibacterial effects during secondary bacterial infections (1).
Survival after secondary challenge.As a measure of the syn-ergistic impact of virus and bacteria on disease severity, mortality
FIG 2Lung viral titers after secondary challenge. Viral load from groups of mice infected with swine isolates of influenza A virus, followed 5 days later with sublethal doses of bacteria (S. pyogenesorS. aureus) or PBS for controls were determined using MDCK monolayers. All groups contained 5 mice, except PR8-S. pyogenesat 48 h (n⫽4 mice), CO77-PBS at 0 h (n⫽4 mice), IA85-PBS at 48 h (n⫽4 mice), and IA85-S. aureusat 24 h (n⫽4 mice). Data are presented as box-and-whisker plots, where the whisker represents the 25th to 75th percentile and standard deviation, and the horizontal line indicates the mean. (A) Viruses from group 1: virulent PB1-F2; (B) viruses from group 2: avirulent PB1-F2; (C) viruses from group 3: truncated PB1-F2. *,P⬍0.05 by ANOVA (with Dunn’s correction) versus the corresponding PBS group (each time point examined individually). ND indicates not done, as no animals remained in this group at sampling.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.134.451.66.533.2]after influenza virus-bacterium superinfection was assessed (Fig. 4). Inoculation with PBS served as a control that demon-strated the sublethal effect of each bacterial species alone on the murine host (Fig. 4A). All viruses used in the superinfection model were delivered at a sublethal dose (0.25 MLD50), with 100%
survival demonstrated in mice receiving⬍1 MLD50for each virus
individually (Table 1). Thus, lethality observed in this study (Fig. 4B,C, AndD) is associated with influenza virus-bacterium super-infection.
Our data demonstrate that the group 1 viruses PR8, GE81, and TX07 supported lethal superinfections withS. pneumoniae,S. au-reus, andS. pyogenes(Fig. 4B). Specifically, survival was 20% or less regardless of the bacterial species used to superinfect. The one exception within the group 1 viruses was the CO77 virus, which did not express virulence-associated amino acids at two key sites: 66 and 82 (Fig. 1). Consequently, this virus demonstrated various
degrees of survival based on bacterial species, ranging from 100%, 40%, and 0% withS. aureus,S. pyogenes, andS. pneumoniae, re-spectively. Of note, the TX07-S. pyogenessuperinfection repre-sented the most lethal virus-bacterium combination used in this study, with 0% survival by 48 h after bacterial challenge. TX07 was the only virus studied that has the N66S mutation found to be associated with increased virulence of the 1918 pandemic strain (14).
As predicted, a decrease in the number of virulence-associated amino acids expressed (group 2) improved survival after second-ary bacterial infection, with all three bacterial species demonstrat-ing 80% survival or better (Fig. 4C). Surprisingly, the improved survival observed after TX98 primary infection contrasted with the bacterial titers detected after superinfection (Fig. 3B). Specif-ically, for this group 2 virus, survival was 80% after eitherS. aureus
orS. pyogenessuperinfection, despite a statistically significant
in-FIG 3Lung bacterial titers after secondary challenge. Bacterial loads were assessed from groups of 5 mice infected with swine influenza A virus isolates followed 5 days later with bacteria (S. pneumoniae,S. aureus, orS. pyogenes). All groups contained 5 mice, except PR8-S. pyogenesat 48 h (n⫽4 mice) and IA85-S. pyogenes at 24 h (n⫽4 mice). Data are presented as box-and-whisker plots, where the whisker represents the 25th to 75th percentile and standard deviation, while the horizontal line indicates the mean. (A) Viruses from group 1: virulent PB1-F2; (B) viruses from group 2: avirulent PB1-F2; (C) viruses from group 3: truncated PB1-F2. The PBS columns are identical for each pathogen in each panel (A, B, and C) and were included in each panel for ease of comparison. *,P⬍0.05 by ANOVA (with Dunn’s correction) versus the corresponding PBS group (each time point examined individually); **,P⬍0.05 by ANOVA (with Dunn’s correction) versus corresponding TX07 group; †,P⬍0.05 by ANOVA (with Dunn’s correction) versus corresponding TX98 group. ND indicates not done, as no animals remained in this group at sampling.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.110.475.77.443.2]crease in bacteria present at 48 h postinoculation. This paradoxi-cal finding indicates that after TX98 infection, clearance of the bacteria is still effective, and the exact mechanism associated with this observation is unknown.
Interestingly, a similar observation was made for S. pneu-moniae andS. aureus when the primary inoculation was with group 3 viruses expressing a truncated PB1-F2 protein (Fig. 4D). For these two bacterial species, evaluation of lung titers demon-strates thatS. pneumoniaebacteria remain at statistically
signifi-cant levels after NC08 at both 24 and 48 h postinoculation (Fig. 3C;P⬍0.05) and at statistically significant levels forS. aureusafter NC08 and IA85 at 48 h postinoculation (P⬍0.05). Despite these increased titers, survival was 80% (S. pneumoniae) and 100% (S. aureus) with these virus-bacterium superinfection combinations. Furthermore, within the IA85-S. pneumoniae superinfection group, there was a noticeable increase in bacterial titers between 24 and 48 h postinoculation, which contrasts with the 100% sur-vival observed within this group. Interestingly, secondary infec-tion using group 3 viruses andS. pyogenesdemonstrated a statis-tically significant difference (P⬍0.05 compared toS. pneumoniae
andS. aureus) in survival (20% with NC08 and 60% with IA85;
Fig. 4D) that was better associated with the statistically significant levels ofS. pyogenesdetected within the lungs of mice (Fig. 3C) after primary infection with NC08 (24 h;P⬍0.05) and IA85 (48 h;
P⬍0.05). We interpret this finding to indicate that along with host contributions to the severity of these superinfections, there are contributions for the specific bacteria that are associated with disease severity.
To summarize these survival data, all three bacterial species were grouped based on the presence of predicted provirulence amino acids expressed by the challenge virus (Fig. 5). These data demonstrate that as the number of PB1-F2 virulence-associated residues expressed increases, overall survival drops from⬃80% (0 residues) to⬍10% (4 residues), regardless of the bacterial species that superinfects. We conclude that the amino acid residues 62L, 66S, 75R, 79R, and 82L are molecular signatures of the virulence of swine influenza viruses in the setting of bacterial superinfection. Furthermore, we suggest that surveillance of internal influenza virus genes, for example, PB1-F2, should be expanded to identify the potential genetic signatures associated with increased severity of superinfections.
DISCUSSION
Influenza virus surveillance focuses primarily on changes in the HA and NA proteins expressed at the viral surface (59). This sur-veillance is critical for vaccine selection (23), as HA and NA are the major targets for vaccine-induced protective immunity (18). Since the virulence of influenza viruses is a multigenic trait (7,8,
21), surveillance of additional viral genes may allow us to better predict the severity of infections associated with circulating influ-enza viruses (45). Furthermore, a description of distinct contribu-tions of viral virulence factors toward deadly superinfeccontribu-tions will
FIG 4Survival after secondary challenge. Mice were infected intranasally with 0.25 LD50of influenza virus and followed 5 days later with a sublethal dose of
bacteria (S. pneumoniae,S. aureus, orS. pyogenes) and monitored for survival for 9 days postsecondary challenge. All groups contained 5 mice, except PR8-S. pneumoniae(n⫽10 mice). (A) Mice that received PBS at day 0 followed by the individual bacterial species at day 5; (B) viruses from group 1: virulent PB1-F2; (C) viruses from group 2: avirulent PB1-F2; (D) viruses from group 3: trun-cated PB1-F2. *,P⬍0.05 by log rank test on Kaplan-Meier data versusS. aureusgroup; **,P⬍0.05 by log rank test on Kaplan-Meier data versus both groups.
FIG 5Contribution of the number of inflammatory PB1-F2 amino acids toward survival after secondary bacterial infection. Data presented inFig. 4are grouped based on survival after inoculation with swine influenza virus isolates that express either 0, 2, 3, or 4 provirulence amino acids, regardless of the secondary bacterial species delivered (S. pneumoniae,S. aureus, orS. pyogenes).
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.44.284.61.505.2] [image:6.585.337.506.64.182.2]improve our ability to predict the overall impact of an influenza virus that causes an epidemic or a pandemic. We designed exper-iments to evaluate the potential impact that single, naturally oc-curring variants in PB1-F2 protein can have on predicting the severity of secondary bacterial infections with three clinically rel-evant Gram-positive bacterial species (3–5,44,52). Our findings demonstrate that expression of naturally occurring PB1-F2 vari-ants correlates with the severity of secondary complications ob-served, including lung bacterial titers and survival after superin-fection. We propose that viral contributions toward secondary bacterial complications can be identified based on viral genotype, and PB1-F2 represents an initial protein to consider as we move toward defining the mechanism of these natural, lethal interac-tions.
The current study incorporated eight distinct influenza virus isolates that were separated into three groups based on PB1-F2 sequence, and selection was based on previous performance of laboratory-generated viruses that differ solely in the PB1-F2 gene expressed (1,33,62). Viruses selected for this study were of swine origin, as this species acts as an intermediate host for human in-fluenza pandemics (46,47) and represents a population within which influenza viruses can evolve prior to emergence in humans (55). The first group consisted of viruses expressing either three or four of the five virulence-associated amino acid residues identified in PB1-F2. Considering the viral titers, bacterial titers, and sur-vival profiles observed, we conclude that viruses within group 1 demonstrated the most consistent potential to induce a superin-fection with a Gram-positive respiratory pathogen. In fact, the severe disease associated with the TX07 virus for all three bacterial species suggests that the recently defined virulence-associated N66S mutation (14) also represents a signature for secondary bac-terial complications. Interestingly, the data presented here tie to-gether independent observations demonstrating that the 1918 pandemic PB1-F2 primes strongly for secondary bacterial pneu-monia (33) and that the N66S mutation in the 1918 strain in-creases the virulence associated with the virus alone (14). The 1918 pandemic strain and select avian viruses, predominantly of the H2 subtype, are the only influenza viruses known to encode all 5 amino acids that have been associated with enhanced bacterial superinfection (1). These latter strains may represent significant pandemic threats.
A recent report demonstrated the significant gap in our sur-veillance within the swine population (11), and this deficiency is even more pronounced when one considers surveillance of inter-nal influenza virus genes, like the PB1-F2 gene (9,16,48,55,56). In addition to our observations with group 1 viruses, our conclu-sion that internal viral genes, like the PB1-F2 gene, can be used to identify the potential for serious disease outcomes after superin-fection is strengthened by our observations with the group 2 (avir-ulent PB1-F2) and group 3 (truncated PB1-F2) viruses. Specifi-cally, bacterial titers and survival were improved in animals that are initially exposed to viruses within these 2 groups relative to group 1 viruses. The lone exception to this improved survival was observed when group 3 influenza viruses (NC08 and IA85) were inoculated prior toS. pyogenessuperinfection. This paradoxical finding that a truncated PB1-F2 can predispose toward superin-fection withS. pyogenesis even more interesting when it is consid-ered along with clinical reports of increases in invasiveS. pyogenes
(2,4,5,42,60) in association with currently circulating viruses that express a truncated PB1-F2 (41). While these findings do not
directly implicate the PB1-F2 gene in the development of these superinfections, they serve as an initial justification for the in-creased surveillance of naturally occurring influenza virus viru-lence factors, including PB1-F2, NS1, and PB2, as contributors to secondary bacterial complications.
Likewise, our findings complement recent evidence that an avirulent PB1-F2 protein may limit bacterial outgrowth and may even act through an unknown mechanism as an antibacterial agent that reduces influenza virus-bacterium superinfection. A report from our group recently demonstrated that a peptide with a single substitution at position 82 showed an increase in survival whenS. pneumoniaewas the secondary invader (1). The data pre-sented here further suggest the importance of position 82 in the context of a broad spectrum of secondary bacterial invaders. Thus, it appears that the anti-inflammatory properties associated with the amino acid at position 82 can improve survival and/or delay time to death, even if the remaining amino acids predict a proin-flammatory phenotype (CO77).
The experimental approach and data presented within this study indicate that there are differences in the interaction between specific viruses and bacteria, but not all of these differences can be attributed to PB1-F2 expression. In fact, other influenza virus genes can contribute to the virulence associated with primary in-fluenza virus infection, including PB2 (10,28,43) and NS1 (6,22,
41,49). Viruses used in the current study were selected based on PB1-F2 genotype, without consideration for other viral genes ex-pressed by these viruses. The use of these disparate, naturally oc-curring viruses found in the swine reservoir in this context com-plements prior work in theS. pneumoniaemodel, where isogenic laboratory strains of influenza virus were altered so that the only differences were in the PB1-F2 expressed (1,32–34,38). Under-standing the contributions from PB1-F2 and other virulence fac-tors toward death after superinfection is especially important when studying mutations associated with increased transmissibil-ity of viruses that have pandemic potential, like H5N1 (19). Spe-cifically, if natural virus reassortants obtain the ability to circulate in humans, we hypothesize that genetic contributions toward sec-ondary bacterial infections can be used to predict the severity of this pandemic event and allow for the recommendation of thera-peutic interventions, in particular the selection of appropriate an-tibiotics (35). Future experiments that will directly evaluate the contributions of additional virulence factors expressed by these viruses toward outcomes from these superinfections are currently being designed.
In addition, the exact mechanism for enhanced bacterial dis-ease after influenza virus infection is unclear, but it likely involves host immune responses toward the virus itself. Host contributions toward prevention of secondary bacterial infections are noted in groups where lung bacterial titers remain high but overall survival remains⬎80%, for example,S. pyogenesafter TX98 andS. pneu-moniaeafter either NC08 or IA85. Improved survival despite high bacterial titers cannot be fully explained by the HA, NA, or PB1-F2 genotype, indicating that additional factors contribute to the out-comes observed. The additional factors dictating these outout-comes could be provided by either the host, the virus, or the bacterial species. For example, recent evaluation of host responses demon-strates that immune contributions to disease severity can be ob-served in the form of cytokines associated with severe disease (50,
51,53), with a recently proposed mechanism of interferon antag-onism through interference with the retinoic acid-inducible gene
on November 7, 2019 by guest
http://jvi.asm.org/
I (RIG-I)/mitochondrial antiviral signaling protein (MAVS) com-plex (17,54). Following PB1-F2 expressionin vitroorin vivo, this interaction with RIG-I/MAVS prevents activation of interferon regulatory factor 3 (IRF-3), with the result that beta interferon (IFN-) induction in response to the virus is inhibited, more in-filtration of immune cells into the lungs occurs, and morbidity increases. Thus, PB1-F2 appears to be a multifunctional peptide with anti-interferon activity similar to the nonstructural protein NS1 (22). It is unclear whether this interferon inhibitory activity contributes to the enhanced susceptibility to bacteria following influenza virus infection or if a separate function is involved. Fur-thermore, it is unclear at this time whether the specific amino acid residues examined in this study are related to the proposed IFN antagonism or if they act through a separate mechanism.
While these findings are not complete in their description of the contributions of the virus, bacteria, and host toward superin-fection pathogenesis, they provide initial evidence that molecular signatures present in an internal gene from naturally occurring swine influenza viruses can be monitored to predict the severity of secondary bacterial infections. As evidence, our observation ofS. pyogenessuperinfection after inoculation with viruses expressing truncated PB1-F2 variants that would be predicted to be avirulent demonstrates that consideration of viral virulence within primary influenza virus infection models alone may not allow for complete appreciation of the outcomes of dynamic, polymicrobial diseases, like the ones modeled here. Continued evaluation in models like these will allow us to define the impact of predicted influenza virus virulence genes, including the PB1-F2 gene, toward survival after superinfection. In conclusion, while we have been able to associate naturally occurring PB1-F2 variants with outcomes after superin-fection with three clinically relevant bacterial species, there is a lot more to be done as we identify the viral, bacterial, and host factors that directly modulate the disease severity observed.
ACKNOWLEDGMENTS
The authors acknowledge Michael S. Chaussee (University of South Da-kota [USD], Vermillion, SD) for providing the MGAS315 strain of Strep-tococcus pyogenesbacterium that was used in this study.
Funding was provided by the USD Foundation, the Division of Basic Biomedical Sciences, The U. Discover Program (M.J.S.), the SSOM Fac-ulty Research Program (V.C.H.), the USD Inside TRACK program (V.C.H.), and the American Lebanese Syrian Associated Charities (AL-SAC).
REFERENCES
1.Alymova IV, et al.2011. Immunopathogenic and antibacterial effects of H3N2 influenza A virus PB1-F2 map to amino acid residues 62, 75, 79, and 82. J. Virol.85:12324 –12333.
2.Ampofo K, et al.2010. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr. Infect. Dis. J.29:905–909.
3.Anonymous. 2007. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb. Mortal. Wkly. Rep.56:325–329.
4.Anonymous.2009. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep.58:1071–1074. 5. Anonymous.2011. Severe illness from 2009 pandemic influenza A
(H1N1)—Utah, 2009-10 influenza season. MMWR Morb. Mortal. Wkly. Rep.60:1310 –1314.
6.Billharz R, et al.2009. The NS1 protein of the 1918 pandemic influenza virus blocks host interferon and lipid metabolism pathways. J. Virol.83: 10557–10570.
7.Blazejewska P, et al.2011. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virol-ogy412:36 – 45.
8.Bogs J, et al. 2010. Highly pathogenic H5N1 influenza viruses carry virulence determinants beyond the polybasic hemagglutinin cleavage site. PLoS One5:e11826. doi:10.1371/journal.pone.0011826.
9.Brockwell-Staats C, Webster RG, Webby RJ.2009. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influenza Other Respi. Viruses3:207–213.
10. Bussey KA, Bousse TL, Desmet EA, Kim B, Takimoto T.2010. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J. Virol.84:4395– 4406.
11. Butler D.2012. Flu surveillance lacking. Nature483:520 –522. 12. Chaussee MS, et al.2011. Inactivated and live, attenuated influenza
vac-cines protect mice against influenza:Streptococcus pyogenes superinfec-tions. Vaccine29:3773–3781.
13. Chen CJ, et al.2010. Differential localization and function of PB1-F2 derived from different strains of influenza A virus. J. Virol.84:10051– 10062.
14. Conenello GM, et al.2011. A single N66S mutation in the PB1-F2 protein of influenza A virus increases virulence by inhibiting the early interferon responsein vivo. J. Virol.85:652– 662.
15. Conenello GM, Zamarin D, Perrone LA, Tumpey T, Palese P.2007. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog.3:e141. doi: 10.1371/journal.ppat.0030141.
16.Ducatez MF, et al. 2011. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg. Infect. Dis.17:1624 –1629.
17. Dudek SE, et al.2011. The influenza virus PB1-F2 protein has interferon antagonistic activity. Biol. Chem.392:1135–1144.
18. Eichelberger M, et al.2008. FDA/NIH/WHO public workshop on im-mune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10-11, 2007. Vaccine26:4299 – 4303.
19. Fouchier RA, et al.2012. Pause on avian flu transmission research. Sci-ence335:400 – 401.
20. Francis KP, et al.2001. Visualizing pneumococcal infections in the lungs of live mice using bioluminescentStreptococcus pneumoniaetransformed with a novel Gram-positive lux transposon. Infect. Immun.69:3350 – 3358.
21. Fukuyama S, Kawaoka Y.2011. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr. Opin. Immu-nol.23:481– 486.
22. Garcia-Sastre A, et al.1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology252:324 –330. 23. Gerdil C.2003. The annual production cycle for influenza vaccine.
Vac-cine21:1776 –1779.
24. Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW.2003. The influenza A virus PB1-F2 protein targets the inner mitochondrial mem-brane via a predicted basic amphipathic helix that disrupts mitochondrial function. J. Virol.77:7214 –7224.
25. Henkel M, et al.2010. The proapoptotic influenza A virus protein PB1-F2 forms a nonselective ion channel. PLoS One5:e11112. doi:10.1371/ journal.pone.0011112.
26. Huber VC, McCullers JA.2006. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J. Infect. Dis.193: 677– 684.
27. Iverson AR, et al.2011. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J. Infect. Dis.203:880 – 888.
28. Jagger BW, et al.2010. The PB2-E627K mutation attenuates viruses containing the 2009 H1N1 influenza pandemic polymerase. mBio1(1): e00067–10. doi:10.1128/mBio.00067-10.
29. Le Goffic R, et al.2010. Influenza A virus protein PB1-F2 exacerbates IFN-beta expression of human respiratory epithelial cells. J. Immunol.
185:4812– 4823.
30. Ma W, et al.2011. Pathogenicity of swine influenza viruses possessing an avian or swine-origin PB2 polymerase gene evaluated in mouse and pig models. Virology410:1– 6.
31. Mazur I, et al.2008. The proapoptotic influenza A virus protein PB1-F2 regulates viral polymerase activity by interaction with the PB1 protein. Cell Microbiol.10:1140 –1152.
32. McAuley JL, et al.2010. PB1-F2 proteins from H5N1 and 20 century
on November 7, 2019 by guest
http://jvi.asm.org/
pandemic influenza viruses cause immunopathology. PLoS Pathog.
6:e1001014. doi:10.1371/journal.ppat.1001014.
33. McAuley JL, et al.2007. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe2:240 –249.
34. McAuley JL, Zhang K, McCullers JA.2010. The effects of influenza A virus PB1-F2 protein on polymerase activity are strain specific and do not impact pathogenesis. J. Virol.84:558 –564.
35. McCullers JA.2008. Planning for an influenza pandemic: thinking be-yond the virus. J. Infect. Dis.198:945–947.
36. McCullers JA, Bartmess KC.2003. Role of neuraminidase in lethal syn-ergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis.187:1000 –1009.
37. McCullers JA, Huber VC.2012. Correlates of vaccine protection from influenza and its complications. Hum. Vaccin. Immunother.8:34 – 44. 38. McCullers JA, et al.2010. Influenza enhances susceptibility to natural
acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis.202:1287–1295.
39. McCullers JA, Rehg JE.2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis.186:341–350. 40. Mitzner D, et al.2009. Phosphorylation of the influenza A virus protein
PB1-F2 by PKC is crucial for apoptosis promoting functions in mono-cytes. Cell Microbiol.11:1502–1516.
41. Ozawa M, et al.2011. Impact of amino acid mutations in PB2, PB1-F2, and NS1 on the replication and pathogenicity of pandemic (H1N1) 2009 influenza viruses. J. Virol.85:4596 – 4601.
42. Parola P, et al.2011. Letter to the editor. Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill.16:pii⫽19816.
43. Ping J, et al.2010. PB2 and hemagglutinin mutations are major determi-nants of host range and virulence in mouse-adapted influenza A virus. J. Virol.84:10606 –10618.
44. Rothberg MB, Haessler SD.2010. Complications of seasonal and pan-demic influenza. Crit. Care Med. 38:e91– e97. doi:10.1097/ CCM.0b013e3181c92eeb.
45. Schnitzler SU, Schnitzler P.2009. An update on swine-origin influenza virus A/H1N1: a review. Virus Genes39:279 –292.
46. Scholtissek C.1987. Molecular aspects of the epidemiology of virus dis-ease. Experientia43:1197–1201.
47. Scholtissek C, Rohde W, Von HV, Rott R.1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology87:13–20.
48. Shinde V, et al.2009. Triple-reassortant swine influenza A (H1) in hu-mans in the United States, 2005-2009. N. Engl. J. Med.360:2616 –2625. 49. Spesock A, et al.2011. The virulence of 1997 H5N1 influenza viruses in
the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol.85:7048 –7058.
50. Sun K, Metzger DW.2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med.
14:558 –564.
51. Sun K, Torres L, Metzger DW.2010. A detrimental effect of interleu-k10 on protective pulmonary humoral immunity during primary in-fluenza A virus infection. J. Virol.84:5007–5014.
52. Tasher D, et al.2011. Invasive bacterial infections in relation to influenza outbreaks, 2006-2010. Clin. Infect. Dis.53:1199 –1207.
53. van der Sluijs KF, et al.2004. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza in-fection. J. Immunol.172:7603–7609.
54. Varga ZT, et al.2011. The influenza virus protein PB1-F2 inhibits the induction of type I interferon at the level of the MAVS adaptor protein. PLoS Pathog.7:e1002067. doi:10.1371/journal.ppat.1002067.
55. Vijaykrishna D, et al.2010. Reassortment of pandemic H1N1/2009 in-fluenza A virus in swine. Science328:1529.
56. Vijaykrishna D, et al.2011. Long-term evolution and transmission dy-namics of swine influenza A virus. Nature473:519 –522.
57. Weeks JN, Boyd KL, Rajam G, Ades EW, McCullers JA.2011. Immu-notherapy with a combination of intravenous immune globulin and p4 peptide rescues mice from postinfluenza pneumococcal pneumonia. An-timicrob. Agents Chemother.55:2276 –2281.
58. Wise HM, et al.2009. A complicated message: identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J. Virol.83:8021– 8031.
59. World Health Organization.2002. WHO manual on animal influenza diagnosis and surveillance, p 1–135.InWebster RG, Cox N, Stohr K (ed). World Health Organization, Geneva, Switzerland.
60. Zakikhany K, et al.2011. Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to Jan-uary 2011. Euro Surveill.16:pii⫽19785.
61. Zamarin D, Garcia-Sastre A, Xiao X, Wang R, Palese P.2005. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog.1:e4. doi:10.1371/journal.ppat.0010004. 62. Zamarin D, Ortigoza MB, Palese P.2006. Influenza A virus PB1-F2
protein contributes to viral pathogenesis in mice. J. Virol.80:7976 –7983.