ABSTRACT
COUSE, JOHN FLOYD. The Role of Estrogen Receptor-a and Estrogen Receptor-b in the Hyperluteinized Mouse Ovary. (under the direction of Robert C. Smart and Kenneth S. Korach)
THE ROLE OF ESTROGEN RECEPTOR-a AND ESTROGEN RECEPTOR-b IN THE HYPERLUTEINIZED MOUSE OVARY
by
JOHN FLOYD COUSE
A dissertation submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the Degree of Doctor of Philosophy
ENVIRONMENTAL AND MOLECULAR TOXICOLOGY
Raleigh 2004
APPROVED BY:
Robert C. Smart, Ph.D. Kenneth S. Korach, Ph.D.
Co-chair of Advisory Committee Co-chair of Advisory Committee
I dedicate this work to …
My wife, Diane, for her support, encouragement and patience during this journey that coincided with our first
four wonderful years of marriage.
and
My Mom and Dad, who instilled in me many lessons for life, but above all taught me to always finish what I start, to
take pride in everything I do and that all hard work is rewarded.
From the point of ignition To the final drive
The point of a journey Is not to arrive
BIOGRAPHY
I am John Floyd Couse, the son of Anne T. (Condon) and Clifton K. Couse. I bear the name of my grandfathers, John Condon and Floyd Couse. I am not sure whether my parents chose my name with the intent of providing me with a lasting impression of my ancestry or to simply satisfy their dilemma of naming a seventh child. Regardless of their reasons, they succeeded on both accounts and I strive to carry on my grandfathers’ names with honor and integrity everyday. I spent the first twenty years of my life in Brownville, New York and graduated from General Brown High School in 1985. Brownville is a blue-collar paper mill town of 1,200 people surrounded by blue-collar paper mill towns of smaller or larger populations. This part of New York where Lake Ontario spills into the St. Lawrence River is sprinkled with sites of spectacular natural beauty and endowed with seasonal changes that come stunningly swift and dramatic. My favorite memories will always be of winter and hearing the hard-packed snow squeak underneath my feet as I walked streets walled in by head-high banks of snow and inhaled the fresh sub-zero air that made your nostrils pinch together.
accomplishment that sums up their view on how one should go about conducting themselves in this world.
I was the first in my family to go to college. This is hardly because I was more worthy than my siblings but due more to my desire to spend time with friends, all of whom were destined for college. Undoubtedly, I otherwise would have entered the military. Instead I traded the winter winds of Lake Ontario for those of Lake Champlain and spent four years at the State University of New York at Plattsburgh to earn a B.S. in microbiology in 1989. From there I enrolled in the School of Public Health at the University of North Carolina at Chapel Hill and earned an M.S. in medial parasitology in 1991. Employment for parasitologists is quite sparse so I chose to follow my father’s career path. I wasn’t much of a welder but felt I could be an effective teacher. Unfortunately, the Teach for America Program, in its second year at the time, did not share my self-assessment and rejected my application. While contemplating a return to New York, a series of circumstances of which I had little active role secured me employment at NIEHS, where I have been since.
ACKNOWLEDGEMENTS
I am indebted to Dr. Ken Korach for providing me the opportunity to undertake my own projects and granting me a level of freedom that few others in my position enjoy. Ken had several offers from renowned investigators to collaborate on studies of the ERKO ovary and a single offer from a technician that came with a promise to work hard but backed by little knowledge in ovarian physiology. I am incredibly grateful to Ken for his confidence in me and equally thankful for his friendship and guidance over the past several years.
I am also indebted to Ms. Mariana Yates. Mariana’s skills at the bench, her ability to learn techniques, and her ability to keep pace with the many projects we simultaneously manage has been invaluable. Mariana’s dedication to this work is best illustrated throughout the extent of the studies I have been able to carry out with success.
I have witnessed the passage of several investigators and trainees through the Receptor Biology Section (RBS) and NIEHS and have been influenced by all of them to some degree. One such person deserves special mention, Dr. Jonathan Lindzey. Jonathan and I not only shared the same laboratory for four years but a common interest in working atmosphere, music and reproductive biology. Equally important, Jonathan taught me endocrinology, experimental design and how to effectively critique and evaluate one’s own work. Thank you Jonathan for the enduring lessons, the wonderful rap sessions at the bar, and for introducing me to The Blues.
I have been fortunate to collaborate and interact with numerous individuals in the field and would like to especially acknowledge Drs. David Schomberg, Madhabananda Sar, Donna Bunch, John Nilson, Dennis Lubahn, Oliver Smithies, George Stancel and Donald McDonnell for their mentoring and encouragement to return to school and work towards this degree. I am also grateful to my committee members, Drs. Robert Smart, Gerald LeBlanc and William Miller for granting me this opportunity and providing guidance over the past four years.
TABLE OF CONTENTS
Page
LIST OF TABLES ix
LIST OF FIGURES x
1. INTRODUCTION 1
1.1 Estrogen receptors and estrogen signaling 2
1.2 Physiology and function of the ovary 6
1.2 Intraovarian role of estrogen signaling 8
1.3 Neuroendocrine role of estrogen signaling in ovarian function 9
1.4 Review of ovarian phenotypes in the estrogen receptor null mice 12
1.3 Hypotheses 15
1.6 References cited 20
2. PREVENTION OF THE POLYCYSTIC OVARIAN PHENOTYPE AND CHARACTERIZATION OF OVULATORY CAPACITY IN THE ESTROGEN RECEPTOR-a KNOCKOUT MOUSE 28
2.1 Abstract 28
2.2 Introduction 29
2.3 Materials and methods 31
2.4 Results 35
2.5 Discussion 44
2.6 References cited 53
3. CHARACTERIZATION OF THE HYPOTHALAMIC-PITUITARY-GONADAL (HPG) AXIS IN ESTROGEN RECEPTOR NULL MICE REVEALS HYPERGONADISM AND ENDOCRINE SEX-REVERSAL IN FEMALES LACKING ERa BUT NOT ERb 58
3.1 Abstract 58
3.2 Introduction 59
3.3 Materials and methods 61
3.4 Results 67
3.5 Discussion 80
3.6 References cited 91
4. FEMALE MICE LACKING ESTROGEN RECEPTOR-b EXHIBIT AN ATTENUATED OVARIAN RESPONSE TO CHRONICALLY ELEVATED LUTEINIZING HORMONE 96
4.1 Abstract 96
4.2 Introduction 97
TABLE OF CONTENTS (Continued)
Page
4.4 Results 103
4.5 Discussion 114
4.6 References cited 123
5. CONCLUSION 127
5.1 References cited 136
APPENDICES 138
Appendix A: Publications authored or co-authored by John F. Couse 139
Appendix B: Selected reviews authored or co-authored by John F. Couse 142
Appendix C: Invited platform presentations by John F. Couse 143
LIST OF TABLES Table 1.1 Ovarian phenotypes in the ERKO mice
Table 2.1 Oocyte yield after superovulation of wild-type and aERKO mice
Table 2.2 Results of in vitro fertilization of oocytes collected from immature control (wild-type and heterozygous) and aERKO mice after superovulation
Table 3.1 Specifications of all probes and PCR primers used
Table 3.2 Plasma hormone levels in untreated adult females or in those following treatment with a GnRH antagonist
Table 4.1 Oligonucleotide primers and probes used for semi-quantitative RTPCR Table 4.2 Summary of pathology observed in wild-type (WT), WTLHCTP, bERKO, and
LIST OF FIGURES
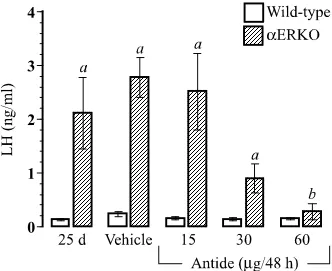
Figure 2.1 The efficacy of prolonged GnRH antagonist treatment in reducing serum luteinizing hormone (LH) in aERKO females
Figure 2.2 Prevention of the aERKO ovarian phenotype after prolonged treatment with a GnRH antagonist
Figure 2.3 Gonadotropin receptor mRNA levels after prolonged treatment with a GnRH antagonist
Figure 2.4 Ovarian histology in immature wild-type and aERKO females after superovulation
Figure 2.5 Ribonuclease protection assay (RPA) for markers of follicular maturation in immature wild-type and aERKO ovaries
Figure 2.6 Ribonuclease protection assay (RPA) for markers of ovulation in immature wild-type and aERKO ovaries
Figure 2.7 Serum progesterone in immature wild-type and aERKO females after superovulation
Figure 3.1 Gonadotropin-related gene expression in the pituitary of ERKO females Figure 3.2 Prl gene expression in the pituitary of ERKO females
Figure 3.3 Two-cell, Two-gonadotropin paradigm of steroidogenesis in the ovary Figure 3.4 Gene expression assays for steroidogenic components in the ERKO ovary Figure 3.5 Effect of a GnRH antagonist on pituitary and ovarian gene expression in
wild-type and aERKO females
Figure 3.6 Effect of flutamide on ovarian gene expression in aERKO females
Figure 3.7 Evaluation of Inha, Inhba, Inhbb and Fst expression in pituitary and ovary of ERKO females
Figure 4.1 WTLHCTP and bERKOLHCTP females exhibit increased plasma LH and sex steroids
LIST OF FIGURES (CONTINUED)
Figure 4.3 WTLHCTP, bERKO and bERKOLHCTP ovaries all exhibit interstitial cell hyperplasia
Figure 4.4 Estrogen receptor genotype determines the ovarian gene expression pattern that occurs during chronic LH stimulation
Figure 4.5 Females lacking either ER type exhibit attenuated LH-induced Lhcgr
1. INTRODUCTION
The ovary is an endocrine organ and produces the bulk of circulating estradiol in the mammalian female. Estradiol is principal to the manifestations of puberty, exemplified by dramatic transformations in the reproductive tract, breasts and central nervous system. The cyclic fluctuations in circulating estradiol that mark the estrous cycle of the rodent and the menstrual cycle of the human are remarkably predictable and obligatory to fertility. The role of estradiol in maintaining the health and function of the female reproductive tract, mammary glands and multitude of non-reproductive tissues continues to be well characterized. However, our knowledge of a comparative role for estradiol in ovarian function or even within the ovary itself has remained scant. Perhaps this dearth in understanding is due to the difficulties inherent to determining the role of an endocrine factor within the very tissue from which it is produced. For example, to study the contribution of estradiol in uterine or mammary gland function in the laboratory animal, one is able to rid the animal of endogenous estradiol via surgical removal of the ovary (ovariectomy). This allows investigators to observe the subsequent effects of a lack of estradiol on the tissue of interest as well as re-administer exogenous estrogens or other hormones in a controlled fashion. This classical ablation model has been employed for over 100 years (1, 2) and has considerably advanced the field of reproductive endocrinology, but is of course not applicable to the in vivo study of estrogen action within the ovary itself. However, the past two decades have witnessed the advancement of two laboratory techniques that have since revolutionized the “ablation” model as an investigative tool. The marriage of targeted homologous recombination with in vitro
targeting allows for the study of a particular intracellular component such as a hormone receptor. Prior to these advancements, such studies were impossible or relied on the use of pharmacological agents, which are accompanied by their own inherent limitations. The work presented herein is focused on our efforts to better determine the role of estradiol in ovarian function via the study of the estrogen receptor knockout (ERKO) mice.
The ERKO mice were developed using the techniques described above and as a group are comprised of three individual lines, those lacking a functional Esr1 gene (aERKO), those lacking a functional Esr2 gene (bERKO), and those lacking both (abERKO). The latter were generated by cross breeding the individual ERKO lines. Interestingly, females from each ERKO line exhibit a series of unique ovarian phenotypes. The limited descriptions of the ERKO ovarian phenotypes that were published prior to the work presented herein were the foundation for the hypotheses I have addressed, and therefore are summarized below. Also, the estrogen signaling system, the physiology of the ovary, and those estrogen actions in the hypothalamic-pituitary axis that impinge upon ovarian function are reviewed. Finally, I close this introduction with an outline of the hypotheses that led to the investigative work presented in the chapters that follow.
1.1 Estrogen receptors and estrogen signaling
Prior to 1996, a single form of estrogen receptor (ER) was believed to mediate all nuclear functions of estradiol. A revision of this paradigm was compulsory upon the discovery of a second ER in multiple species (3-5). The newly discovered receptor was termed ERb and the classical form renamed as ERa. The two ERs are products of distinct genes, Esr1 (ERa) and Esr2 (ERb) that are located on separate chromosomes. Hence, the estrogen signaling system now shares the distinction of having multiple receptor forms, as was previously described for thyroid hormone, retinoids, mineralicorticoids and progesterone (6).
molecular weight of 66 kDa (7). Multiple regulatory sequences in the 5’-untranslated region of the human and rat Esr1 gene have been described yet only a single open reading frame exists (8-10). Naturally occurring variants and mutations of the Esr1 transcript have been detected in normal and neoplastic tissues of several species (11-13), although in vivo evidence of correlating protein products remains controversial. The coding portion of the mouse Esr2 (ERb) gene yields multiple transcripts of varied size, at least one of which encodes a 527-530 amino acid protein with an approximate molecular weight of 60 kDa (3, 5, 14, 15). Most of the difference in size between the two receptors is due to a shorter N’-terminus in the ERb. Similar to ERa, a number of Esr2 splice variants have been described, including an 18 amino acid insertion in the C’-terminal region of ERb in the rat (16, 17), human and mouse (18).
The ERs are Class I members of the nuclear hormone receptor superfamily, defined as ligand-inducible transcription factors that interact with a palindromic hormone response element (HRE) located in the regulatory region of target genes (6). The modernized “two-step mechanism” of ER action states that estradiol diffuses through the nuclear membrane and binds to the ER, releasing it from an inactive complex and allowing ligand-bound receptors to homodimerize and associate with the estrogen response element (ERE) in the promoter region of the target gene (19). This complex then recruits co-regulator proteins as well as interacts with the transcriptional machinery to ultimately allow for increased transcription of the target gene (20-22). Alternate pathways of transactivation by steroid receptors have been described in recent years, including transactivation by ligand bound ER in the absence of ERE binding but instead via interaction with other DNA tethered transcription factors, such as AP-1 (23, 24). In addition, ligand-independent activation of ER via pathways that stimulate cellular kinases and phosphatases has been demonstrated
in vitro and in vivo (21). These discoveries strongly support the importance of the ER and its ability to provide diverse physiological functions even in the absence of ligand.
ERa and ERb share several functional properties when evaluated in vitro. For example, both receptors recruit the co-activator SRC-1 and are equally susceptible to inhibition by several antiestrogens (raloxifene, ICI 164,384 and EM-800) when acting on a basal promoter linked to a consensus ERE (5). However the agonist activity of 4-hydroxytamoxifen appears to be unique to ERa, although even this is dependent on the cell and promoter context (5, 34). Furthermore, estrogen antagonists that block the transactivational activity of an ERa/AP-1 complex behave as agonists when bound to ERb/AP-1 in vitro (35). The ability to synthesize non-steroidal ligands that are receptor selective in terms of binding and agonist/antagonist activities provides further evidence of distinct structural and functional differences between ERa and ERb (36). Nonetheless, no data exists to date that would indicate a genomic response solely mediated by ERb. In contrast, ERa is essential in mediating the positive actions of estradiol on multiple genes known to possess a functional ERE, as confirmed by their reduced expression in the aERKO mice. Furthermore, in vitro experiments have indicated the possibility of cooperative activity between the two receptors, in the form of a heterodimer (5, 37-39). The in vitro transactivational activity of the heterodimer in mammalian cell transfection assays lies between that of the more active ERa homodimer and the less active ERb homodimer (5, 37, 38).
Normal and neoplastic human mammary tissue and cell lines express detectable ERb (15, 48-51) yet the mouse mammary gland appears to predominantly express ERa (40). Furthermore, when both Esr genes are expressed in a particular tissue, the two receptors are often present in different cell types. In the ovary, ERb is localized to the granulosa cells of maturing follicles, whereas ERa is detectable in the surrounding thecal cells (52-54). In the rat prostate, ERa and ERb are detectable in the stroma and epithelium, respectively (3). To date, the best illustration of ERa/ERb co-localization is in certain regions of the rat forebrain (55).
1.2 Physiology and function of the ovary
The adult ovary serves two major functions: 1) to foster maturation of female germ cells toward ovulation and fertilization, and 2) to synthesize the steroid and peptide secretions necessary to stimulate the processes in the reproductive tract, mammary gland and central nervous system that are obligatory for the establishment and maintenance of pregnancy. The functional units of the ovary can be divided into three broad categories: the follicles, corpora lutea, and the interstitium (56, 57). All three possess the capacity to synthesize hormonal factors, most especially steroids, in response to gonadotropins from the anterior pituitary. The maturing follicle can be further divided into three main functional units: the outermost thecum, which surrounds multiple layers of granulosa cells, which together act to encase a single germ cell (oocyte) at the core. The overall size of the follicle and the number of cells composing the thecal and granulosa cell compartments is dependent on the stage of follicle maturation. The corpus luteum is a clearly defined and vascularized structure formed from terminally differentiated thecal and granulosa cells after follicle rupture. Finally, the interstitium is composed of fibroblast like cells that are recruited to form the thecal or granulosa units of the follicle as well as secondary thecal cells derived from atretic follicles and regressed corpora lutea.
The luteal phase that follows is marked by secretion of large amounts of progesterone and estradiol that are necessary for the maintenance of pregnancy. During the follicular phase, follicles are categorized based on size, gonadotropin responsiveness and steroidogenic capacity. The quiescent primordial follicles compose the most prevalent stage at any one time and provide the pool from which follicles will be selected for maturation. Primordial follicles are characterized by an ooctye arrested at the diplotene stage of the first meiotic division surrounded by a single layer of cuboidal granulosa cells. Recruitment of primordial follicles toward the primary follicle stage marks the beginning of the follicular phase although the factors involved are not well understood. Henceforth, each stage is characterized by dramatic changes in the structure and functional capabilities of the follicle (57-61). Follicle-stimulating hormone (FSH) is the principal stimulatory factor for progression towards the secondary or pre-antral stage, which is marked by a dramatic increase in oocyte size, granulosa cell proliferation to form several concentric layers around the oocyte, and formation of a surrounding thecal cell layer (57). A basement membrane separates the vascularized thecal layer from the granulosa cells and ovum, making the latter two dependent on the passive movement of hormonal factors (57). As the follicle continues to mature, oocyte growth ceases and granulosa cell proliferation slows but the follicle continues to enlarge due to the formation of a fluid-filled antrum. The large antral follicle then acquires expression of the luteinizing hormone receptor (LH-R) allowing it to respond to a large bolus of luteinizing hormone (LH) from the anterior pituitary, which causes ovulation and hence terminates the follicular phase and begins the luteal phase of the ovarian cycle by directing the formation of a corpus luteum at each site of follicle rupture (62).
necessary for the synthesis of androstenedione from cholesterol. This requires the successive enzymatic actions of cytochrome P450-side-chain cleavage enzyme (P450scc),
3b-hydroxysteroid dehydrogenase/D5-D4-isomerase and cytochrome P450 17a
-hydroxylase/C17-20-lyase (P45017a) (57). In turn, granulosa cells constitutively express
FSH-receptor (FSH-R) and respond to FSH by up-regulating the components necessary for conversion of androstenedione to estradiol. This requires the successive enzymatic actions of cytochrome P450-aromatase and 17b-hydroxysteroid-dehydrogenase type I. Granulosa cells lack P45017a activity and are therefore dependent upon the thecum for the
androgen precursors necessary for estradiol synthesis. Ultimately, enormous amounts of estradiol are released into the antral fluid and diffuse back through the basement membrane to enter the general circulation. Recently, the “two-cell, two-gonadotropin” paradigm has been challenged to incorporate a role for the oocyte in regulating granulosa cell steroidogenesis as data indicate that oocyte-derived secretions are inhibitory to estradiol synthesis (63-65).
1.3 Intraovarian role of estrogen signaling
involved remains unclear. ERa and ERb are expressed in a distinct pattern in the mammalian ovary that is conserved among several species. E Rb transcripts and immunoreactivity are predominantly localized to the granulosa cells of growing follicles, whereas ERa appears limited to the interstitium and thecal cells (3, 14, 53, 85). During luteinization, ERb is rapidly decreased in the terminally differentiated somatic cells (14, 86) whereas ERa levels increase in the corpus luteum (87).
1.4 Neuroendocrine role of estrogen signaling in ovarian function
The neuroendocrine system undergoes a process of sexual differentiation and maturation that is analogous to the reproductive tract. Sexual differentiation of the neuroendocrine system is best illustrated by the unique ability of the female hypothalamus to induce an LH surge in response to a rise in serum estradiol (88, 89). Sexual maturation of the neuroendocrine system may be defined as the acquisition of pituitary responsiveness to hypothalamic factors and ovarian steroids and the onset of steroid induced sexual behavior. Sex steroid hormone receptors are widely distributed throughout regions of the brain (90, 91) and the heterogeneous cell types of the pituitary (92), indicating each is a target of steroid hormone action. The organizational effects of steroids on the developing brain are permanent and fix the system’s sensitivity to the
activational effects of steroids during adulthood (93).
pituitary via the pulsatile secretion of gonadotropin-releasing hormone (GnRH), which flows by means of the portal vessels to the anterior pituitary and stimulates the gonadotrophs via the cell-surface GnRH-receptor to synthesize and secrete FSH and LH. The gonadotropins are dimers of a common a-glycoprotein subunit (Cga) and a distinct b-subunit, either LHb (Lhb) or FSHb (Fshb) that confers specificity to the dimeric hormone (95). As described above, the actions of FSH and LH are critical to folliculogenesis and estradiol production via the “two-cell, two-gonadotropin” paradigm in the ovary. As follicles in the ovary approach ovulation, rising levels of ovarian-derived estradiol and peptide hormones feedback upon the hypothalamus and pituitary to negatively regulate further gonadotropin secretion (96). This series of endocrine feedback loops is collectively termed the hypothalamic-pituitary-gonadal (HPG) axis and is critical to the maintenance of gonadal homeostasis in both mammalian sexes. However, a poorly understood sexual dimorphism in the female hypothalamus and pituitary provides for a transient switch to a positive response to rising estradiol levels which causes the release of an enormous LH surge that stimulates the ovary to ovulate and is the hallmark of the female reproductive cycle. The pulsatile nature of GnRH secretion from the hypothalamus and hence gonadotropin secretion from the anterior pituitary is critical to proper function of their respective target tissues. Constant stimulation of the anterior pituitary with GnRH leads to a refractory state and gonadotropin secretion is decreased significantly. In turn, chronic gonadotropin stimulation of the ovary is detrimental to folliculogenesis and is the focus of the studies herein.
feedback, although this may not be true in all species (95-97, 100). In the rodent, castration is principally thought to cause an increased GnRH pulse frequency that then manifests as increased plasma gonadotropins (95, 101-104). Mice possessing a transgenic reporter gene under the regulation of a Cga or Lhb gene promoter fragment exhibit increased reporter activity after ovariectomy in the anterior pituitary that is reduced by estradiol despite the absence of ERa binding in the transgenic promoter (105). Furthermore, the post-ovariectomy rise in reporter activity in the anterior pituitary is prevented by administration of a GnRH antagonist indicating the primary effect of ovariectomy is dysregulation of hypothalamic GnRH secretion.
1.5 Review of ovarian phenotypes in the estrogen receptor null mice
A detailed description of the targeting scheme employed to disrupt the mouse Esr1
and Esr2 genes can be found in the initial descriptions of the aERKO (109) and bERKO mice (110). To generate the aERKO, a 1.8 kb insert possessing the gene for neomycin
(neo) resistance under control of the phosphoglycerate kinase (PGK) promoter and
including a PGK-polyadenylation signal was inserted into the Not I site of an Esr1 exon 2 clone from a genomic 129/J mouse library. The insert was placed into a replacement W -type targeting vector (111) with the appropriate Esr1 flanking sequences. Upon homologous recombination in mouse embryonic stem cells (129/J), the neo insert was inserted approximately 270 bp downstream of the Esr1 translation start site and thereby inhibits ERa protein synthesis. To generate the bERKO, a genomic clone possessing the first three exons of the mouse Esr2 gene was selected from a 129/SvJ mouse library. A replacement W-type targeting vector was generated to include Esr2 5’ and 3’ flanking sequences and a PGK promoter-regulated neo gene inserted in the reverse orientation in the Pst I site of exon 3. Homologous recombination of the Esr2 gene resulted in disruption of the sequences coding for the first zinc finger of the ERb protein. For both lines, standard protocols of clone selection and blastocyst (C57BL/6J) injection were used to generate chimeric mice possessing the respective disrupted gene. Breeding of mice heterozygous for the respective gene disruption results in a Mendelian distribution of genotypes and a balanced sex ratio in both ERKO lines (109, 110).
gonadotropin secretion may begin prematurely in females lacking ERa. The most striking ovarian phenotypes in each ERKO line become obvious shortly after sexual maturity. aERKO females are anovulatory and exhibit a distinct ovarian phenotype of enlarged, hemorrhagic and cystic follicles; increased Fshr and Lhcgr expression; and elevated estradiol synthesis. The cystic follicles accumulate over time and generate an ovary that grossly resembles a bundle of grapes. This signature phenotype of the aERKO female is 100% penetrant. Thecal cells of the aERKO cystic follicles are hypertrophied, suggesting that the LH response is preserved in the absence of ERa. Furthermore, growing follicles in the pre- to small antral stage are present in aERKO ovaries indicating that ERa is not required for the recruitment of primordial follicles and the initial stages of the follicular phase of the ovarian cycle. Nonetheless, an evaluation of numerous aERKO ovaries reveals the complete absence of corpora lutea, indicating an inability to spontaneously ovulate and therefore a primary cause of infertility.
Adult bERKO females exhibit grossly normal ovaries but are sub-fertile during continuous mating, as evidenced by the production of fewer litters and less pups per litter compared to wild-type (110). Sexually mature bERKO females exhibit ovaries with a relatively normal interstitial compartment and follicles at progressive stages of the follicular phase. Therefore, the functions of ERb are not essential for the establishment of germ cell number or follicle development in the ovary. Several of the speculated intraovarian roles of estradiol discussed above, including a postulated role in granulosa cell proliferation (69-72, 82), are not overtly impaired in the bERKO ovary. However, there are indications of increased follicular atresia and a paucity of corpora lutea in the bERKO ovary when compared to the wild-type (110), suggesting that subfertility may be due to a reduced ovulatory frequency.
around 20 d of age. Also similar to the aERKO is the lack of corpora lutea or any indication of spontaneous ovulation in the abERKO ovary. However, there are two phenotypes that distinguish the abERKO ovary from the others. First, abERKO ovaries fail to exhibit the hemorrhagic and cystic follicles characteristic of the aERKO; and secondly, abERKO ovaries exhibit follicle “ghosts” that lack any evidence of a germ cell but possess intra-luminal somatic cells that are Sertoli-like in appearance (112). Along with the appearance of these Sertoli-like cells is an increased expression of the Sox9 gene, a transcription factor that is known to be essential for Sertoli-cell development in the fetal testis. This phenotype of “sex-reversal” is especially unique to the abERKO ovary because it appears to commence post-pubertally (112).
Anovulation and compromised folliculogenesis similar to that observed in ERKO females is described in several “knock-out” lines, including mice null for Cyp19 (113-115),
Fshb (116), Fshr (117), Pgr (118), Ptgs2 (119), Gdf9 (120), Igf1 (121), Ccnd2 (122) and
Cnx37 (123); indicating that the process of folliculogenesis and successful ovulation is extremely complex and dependent upon the synchronous actions of both intra- and extraovarian physiological systems.
1.6Hypotheses
Chapter 2. Prevention of the polycystic ovarian phenotype and characterization of
ovulatory capacity in the estrogen receptor-a knockout mouse. The aERKO ovarian
and cystic follicles are not apparent in females prior to 35 d of age and in fact the ovaries of young aERKO females appear relatively normal. Secondly, J. H. Nilson and colleagues were concurrently describing a transgenic mouse they generated with the specific intent of increasing plasma LH levels, the LHbCTP line (124, 125). The ovarian phenotypes of the LHbCTP females are strikingly similar to those of the aERKO in every aspect described for up to this point. Therefore, under the assumption that LHbCTP ovaries still possess normal ERa expression yet exhibit ovarian phenotypes indistinguishable from those of ERa-null females, I hypothesized the following: the cystic and hemorrhagic follicles in the aERKO ovary are secondary to the chronically elevated LH that results
from the loss of ERa functions in the hypothalamus and pituitary and not within the ovary
itself. Chapter 2 describes the finding that adult aERKO females do indeed possess
plasma LH levels that are comparable to the LHbCTP mouse and that a reduction in plasma LH in the aERKO via treatments with a GnRH-antagonist totally prevent the hemorrhagic and cystic ovarian phenotype. The effectiveness of a GnRH-antagonist to “rescue” plasma LH levels in the aERKO female was equally important and should not be disregarded as it strongly indicates the hypothalamus and not the pituitary as the primary site of estradiol mediated negative feedback and that this action is ERa -dependent. Consequent to this first set of experiments was a second hypothesis: aERKO ovaries will respond normally to superovulation with a controlled dose of gonadotropins
if administered prior to the onset of the LH-induced hemorrhagic and cystic phenotype.
Chapter 3. Characterization of the hypothalamic-pituitary-gonadal (HPG) axis in
estrogen receptor null mice reveals hypergonadism and endocrine sex-reversal in females
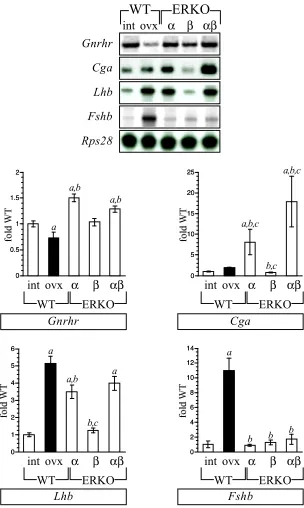
lacking ERa but not ERb. Chapter 3 is the first introduction of the bERKO and abERKO mice to these studies as these models were not available earlier for extensive experimentation. Much of the work presented in this chapter is descriptive rather than experimental. I felt it was absolutely imperative to determine and compare the reproductive endocrine milieu in each of the individual ERKO lines before forming hypotheses concerning the respective causes or designing the necessary experiments to address the ovarian phenotypes in each. Initially, a comparative evaluation of plasma gonadotropin and gonadal steroid levels among age-matched females from all three ERKO lines was completed. Secondly, gene expression assays for the components most relevant to determining the reproductive endocrine milieu were designed to include evaluation of the GnRH-receptor and gonadotropin subunits in the pituitary, and the enzymatic components of the “two-cell, two-gonadotropin” paradigm of estradiol synthesis in the ovary. Overall, aERKO and abERKO females exhibit elevated expression of the LH subunits in the pituitary and correlating increases in plasma LH. This was the first description of aERKO-like elevated LH in abERKO females and was especially intriguing since abERKO ovaries do not exhibit hemorrhagic and cystic follicles. bERKO females exhibit a normal profile of plasma gonadotropin and subunit gene expression. Also, plasma FSH and pituitary Fshb expression are normal in all three ERKO lines. Evaluation of the steroidogenic capacity of the ERKO ovaries indicated increased enzyme expression in the aERKO ovary only and correlating increases in plasma androstenedione and estradiol, the respective products of thecal and granulosa cells. These latter studies were the first to indicate that neither ERa nor ERb are critical to the positive regulation of steroidogenic enzyme expression in the ovary.
of the ovary, now known to manifest as increased steroidogenesis as well as cystic follicles. This indeed proved to be true and indicated that elevated androstenedione and estradiol synthesis by the aERKO ovary was more attributable to the loss of ERa in the hypothalamus. An especially novel discovery was that aERKO and abERKO females possessed extraordinarily high levels of plasma testosterone. Although the “cell,
two-gonadotropin” paradigm of steroidogenesis in the ovary does not provide for significant
testosterone synthesis, the murine form of the enzyme 17b-HSD I, which normally converts estrone to estradiol in the granulosa cells is reportedly able to convert androstenedione to testosterone (126). Having shown that aERKO females possess 3-fold higher than normal plasma androstenedione levels, I hypothesized the following: the abnormal capacity of the ERa-null ovary to synthesize testosterone is primarily due to
excessive LH-induction of CYP17 activity and subsequent androstenedione synthesis. As
described in chapter 3, this hypothesis proved to be false. Instead, it was found that aERKO and abERKO ovaries express unusually high levels of the enzyme 17b-HSD III, which also converts androstenedione to testosterone but is considered unique to the Leydig cells of the male testis. Furthermore, ectopic Hsd17b3 expression in aERKO ovaries is LH-dependent. This finding represented a second illustration of “sex-reversal” in the abERKO ovary but perhaps more importantly, the first such description in ovaries lacking ERa only.
Chapter 4. Female mice lacking estrogen receptor-b exhibit an attenuated response to
chronically elevated luteinizing hormone. At least two aspects of the ERKO ovarian
ERb may play a role in the manifestations of elevated LH in the aERKO ovary. Soon after, two additional observations provided compelling support that ERb may indeed be involved in LH-signaling within the ovary. First, bERKO females do not properly respond to human choriogonadotropin (hCG), an LH-analog, when used to superovulate the ovary (110); and second, abERKO females possess elevated plasma LH levels that surpass aERKO females yet do not exhibit cystic and hemorrhagic follicles (this was shown in chapter 3 studies (112, 127). These observations culminated in the following hypothesis: the intraovarian actions of ERb are involved in the aberrant phenotypes that occur during LH-hyperstimulation of the ovary. In order to experimentally test this hypothesis it was necessary to generate female mice lacking ERb but possessing chronically elevated plasma LH. Because LH has an extremely transient half-life in plasma, it would be quite difficult to mimic the aERKO levels in the bERKO with exogenous hormone treatments. Therefore, I felt the most effective approach was to cross the bERKO and the earlier described LHbCTP mice to generate a bERKOLHCTP line
of females. This chapter thoroughly describes the ovarian phenotypes present in these females. Two particularly outstanding observations were made, the first being that the LH-induced cystic and hemorrhagic follicles of the aERKO and LHbCTP do not occur in ovaries lacking functional ERb. Secondly, implied in the above hypothesis and based on observations in the aERKO is that ectopic Hsd17b3 expression in the mouse ovary is the consequence of LH-hyperstimulation and therefore should be observed in the LHbCTP
and bERKOLHCTP ovaries as well. However, a comparative analysis of all genotypes
1.7 References cited
1. Knauer E 1900 Die ovarientransplantation. Arch Gynakol 60:322-376
2. Marshall FHA, Jolly WA 1905 Contributions to the physiology of mammalian reproduction. Phil Trans Roy Soc B 198:99-142
3. Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925-5930
4. Mosselman S, Polman J, Dijkema R 1996 ERb: identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49-53
5. Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V 1997 Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor b. Mol Endocrinol 11:353-365
6. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835-839
7. White R, Lees JA, Needham M, Ham J, Parker M 1987 Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol 1:735-744
8. Keaveney M, Klug J, Gannon F 1992 Sequence analysis of the 5' flanking region of the human estrogen receptor gene. J DNA Seq Map 2:347-358
9. Grandien K, Berkenstam A, Gustafsson J-Å 1997 The estrogen receptor gene: promoter organization and expression. Inter J Biochem Cell Biol 29:1343-1369 10. Fasco MJ 1998 Estrogen receptor mRNA splice variants produced from the distal
and proximal promoter transcripts. Mol Cell Endocrinol 138:51-59
11. Miksicek RJ 1994 Steroid receptor variants and their potential role in cancer. Sem Cancer Biol 5:369-379
12. Murphy LC, Dotzlaw H, Leygue E, Douglas D, Coutts A, Watson PH 1997 Estrogen receptor variants and mutations. J Steroid Biochem Mol Bio 62:363-372 13. Sluyser M 1995 Mutations in the estrogen receptor gene. Human Mut 6:97-103 14. Byers M, Kuiper GG, Gustafsson J-Å, Park-Sarge OK 1997 Estrogen receptor-b
mRNA expression in rat ovary: down-regulation by gonadotropins. Mol Endocrinol 11:172-82
15. Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson J-Å 1997 Human estrogen receptor-b gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 82:4258-65
16. Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA 1998 Identification of estrogen receptor b2, a functional variant of estrogen receptor b expressed in normal rat tissues. Endocrinology 139:1082-1092
17. Chu S, Fuller PJ 1997 Identification of a splice variant of the rat estrogen receptor b gene. Mol Cell Endocrinol 132:195-199
19. Parker MG 1995 Structure and function of estrogen receptors. Vitam Horm 51:267-287
20. White R, Parker MG 1998 Molecular mechanisms of steroid hormone action. Endocr Rel Cancer 5:1-14
21. Weigel NL, Zhang Y 1998 Ligand-independent activation of steroid hormone receptors. J Mol Med 76:469-479
22. Tsai M-J, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451-486
23. Webb P, Lopez GN, Uht R, Kushner PJ 1995 Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin of the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443-456
24. Sukovich DA, Mukherjee R, Benfield PA 1994 A novel cell-specific mechanism for estrogen receptor mediated gene activation in the absence of an estrogen-responsive element. Mol Cell Biol 14:7134-7143
25. Ponglikitmongkol M, Green S, Chambon P 1988 Genomic organization of the human oestrogen receptor gene. EMBO J 7:3385-3388
26. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-Å 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors a and b. Endocrinology 138:863-70
27. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA 1998 Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139:4252-63
28. Montano MM, Muller V, Trobaugh A, Katzenellenbogen BS 1995 The carboxy-terminal F domain of the human estrogen receptor: role in the transcriptional activity of the receptor and the effectiveness of antiestrogens as estrogen antagonists. Mol Endocrinol 9:814-825
29. Peters GA, Khan SA 1999 Estrogen receptor domains E and F: role in dimerization and interaction with coactivator RIP-140. Mol Endocrinol 13:286-296
30. Picard D, Kumar V, Chambon P, Yamamoto KR 1990 Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul 1:291-299
31. Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P 1992 Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J 11:1-14
32. Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai M-J, O'Malley BW 1997 Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Rec Prog Horm Res 52:141-165
33. Giguere V, Tremblay A, Tremblay GB 1998 Estrogen receptor b: re-evaluation of estrogen and antiestrogen signaling. Steroids 63:335-339
35. Paech K, Webb P, Kuiper GGJM, Nilsson S, Gustafsson J-Å, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERa and ERb at AP1 sites. Science 277:1508-1510
36. Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS 1999 Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-a or estrogen receptor-b. Endocrinology 140:800-4
37. Pettersson K, Grandien K, Kuiper GGJM, Gustafsson J-Å 1997 Mouse estrogen receptor b forms estrogen response element binding heterodimers with estrogen receptor a. Mol Endocrinol 11:1486-1496
38. Cowley SM, Hoare S, Mosselman S, Parker MG 1997 Estrogen receptors a and b form heterodimers on DNA. J Biol Chem 272:19858-19862
39. Ogawa S, Inoue S, Orimo A, Hosoi T, Ouchi Y, Muramatsu M 1998 Cross-inhibition of both estrogen receptor a and b pathways by each dominant negative mutant. FEBS Lett 423:129-132
40. Couse JF, Lindzey J, Grandien K, Gustafsson J-Å, Korach KS 1997 Tissue distribution and quantitative analysis of estrogen receptor-a (ERa) and estrogen receptor-b (ERb) messenger ribonucleic acid in the wild-type and ERa-knockout mouse. Endocrinology 138:4613-4621
41. Brandenberger AW, Tee MK, Jaffe RB 1998 Estrogen receptor alpha (ER-a) and beta (ER-b) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-b in neoplastic tissues. J Clin Endocrinol Metab 83:1025-8
42. Arts J, Kuiper GGJM, Janssen JMMF, Gustafsson J-Å, Lowik CWGM, Pols HAP, Van Leeuwen JPTM 1997 Differential expression of estrogen receptors a and b mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology 138:5067-5069
43. Pau CY, Pau K-YF, Spies HG 1998 Putative estrogen receptor b and a mRNA expression in male and female rhesus maques. Mol Cell Endocrinol 146:59-68
44. Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388:507-25
45. Wilson ME, Price Jr. RH, Handa RJ 1998 Estrogen receptor-b messenger ribonucleic acid expression in the pituitary gland. Endocrinology 139:5151-5156
46. Mitchner NA, Garlick C, Ben-Jonathan N 1998 Cellular distribution and gene regulation of estrogen receptors a and b in the rat pituitary gland. Endocrinology 139:3976-3983
47. Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws Jr. ER 1998 Selective expression of estrogen receptor a and b isoforms in human pituitary tumors. J Clin Endocrinol Metab 83:3965-3972
48. Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su J-L, Kliewer SA, Lehmann J, Willson TM 1998 Cloning and characterization of human estrogen receptor b isoforms. Biochem Biophys Res Comm 247:75-78
50. Dotzlaw H, Leygue E, Watson PH, Murphy LC 1996 Expression of estrogen receptor-beta in human breast tumors. J Clin Endocrinol Metab 827:2371-2374 51. Dotzlaw H, Leygue E, Watson PH, Murphy LC 1999 Estrogen receptor-b
messenger RNA expression in human breast tumor biopsies: relationship to steroid receptor status and regulation by progestins. Cancer Res 59:529-532
52. Rosenfeld CS, Yuan X, Manikkam M, Calder MD, Garverick HA, Lubahn DB 1999 Cloning, sequencing, and localization of bovine estrogen receptor-b within the ovarian follicle. Biol Reprod 60:691-7
53. Sar M, Welsch F 1999 Differential expression of estrogen receptor-b and estrogen receptor-a in the rat ovary. Endocrinology 140:963-971
54. Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, Tsutsumi O, Taketani Y, Muramatsu M 1999 Differential immunolocalization of estrogen receptor a and b in rat ovary and uterus. J Mol Endocrinol 22:37-44
55. Shughrue PJ, Scrimo PJ, Merchenthaler I 1998 Evidence for the colocalization of estrogen receptor-b mRNA and estrogen receptor-a immunoreactivity in neurons of the rat forebrain. Endocrinology 139:5267-5270
56. Baird DT 1984 The ovary. In: Austin CR, Short RV (eds) Reproduction in
Mammals: Hormonal Control of Reproduction. Cambrige University Press,
Cambridge, pp 91-114
57. Carr BR 1998 Disorders of the ovaries and female reproductive tract. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds) Williams Textbook of Endocrinology, 9th ed. W. B. Saunders Company, Philadelphia, pp 751-817
58. Richards JS 1980 Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev 60:51-89
59. Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725-751
60. Magoffin DA 1991 Regulation of differentiated functions in ovarian theca cells. Sem Reprod Endocrinol 9:321-331
61. Picton H, Briggs D, Gosden R 1998 The molecular basis of oocyte growth and development. Mol Cell Endocrinol 145:27-37
62. Robker RL, Richards JS 1998 Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod 59:476-482
63. Vanderhyden BC, Cohen JN, Morley P 1993 Mouse oocytes regulate granulosa cell steroidogenesis. Endocrinology 133:423-426
64. Vanderhyden BC, Macdonald EA 1998 Mouse oocytes regulate granulosa cell steroidogenesis throughout follicular development. Biol Reprod 59:1296-1301
65. Vanderhyden BC, Tonary AM 1995 Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by a factor(s) secreted by the ooctye. Biol Reprod 53:1243-1250
66. Pencharz RI 1940 Effect of estrogens and androgens alone and in combination with chorionic gonadotropin on the ovary of the hypophysectomized rat. Science 91:554-555
68. Richards JS 1975 Estradiol receptor content in rat granulosa cells during follicular development: modification by estradiol and gonadotropins. Endocrinology 97:1174-1184
69. Rao MC, Midgley ARJ, Richards JS 1978 Hormonal regulation of ovarian cellular proliferation. Cell 14:71-78
70. Goldenberg RL, Vaitukaitis JL, Ross GT 1972 Estrogen and follicle stimulation hormone interactions on follicle growth in rats. Endocrinology 90:1492-1498
71. Bley MA, Saragueta PE, Baranao JL 1997 Concerted stimulation of rat granulosa cell deoxyribonucleic acid synthesis by sex steroids and follicle-stimulating hormone. J Steroid Biochem Mol Biol 62:11-19
72. Reilly CM, Cannady WE, Mahesh VB, Stopper VS, De Sevilla LM, Mills TM 1996 Duration of estrogen exposure prior to follicle-stimulating hormone stimulation is critical to granulosa cell growth and differentiation in rats. Biol Reprod 54:1336-42 73. Burghardt RC, Anderson E 1981 Hormonal modulation of gap junctions in rat
ovarian follicles. Cell Tissue Res 214:181-193
74. Hernandez ER, Roberts CT, Jr., LeRoith D, Adashi EY 1989 Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5'-untranslated mRNA variant representation and hormonal regulation. Endocrinology 125:572-4 75. Kaipia A, Hsueh AJ 1997 Regulation of ovarian follicle atresia. Annu Rev Physiol
59:349-363
76. Hsueh AJW, Billig H, Tsafriri A 1994 Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 15:707-724
77. Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley ARJ, Reichert LEJ 1976 Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology 99:1562-1570 78. Tonetta SA, diZerga GS 1989 Intragonadal regulation of follicular maturation. Endocr
Rev 10:205-229
79. Tonetta SA, Ireland JJ 1984 Effect of cyanoketone on follicle-stimulating hormone (FSH) induction of receptors for FSH in granulosa cells of the rat. Biol Reprod 31:487-493
80. Wang XN, Greenwald GS 1993 Hypophysectomy of the cyclic mouse. II. Effects of follicle-stimulating hormone (FSH) and luteinizing hormone on folliculogenesis, FSH and human chorionic gonadotropin receptors, and steroidogenesis. Biol Reprod 48:595-605
81. Wang XN, Greenwald GS 1993 Synergistic effects of steroids with FSH on folliculogenesis, steroidogenesis and FSH- and hCG-receptors in hypophysectomized mice. J Reprod Fertil 99:403-413
82. Kessel B, Liu YX, Jia XC, Hsueh AJ 1985 Autocrine role of estrogens in the augmentation of luteinizing hormone receptor formation in cultured rat granulosa cells. Biol Reprod 32:1038-1050
83. Farookhi R, Desjardins J 1986 Luteinizing hormone receptor induction in dispersed granulosa cells requires estrogen. Mol Cell Endocrinol 47:13-24
85. Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS 1999 Targeted disruption of the estrogen receptor-a gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140:2733-44
86. Misao R, Nakanishi Y, Sun WS, Fujimoto J, Iwagaki S, Hirose R, Tamaya T 1999 Expression of the oestrogen receptor a and b mRNA in corpus luteum of human subjects. Mol Hum Reprod 5:17-21
87. Frasor J, Gibori G 2003 Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab 14:118-23
88. MacLusky NJ, Leiberburg I, McEwen BS 1979 Development of steroid receptor systems in the rodent brain. In: Hamilton TH, Clark JH, Sadler WA (eds) Ontogeny
of Receptors and Reproductive Hormone Action. Raven Press, New York, pp
393-402
89. McEwen BS 1992 Effects of the steroid/thyroid hormone family on neural and behavioral plasticity. In: Nemeroff CB (ed) Neuroendocrinology. CRC Press, Boca Raton, pp 333-351
90. McEwen BS 1992 Steroid Hormones: effect on brain development and function. Horm Res 37 (suppl 3):1-10
91. Blaustein JD 1994 Estrogen receptors in neurons: new subcellular locations and functional implications. Endocrine 2:249-258
92. Stefaneanu L 1997 Pituitary sex steroid receptors: localization and function. Endocr Path 8:91-108
93. Kawata M 1995 Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res 24:1-46
94. Karsch FJ 1984 The hypothalamus and anterior pituitary gland. In: Austin CR, Short RV (eds) Reproduction in Mammals: Hormonal Control of Reproduction.
Cambridge University Press, Cambridge, pp 1-20
95. Gharib SD, Wierman ME, Shupnik MA, Chin WW 1990 Molecular biology of the pituitary gonadotropins. Endocr Rev 11:177-199
96. Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279-286
97. Haisenleder DJ, Dalkin AC, Marshall JC 1994 Regulation of gonadotropin gene expression. In: Knobil E, Neill JD (eds) The Physiology of Reproduction, Second Edition ed. Raven Press, Ltd., New York, pp 1793-1813
98. Fink G 1988 Gonadotropin secretion and its control. In: Knobil E, Neill JD (eds) The
Physiology of Reproduction. Raven Press, New York, pp 1349-1377
99. Yamamoto M, Diebell ND, Bogdanove EM 1970 Analysis of initial and delayed effects of orchidectomy and ovariectomy on pituitary LH levels in adult and immature rats. Endocrinology 86:1102-1111
100. Shupnik MA 1996 Gonadal hormone feedback on pituitary gonadotropin genes. TEM 7:272-276
102. Levine JE, Ramirez VD 1982 LHRH release during the rat estrous cycle and after ovariectomy, as estimated by push-pull cannulae. Endocrinology 111:1439-1448 103. Rodin DA, Lalloz MR, Clayton RN 1989 Gonadotropin-releasing hormone regulates
follicle-stimulating hormone beta-subunit gene expression in the male rat. Endocrinology 125:1282-9
104. Lalloz MRA, Detta A, Clayton RN 1988 Gonadotropin-releasing hormone is required for enhanced luteinizing hormone subunit gene expression in vivo. Endocrinology 122:1681-1688
105. Keri RA, Wolfe MW, Sauders TL, Anderson I, Kendall SK, Wagner T, Yeung J, Gorski J, Nett TM, Camper SA, Nilson JH 1994 The proximal promoter of the bovine luteinizing hormone b-subunit gene confers gonadotrope-specific expression and regulation by gonadotropin-releasing hormone, testosterone, and 17 b-estradiol in transgenic mice. Mol Endocrinol 8:1807-1816
106. Stefaneanu L, Kovacs K, Horvath E, Lloyd RV, Buchfelder M, Fahlbusch R, Smyth H 1994 In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab 78:83-88
107. Sar M, Parikh I 1986 Immunohistochemical localization of estrogen receptor in the brain, pituitary and uterus with monoclonal antibodies. J Steroid Biochem 24:497-503
108. Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292-308.
109. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162-11166
110. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677-15682
111. Bronson SK, Smithies O 1994 Altering mice by homologous recombination using embyronic stem cells. J Biol Chem 269:27155-27158
112. Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS 1999 Postnatal sex reversal of the ovaries in mice lacking estrogen receptors a and b. Science 286:2328-31
113. Fisher CR, Graves KH, Parlow AF, Simpson ER 1998 Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965-6970
114. Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK 2000 An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology 141:2614-23.
115. Britt KL, Kerr J, O'Donnell L, Jones MEE, Drummond AE, Davis SR, Simpson ER, Findley JK 2002 Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 16:1389-1397
117. Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P 1998 Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A 95:13612-7
118. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CAJ, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266-2278
119. Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R 1999 Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology 140:2685-95
120. Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM 1999 Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 13:1018-34
121. Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstatiadis A 1996 Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10:903-918
122. Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA 1996 Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470-474
123. Simon AM, Goodenough DA, Li E, Paul DL 1997 Female infertility in mice lacking connexin 37. Nature 385:525-529
124. Nilson JH, Abbud RA, Keri RA, Quirk CC 2000 Chronic hypersecretion of luteinizing hormone in transgenic mice disrupts both ovarian and pituitary function, with some effects modified by the genetic background. Rec Prog Horm Res 55:69-89; discussion 89-91
125. Mann RJ, Keri RA, Nilson JH 2003 Consequences of elevated luteinizing hormone on diverse physiological systems: use of the LH bCTP transgenic mouse as a model of ovarian hyperstimulation-induced pathophysiology. Rec Prog Horm Res 58:343-75
126. Nokelainen P, Puranen T, Peltoketo H, Orava M, Vihko P, Vihko R 1996 Molecular cloning of mouse 17b-hydroxysteroid dehydrogenase type 1 and characterization of enzyme activity. Eur J Biochem 236:482-90
2. PREVENTION OF THE POLYCYSTIC OVARIAN PHENOTYPE AND CHARACTERIZATION OF OVULATORY CAPACITY IN THE ESTROGEN RECEPTOR-a KNOCKOUT MOUSE
2.1 Abstract
ribonuclease protection assays to assess the mRNA levels of several markers of follicular maturation and ovulation, including Esr2 (ERb), Lhcgr (LH-receptor), Ccnd2 (cyclin-D2), Cyp11a (P450-side chain cleavage enzyme), Ptgs2 (prostaglandin synthase-2) and
Pgr (progesterone receptor). No marked differences in the expression pattern for these genes during the superovulation regimen were observed in the immature aERKO ovary compared to that of the wild-type. Serum progesterone levels just prior to ovulation were slightly lower in the aERKO compared to wild-type. These studies indicate that treatment of aERKO females with a GnRH antagonist decreased the serum LH levels to within the wild-type range and concurrently prevented development of the characteristic ovarian phenotype of cystic and hemorrhagic follicles. Furthermore, a lack of functional ERa within the ovary had no effect on the regulation of several genes required for follicular maturation and ovulation. However, the reduced numbers of ovulations following the administration of exogenous gonadotropins in the aERKO suggests an intraovarian role for ERa in follicular development and ovulation.
2.2 Introduction
the anterior pituitary are at least partially regulated by gonadal steroids via classical feedback mechanisms acting at both hypothalamic and pituitary sites (18). Several studies have demonstrated the ability of estradiol to suppress transcription of the gonadotropin subunit genes as well as the synthesis and secretion of the dimeric hormones (18). Therefore, ovarian function depends on a multitude of endocrine and local actions of estradiol that act in concert with the pituitary gonadotropins to provide for proper steroid production and folliculogenesis.
The majority of documented biological actions of estradiol are mediated via the estrogen receptor (ER), a class I member of the thyroid/steroid hormone receptor superfamily of ligand-inducible transcription factors (19). Previous studies have reported the presence of high-affinity estrogen binding sites in the ovary of the rodent and other species (3, 20, 21). However, two forms of nuclear ER are now known to exist, the well-described ERa and the newly discovered ERb. Several studies have demonstrated the presence of the respective mRNAs for both ERa and ERb in ovaries of the mouse (22, 23), rat (24, 25), cow (26), and human (27, 28). Furthermore, studies employing in situ
hybridization (24, 25) and immunohistochemistry (26, 29) indicate a distinct expression pattern for the two ERs in the ovary, in which ERa is highly expressed in the interstitial/thecal compartment whereas ERb is confined to the granulosa cells of growing follicles.
The conclusions of several past studies concerning the role of ERa in the adult female were confirmed in the initial descriptions of the aERKO mouse, including estrogen insensitivity of the reproductive tract (30, 31) and hypothalamic-pituitary axis (32). The aERKO females exhibit ovaries characterized by the presence of multiple hemorrhagic and cystic follicles with no evidence of spontaneous ovulation (30, 33). This phenotype occurs despite a relatively normal expression pattern for the ERb gene in ovaries of aERKO mice (23, 33). In aERKO females, disruption of the negative feedback actions of estradiol in the hypothalamic-pituitary axis results in elevated levels of gonadotropin subunit mRNAs in the pituitary (32) and serum luteinizing hormone (LH) (34). Therefore, the aERKO ovarian phenotype may be the result of a lack of ERa-mediated action either within the ovary and/or at the level of the hypothalamic-pituitary axis. Herein, we describe studies demonstrating the prevention of the adult aERKO ovarian phenotype when pituitary influence is reduced through the use of a gonadotropin-releasing hormone (GnRH) antagonist. We also describe the ability of the immature aERKO to ovulate and exhibit a relatively normal regulation of several genes critical to folliculogenesis and ovulation when stimulated with exogenous gonadotropins.
2.3 Materials and Methods
Gonadotropin-releasing hormone (GnRH) antagonist treatment
maturation and onset of visible aERKO ovarian phenotypes (33) and carried out through to 53 d of age, for a total of 12 treatments. Each group consisted of at least 4 animals/genotype/dose (except for aERKO-vehicle = 3). Body weights were monitored throughout the study with each group showing similar age-related increases regardless of genotype or treatment (body weight (g); Ave. ± SEM) 28 d = 11.9 ± 0.3; 53 d = 18.3 ± 0.2). Animals were sacrificed at 53 days of age, 18-24 h after the final treatment. Serum was processed from whole blood collected from the inferior vena cava. One ovary was immediately snap frozen on dry ice for later RNA extraction and the other was fixed in 10% buffered formalin at 4°C for 6-8 h and then transferred to 70% ethanol at 4°C. For histological analysis, fixed tissues were paraffin embedded, sectioned at 5 mm and stained with hematoxylin and eosin according to standard histological procedures.
Superovulation with exogenous gonadotropins
Superovulation assays were carried out on wild-type and aERKO females at both immature (28 d) and peripubertal (42 d) ages. Each trial (immature = 5; peripubertal = 2) consisted of a single s.c. injection with 2.2 IU pregnant mares’ serum gonadotropin (PMSG) (Sigma) followed 48-52 h later with 3.2 IU human chorionic gonadotropin (hCG) (Sigma). The animals were then sacrificed 16-20 h after the hCG injection and the ovaries and oviduct removed to M-2 medium (Specialty Media, Lavallette, NJ) supplemented with 0.3% hyaluronidase (Sigma). The oocyte/cumulus mass was surgically extracted from the oviduct and the oocytes were counted after enzymatic disassociation from the surrounding cumulus. The ovary/oviduct was then fixed in 10% buffered formalin and prepared for histological analysis as described above.
In vitro fertilization assays