M o d e llin g and C o n tro l o f R e a c tiv e
D is tilla tio n P r o c e s se s
Nicholas C. T. Biller
A thesis subm itted for the degree of D octor of Philosophy of
the University of London
D epartm ent of Chemical Engineering U niversity College London
London W C IE 7JE
ProQuest Number: 10014376
All rights reserved
INFORMATION TO ALL USERS
The quality of this reproduction is dependent upon the quality of the copy submitted.
In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed,
a note will indicate the deletion.
uest.
ProQuest 10014376
Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author.
All rights reserved.
This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC.
ProQuest LLC
789 East Eisenhower Parkway P.O. Box 1346
A b str a c t
R eactive distillation has been applied successfully in in d u stry w here large capital and energy savings have been m ade through th e in tegration of reaction and distillation into one system . O p erating in batch m ode, in either tra y or packed colum ns, offers th e flexibihty required by pharm aceutical and hne chemical industries for producing low volum e/high value p roducts w ith varying specifications. However, regular packed or tra y columns m ay not be suitable for high vacuum operations due to th e pressure drop across th e column section and short p a th distillation m ay be m ore applicable.
T he objective of this thesis is to investigate th e control of reactive distillation in batch colum ns, tra y and packed, and in short p a th colum ns. In order to stu d y control fully, it is necessary to develop rigorous dynam ic models th a t accurately ca p tu re th e process behaviour. T he higher th e degree of rigour, th e m ore accurately th e process conditions and dynam ics are captured. However, m ore rigorous m odels are m ore com putationally expensive to im plem ent and can be prone to num erical errors, introduced for instance during linearisation. Therefore, in this thesis, th e degree of m odelling rigour required for b o th sim ulation and control purposes is explored in detail for tra y and packed batch colum ns and sh o rt-p a th columns.
For b atch tra y colum ns, it is dem o n strated th a t to accurately cap tu re th e change in process conditions, it is necessary to m odel pressure dynam ics and employ a dynam ic energy balance. For packed colum ns, distrib u ted ra te based m odelling is com pared to lum ped equilibrium m odelling and it is found th a t due to th e varying conditions w ithin th e packing, th e efficiency changes, resulting in m ism atch betw een th e two m ethods. T he sh o rt-p a th distillation column which has h ith e rto only been m odelled a t ste a d y -sta te , is m odelled using a dynam ic ra te based m odel, essential for investigating control.
A c k n o w le d g e m e n ts
I would like to th a n k my supervisor, Dr. Eva Sprensen, for her guidance and encouragem ent
th ro u g o u t th e course of this work. I would also like to th a n k th e stu d e n ts and staff, past and present, of th e C o m puter Aided Process Engineering group for th e lively and useful
discussions and th e occasional, b u t necessary, excursions to H untley St. T he financial su p p o rt from th e Engineering and Physical Sciences Research Council and th e C entre for
C o n te n ts
A b s t r a c t 2
A c k n o w l e d g e m e n t s 3
List o f fig u r e s 10
List o f t a b le s 14
1 I n t r o d u c t i o n 15
1.1 M otivation ... 15
1.2 Reactive distillation ... 16
1.3 B atch colum n o p e r a t i o n ... 17
1.3.1 C ontrol of reactive batch d i s t i l l a t i o n ... 18
1.4 S h o rt-p a th colum n o p e r a t i o n ... 19
1.4.1 Falling film e v a p o r a t o r s ... 19
1.4.2 W iped him e v a p o r a t o r s ...20
1.4.3 S h o rt-p a th d i s t i l l a t i o n ... 20
1.5 O bjectives of this w o r k ... 21
1.6 O utline of this t h e s i s ... 22
1.7 M ain c o n tr i b u ti o n s ... 23
C O N T E N T S 5
2 L it e r a t u r e r e v i e w 25
2.1 M odelling of reactive b atch d i s t i ll a t i o n ... 25
2.1.1 In tro d u c tio n ... 25
2.1.2 R eactive batch distillation l i t e r a t u r e ...26
2.1.3 R eactive b atch distillation c o n c lu s io n s ... 40
2.2 M odelling of sh o rt-p a th d i s t i ll a t i o n ...41
2.2.1 In tro d u c tio n ... 41
2.2.2 S h o rt-p a th l i t e r a t u r e ...41
2.2.3 S h o rt-p a th c o n c lu s io n s ... 47
2.3 C o n c lu s io n s ...48
3 M o d e ll in g o f R B D in t r a y c o lu m n s 49 3.1 T ray colum n m o d e llin g ... 49
3.1.1 R igorous m o d e l ...50
3.1.2 A ssum ptions ... 51
3.1.3 In itial c o n d i t i o n s ... 51
3.1.4 In te g ratio n ... 52
3.1.5 Simplified m o d e l ... 53
3.2 C om parison betw een simphfied and rigorous m odels ... 53
3.2.1 E th y l a c e ta te case s t u d y ...53
3.2.2 C ase s tu d y r e s u l t s ...56
C O N T E N T S 6
4 M o d e l l i n g o f R B D in p a c k e d c o lu m n s 64
4.1 R ate-based modelling of packed c o lu m n s ...65
4.1.1 M odelling of m ass and energy t r a n s f e r ... 65
4.1.2 M odelling of h y d ro d y n a m ic s...66
4.1.3 M odelling of chemical r e a c ti o n s ... 67
4.1.4 Packed column m o d e l l i n g ...68
4.2 Reactive b a tc h distillation case s t u d y ... 69
4.2.1 Colum n d e s i g n ...69
4.2.2 C olum n o peration ... 70
4.2.3 Effect of d is c r e tis a tio n ... 71
4.2.4 D eterm ination of H E X ? ... 72
4.3 C om parison betw een ra te based and equilibrium m o d e l s ...80
4.4 Conclusion ... 86
5 C o n t r o l o f r e a c t i v e b a t c h c o lu m n s 88 5.1 I n tr o d u c tio n ... 89
5.1.1 C ontrol of batch distillation c o lu m n s ...91
5.2 C ontrollability m e t h o d s ... 94
5.2.1 Sim ulation controllability analysis ... 94
5.2.2 Frequency response controllability analysis ... 97
5.3 M ethods for controllability a n a ly s is ... 102
5.3.1 M ethod for sim ulation controllability a n a l y s i s ...102
5.3.2 M ethod for frequency response controllability a n a l y s i s ... 102
C O N T E N T S 7
5.4 C ontrollability of b atch distillation c o l u m n s ... 106
5.4.1 Case s tu d i e s ...106
5.4.2 Scaling ... 107
5.4.3 Linear m o d e ls ... 107
5.4.4 Frequency response based c o n tro lla b ility ...110
5.4.5 C ontroller t u n i n g ...I l l 5.4.6 Non-linear m odel s im u l a t io n s ...112
5.5 Effect of r e a c t i o n ... 113
5.5.1 Sim ulation c o n tr o l la b il i ty ... 114
5.5.2 Frequency response controllability ...114
5.6 C o n c lu s io n s ...115
6 M o d e ll in g a n d c o n t r o l o f s h o r t - p a t h c o lu m n s 124 6.1 I n tr o d u c tio n ... 124
6.2 M odelling of sh o rt-p a th evaporators ... 125
6.2.1 M odelling of him p h e n o m e n a ... 126
6.2.2 M odelling of evaporation p h e n o m e n a ...128
6.3 D ynam ic sh o rt-p a th distillation m o d e l ...130
6.3.1 M odelling a s s u m p tio n s ...131
6.3.2 B oundary and initial c o n d itio n s ...134
6.3.3 Num erical s o l u t i o n ... 134
6.4 Case stu d y ... 134
6.4.1 Effect of d is c r e tis a tio n ...136
C O N T E N T S 8
6.4.3 Effect of v is c o s ity ... 139
6.4.4 Effect of feed t e m p e r a t u r e ... 140
6.4.5 Effect of heat of r e a c tio n ... 141
6.4.6 Effect of e f f i c i e n c y ... 141
6.5 C ontrol of sh o rt-p ath d i s t i l l a t i o n ... 142
6.5.1 L i n e a r i s a t i o n ...143
6.5.2 Frequency a n a l y s i s ... 143
6.5.3 Controlled response ... 144
6.6 Conclusion ...145
7 C o n c lu s io n s a n d d ir e c t io n s for f u tu r e w o r k 149 7.1 C o n c lu s io n s ...149
7.1.1 M odelling of reactive batch distillation in tra y colum ns ...150
7.1.2 Modelling of reactive batch distillation in packed c o l u m n s ... 151
7.1.3 C ontrol of reactive batch d i s t i l l a t i o n ...152
7.1.4 M odelling and control of reactive s h o rt-p a th e v a p o r a to r s ... 153
7.2 D irections for future w o r k ...154
7.2.1 M odel v a l i d a t i o n ... 154
7.2.2 M odelling detail ... 154
7.2.3 F u rth er control s tu d i e s ... 155
N o m e n c l a t u r e 157
C O N T E N T S 9
A P r o c e s s M o d e ls 166
A .l Equilibrium tra y model ...166
A .2 R ate-based m odel of packing s e c t i o n ... 169
A .3 Reboiler m o d e l ...173
A .4 C ondenser m o d e l ... 174
A .5 Reflux drum m o d e l ...175
A .6 A ccum ulator m o d e l ... 177
B L in e a r is a t io n a n d s c a lin g m e t h o d s 178 B .l L inearisation of m odel e q u a t i o n s ... 178
L ist o f F ig u res
1.1 Reactive b atch distillation column ... 18
1.2 W iped-Film evaporator ... 20
1.3 S h o rt-p ath e v a p o r a t o r ...21
3.1 D istillate com position (to p ) and distillate ho w rate (b o tto m ) for th e constant
rehux ratio stu d y ... 58
3.2 A ccum ulator com position (to p ) and holdup (b o tto m ) for th e co n stan t rehux ratio stu d y ...59
3.3 D istillate com position (to p ) and distillate h o w rate (b o tto m ) for controlled
com position s t u d y ... 60
3.4 A ccum ulator com position (to p ) and holdup (b o tto m ) for controlled com po
sition s t u d y ... 61
3.5 D istillate how for non-reactive system ... 62
3.6 Reboiler forw ard reaction ra te and ethyl a c e ta te com position (Rigorous Model) ... 62
3.7 D istillate com position (to p ) and distillate h o w rate (b o tto m ) for controlled
com position study w ith reboiler heat input d isturbance ( b o t t o m ) ... 63
4.1 Mass and energy tran sfer betw een phases ... 66
4.2 Vapour com position of E thyl A cetate at 3 hrs (co n stan t rehux ratio ) . . . 74
L I S T OF F I G U R E S 11
4.3 Com position and D istillate Flow rate - (T O P C on stan t reflux ratio - B O T
TO M Controlled C o m p o s it io n ) ... 77
4.4 H E T P packing profile against tim e for Reactive Case S tu d y (C o n sta n t reflux ratio ) ... 78
4.5 M ean H E T P profile for Reactive Case S tudy (C o n sta n t Reflux R atio) . . . 78
4.6 H E T P packing profile against tim e for Reactive Case S tu d y (C ontrolled) . . 79
4.7 M ean H E T P profile for Reactive Case S tudy ( C o n t r o l l e d ) ... 79
4.8 D istillate com position (top) and distillate flow rate (b o tto m ) for constant reflux ratio p o lic y ... 84
4.9 D istillate com position (top) and distillate flow rate (b o tto m ) for controlled com position p o l i c y ... 84
4.10 D istillate com position (to p ), distillate flow rate (m iddle) and reboiler dis tu rb a n c e profile (b o tto m ) for controlled com position policy ...85
5.1 Process for c o n t r o l ... 89
5.2 B atch Column ... 91
5.3 Frequency response of a first order s y s t e m ... 98
5.4 Block diagram of feedback control ... 99
5.5 C ontrollability r e q u ir e m e n ts ... 101
5.6 Com parison of robust m ethod(Sim ple) to sta n d a rd m ethod(R igorous) . . . 104
5.7 In tern al reflux ratio p r o f i l e s ...106
5.8 D istillate com position response to step change in i n p u t s ... 109
5.9 Reboiler te m p e ra tu re response to step change in in p u ts ... 110
5.10 D istillate com position response to unit step change in reflux f l o w ... 116
L I S T O F F I G U R E S 12
5.12 Packed column frequency response of distillate com position to reflux flow
a t 7.5 h r s ...117
5.13 Frequency response of distillate com position to reflux flow for 10 tra y column 118 5.14 Frequency response of distillate com position to reflux flow for 8m packed c o lu m n ...118
5.15 T ray column closed loop response to setpoint change (L inear m odel) . . . . 119
5.16 Packed column closed loop response to setpoint change (L inear m odel) . . 119
5.17 M agnitude com position response (10 tra y colum n) ... 120
5.18 M agnitude com position response (8m packed colum n) ... 120
5.19 C om position controller error in response to 10% step increase in reboiler h eat duty (Rigorous T ray C o l u m n ) ...121
5.20 C om position controller error in response to 10% step increase in reboiler he a t duty (8m Packed Colum n) at 3 hours ...121
5.21 D istillate com position response of reactive and non-reactive tra y colum ns . 122 5.22 Reboiler tem p e ra tu re response of reactive and non-reactive tra y colum ns . . 122
5.23 C om position frequency response of reactive and non-reactive tra y colum ns . 123 6.1 S h o rt-p a th e v a p o r a t o r ... 126
6.2 Top view of mixing bow wave (ad a p ted from M cK enna 1 9 9 5 ) ... 127
6.3 T em p eratu re profile in a wiped film evaporator (L utisan et al 2 0 0 2 ) ... 128
6.4 Cross section of E v a p o r a t o r ... 131
6.5 R eaction S c h e m e ... 135
6.6 Base case profiles: com positions (to p ), te m p e ra tu re ( b o t t o m ) ... 136
L I S T OF F I G U R E S 13
6.8 Effect of feed flow rate on residence t i m e ... 138
6.9 Effect of feed flow rate on reactor y i e l d ... 139
6.10 Effect of film viscosity on reactor y i e l d ... 139
6.11 Effect of viscosity on residence tim e ... 140
6.12 Effect of feed tem p e ra tu re on reactor y i e l d ...140
6.13 E v ap o rato r com position profile w ith no separation of V ...142
6.14 P ro d u c t, B, com position step responses. N on-linear m odel (T O P ), linear m odel ( B O T T O M ) ... 146
6.15 S h o rt-p a th column frequency response of pro d u ct B com position to feed flow 147 6.16 S h o rt-p a th colum n frequency response of controller loop and te m p e ra tu re d i s t u r b a n c e ... 147
6.17 Colum n com position response to a set-point change (controlled scaled linear m o d e l ) ... 148
6.18 Colum n com position response to a feed te m p e ra tu re d istu rb an ce (controlled scaled linear m odel) ...148
A .l Sieve t r a y ... 166
A .2 Packing s e c t i o n ... 169
A .3 R e b o i l e r ...173
A .4 C o n d e n s e r ... 174
L ist o f T ables
2.1 Sum m ary of papers on reactive batch distillation 1979 to present day . . . . 38
2.2 Sum m ary of papers on reactive b atch distillation 1979 to present day (con tinued) 39
2.3 Sum m ary of papers on sh o rt-p ath d i s t i l l a t i o n ... 46
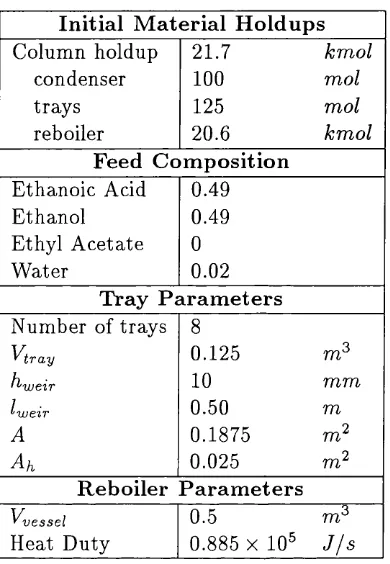
3.1 Colum n p a r a m e te r s ... 56
3.2 C ontroller p a r a m e t e r s ... 57
3.3 A ccum ulator holdup and batch t i m e s ... 63
4.1 Colum n dimensions and packing c h a r a c te r is tic s ... 70
4.2 Com parison betw een level of discretisation for 8m packed c o l u m n ... 72
4.3 Com parison betw een m odelling approaches. (EQ: equilibrium m odel, NEQ: ra te based m odel) ... 83
5.1 C ontrol S c h e m e s ... 93
5.2 Inputs and o u tp u ts for linear m odel (6 T ray column a t 3 hrs Un. point) . . 107
5.3 C ontroller tuning p a r a m e t e r s ...I l l 5.4 In teg rated controller errors ... 113
6.1 Short p a th column configuration ...135
C h a p te r 1
In tr o d u c tio n
This thesis is concerned with the modelling and control o f reactive distillation in tray and packed batch colum ns as well as in short-path colum ns. Reactive distillation offers advantages over separate reaction and separation steps, fo r instance through improved yield, as volatile products are rem oved fro m the re action zone. However, by combining these processes, control is made more difficult. In this chapter, reactive distillation is introduced with its advantages and disadvantages. Then, more specifically, batch operation in packed and tray colum ns and operation in short-path evaporators, is introduced. General com m en ts are made about the control o f these processes. The thesis m otivations, objectives and contributions are outlined. The outline o f the rest o f the thesis is also presented.
1.1
M o t iv a t io n
This thesis is concerned w ith th e modelling and control of reactive distillation. Due to
th e com plexity of combined reaction and separation and th e o p era tio n a l constraints on these processes such as reaction tem p e ra tu re , th ey m ay be difficult to control in practice
(Sprensen and Skogestad, 1994). In this w ork, reactive distillation in tra y and packed colum ns as well as sh o rt-p ath columns are considered. For b a tc h colum ns, th e changing
C H A P T E R 1. I N T R O D U C T I O N 16
process conditions w ith tim e adds a e x tra dimension to th e com plexity of th e control. For
th e sh o rt-p a th column, typically used for th e tre a tm e n t of te m p e ra tu re sensitive products, te m p e ra tu re control is extrem ely im p o rta n t. In order to stu d y th e control of these pro
cesses, it is necessary to develop rigorous dynam ic m odels which accurately cap tu re the
behaviour of th e processes.
1.2
R e a c t iv e d is tilla tio n
Reactive distillation is th e com bination of b o th reaction and sep aratio n into a single unit. This can offer particu lar advantages as reported in th e lite ra tu re by Taylor and K rishna
(2000), D oherty and Buzad (1992) and others:
• Com bining two units into one can lead to significant capital savings
• Im proved conversion of rea c ta n ts, approaching 100% as volatile p ro d u cts are removed
from th e reaction zone
• Low product concentrations in the column section, reducing unw anted side reactions
and leading to higher selectivity
• A zeotropes which would otherw ise be form ed by th e re a c ta n ts /p ro d u c ts can be elim
inated th ro u g h reaction
• Lower reboiler duty w ith exotherm ic reactions, as th e heat of reaction assists in th e vaporisation
However, caution should also be taken when considering reactive distillation for the fol
lowing reasons:
C H A P T E R 1. I N T R O D U C T I O N 17
• If th e reaction is slow, it m ay be m ore econom ical to carry out th e operation in
se p a ra te reaction and separation steps as a large colum n w ith large reflux wiU be required w ith large capital and operating costs to achieve th e required residence
tim e.
• T he coupling of reaction and separation in one unit can result in a m ism atch between
ideal process conditions. T he optim al te m p e ra tu re and pressure for th e reaction m ay be very different to th a t for th e separation. This is also an acute problem in packed
colum ns w here th e selection of the packing is often a com prom ise betw een separation and reaction perform ance.
1.3
B a t c h c o l u m n o p e r a t io n
In th e m an u fa ctu re of low volume, high value chemical p ro d u cts, and in situations where varying specifications of different products are required, th e m otivation for employing b atch op eratio n s is well known. Reactive b atch distillation can be carried out in a column
such as t h a t shown in Figure 1.1. D epending on th e volatilities of th e rea c ta n ts com pared to th e p ro d u cts, th e reaction m ay occur th ro u g h o u t th e column or be confined to th e reboiler. T he sep aratio n section can be either a tra y stack or a packing section. T he
choice depends on th e o p erating conditions, although packed colum ns are particularly suitable if th e reaction is to be heterogeneously catalysed in th e packing. T he reactan ts
m ay be initially charged to the reboiler or one or m ore m ay be fed during sem i-batch o p eratio n . A hom ogeneous cataly st, such as a co n centrated m ineral acid, m ay also be
fed or charged initially. As w ith non-reactive distillation, reactive colum ns are norm ally o p e ra ted a t to ta l reflux un til a ste a d y -sta te profile is developed before distillate w ithdraw al
is s ta rte d . Several prod u ctio n cuts m ay be m ade during th e o p eration which m ay or m ay
C H A P T E R 1. I N T R O D U C T I O N 18
Condenser
Reflux Drum
Reflux Distillate
Distillation Column
Accumulator
iX M " Heat Supply
Reboiler
Figure 1.1: Reactive batch distillation column
1 . 3 . 1 C o n t r o l o f r e a c t i v e b a t c h d i s t i l l a t i o n
T he coupling of reaction and distillation processes creates a m ore complex process which is m ore difficult to control th a n either one on its own. In general, th e use of au to m atic
control system s in batch system s is fairly lim ited, th ere are a num ber of reasons for this:
• It is quite difficult, due to th e tra n sie n t n a tu re of th e process, to control th e unit based only on regulatory or tracking control of certain variables. T he controllers
need to be adjusted according to th e current s ta te of th e process w ith th e aim of
achieving th e desired s ta te at th e end of th e b atch .
• T he desired s ta te at th e end of th e b atch and th e perform ance of th e process will change w ith different charges and process requirem ents which fu rth e r com plicates
C H A P T E R 1. I N T R O D U C T I O N 19
$ T here can be difficulties in observing some s ta te variables, e.g. com position, and if they are to be controlled th ey m ay have to be inferred from q u antities th a t are m ore easily m easured, e.g. tem p e ra tu re .
1.4
S h o r t-p a th c o lu m n o p e r a t io n
It is often not possible to heat m any organic com pounds to a te m p e ra tu re even close
to their norm al boiling points w ithout th erm al decom position occurring. T he degree of decom position will also depend on th e length of tim e th e com pounds are exposed to th e heat source. Vacuum distillation enables th e separation of these kinds of com pounds by keeping th e pressure and hence te m p e ra tu re low. T raditional b atch distillation is some tim es unsuitable for vacuum distillation since th e pressure drop across th e column lim its
the am ount of vacuum achievable in th e still. A m inim um pressure of around 50 m bars (Erdw eg, 1983) can be achieved under ideal conditions. A dditionally, th ere are large res
idence tim es w ithin th e still, which increases th erm al decom position. This has been th e m otivation for developing oth er distillation/ evaporation processes. These include falling film evaporators, wiped-film evaporators and sh o rt-p ath ev aporators.
1 . 4 . 1 F a l l i n g f i l m e v a p o r a t o r s
Falling film evaporators are used successfully in m any industries. T he feed flows down heated walls form ing a film. W hen operated under vacuum and high evaporation rates,
a num ber of problem s can occur. Hot spots form where m ate ria l overheats resulting in
CH A F T E R 1. I N T R O D U C T I O N 20
FEED V A P O U R T O E X T E R N A L
C O N D E N S E R A N D V A C E T 'M PU M P
W IP IN G S Y ST EM
R E S ID U E
I ' i g u r e 1.2: W i p e d - F i l i n e v a j i o r a t o r
1 . 4 . 2 W i p e d film e v a p o r a t o r s
In a wi])e(l-lilni e v a p o r a t o r , s h o w n in F i g u r e 1.2, t h e p r o d u c t is fed t o t h e i n s i d e o f a
s i n g l e t u b e a n d a i n e c h a i i i c a l , r o t a t i n g , w i p e r s p r e a d s a n d m o v e s t h e p r o d u c t s , a v o i d i n g
hot s p o t s . T h e m o r e v o l a t i l e c o m p o n e n t s g e n e r a l l y r u n a g a i n s t t h e p r o d u c t flow, l e a v i n g
t h e e v a p o r a t o r t o a n e x t e r n a l c o n d e n s e r . T h e p r e s s u r e d r o p b e t w e e n t h e e v a p o r a t o r a n d
t h e e x t e r n a l c o n d e n s e r d e t e r m i n e s t h e d e g r e e o f v a c u u m a c h i e v a b l e in t h i s t y p e o f u n i t .
1 . 4 . 3 S h o r t - p a t h d i s t i l l a t i o n
S h o r t - p a t h e v a p o r a t o r , s h o w n in F i g u r e 1.3, is in p r i n c i p l e a w i p e d - f i l m e v a p o r a t o r w i t h
a n i n t e r n a l c o n d e n s e r . T h i s e l i m i n a t e s t h e p r e s s u r e d r o p a s s o c i a t e d w i t h t h e p i p e w o r k
c o n n e c t i n g t h e evaporator a n d t h e c o n d e n s e r . In t l i eor y, t h e r e is n o p r e s s u r e d r o p b e t w e e n t h e e v a p o r a t i n g s u r f a c e a n d t h e c o n d e n s i n g s u r f a c e b e c a u s e t h e d i s t i l l a t i o n g a p is o f t h e
s a m e o r d e r o f m a g n i t u d e as t h e m e a n f r e e p a t h o f t h e e v a j i o r a t i n g m o l e c u l e s a t t h e l ow
21
FEED
C O N D E N SE R
HE A T ING JACKET
WTPING SA'STCM
— COOLING
DISTILLATE R ESIDUE
F i g u r e 1 .X: S i i o r i - j i a t l i e v a p o r a t o r
1.5
O b je c tiv e s o f th is work
R e a c t i v e b a t c h (listi llatioii is a n i n l i e r e n t l y d y n a m i c p r o c e s s s i n c e a m o u n t a n d c o m p o s i t i o n
o f m a t e r i a l w i t h i n t h e c o l u m n c h a n g e s w i t h t i m e a s t h e r e a c t i o n p r o c e e d s a n d p r o d u c t
is w i t h d r a w n . A s a r e s u l t , a d y n a m i c m a t h e m a t i c a l m o d e l is r e q u i r e d t o d e s c r i b e its
o p e r a t i o n . C o n t i n u o u s d i s t i l l a t i o n , sucli a s s h o r t - p a t h d i s t i l l a t i o n , s h o u l d b e d y n a m i c a l l y
m o d e l l e d if t h e m o d e l is t o b e u s e d for c o n t r o l .
T h e o b j e c t i v e o f t h i s t h e s i s is t o i n v e s t i g a t e t h e c o n t r o l o f r e a c t i v e d i s t i l l a t i o n in b a t c h
c o l u m n s , t r a y a n d p a c k e d , a n d in s h o r t p a t h c o l u m n s . C o n t r o l h a s h i t h e r t o o n l y b e e n
s t u d i e d for r e a c t i v e b a t c h d i s t i l l a t i o n in t r a y c o l u m n s , a n d t h e n o n l y e m p l o y i n g s i mp l i f i e d
d y n a m i c m o d e l s w i t h l i n e a r t r a y d y n a m i c s . In o r d e r t o s t u d y c o n t r o l fully, it is n e c e s s a r y
t o d e v e l o p r i g o r o u s d y n a m i c m o d e l s t h a t a c c u r a t e l y c a p t u r e t h e p r o c e s s b e h a v i o u r . For
b a t c h p r o c e s s e s in p a r t i c u l a r , p r o c e s s c o n d i t i o n s c h a n g e w i t h t i m e w h i c h will af f e c t t h e
C H A P T E R 1. I N T R O D U C T I O N 22
T h e higher th e degree of rigour, th e m ore accurately th e process conditions and dynam
ics are captured. However, m ore rigorous models are m ore co m putationally expensive to im plem ent and they require m ore param etric d a ta which m ay be unavailable or uncer
ta in . A dditionally, m ore num erically complex models can be prone to num erical errors, in troduced for instance during hnearisation.
T herefore, in this thesis, th e degree of modeUing rigour required for b o th sim ulation and
control purposes is explored in detail for tra y and packed b a tc h colum ns and sh o rt-p ath
colum ns.
For b atch tra y colum ns, it is dem o n strated th a t to accurately ca p tu re th e change in process conditions, it is necessary to m odel pressure dynam ics and employ a dynam ic energy balance. For packed colum ns, d istrib u ted ra te based m odelhng is com pared to lum ped equilibrium m odelling and it is found th a t due to th e varying conditions within th e packing, the efficiency changes, resulting in m ism atch betw een th e tw o m ethods. T he
sh o rt-p a th distillation column which has h ith e rto only been m odelled at ste a d y -sta te , is
m odelled using a dynam ic ra te based m odel, essential for investigating control.
H aving developed th e dynam ic models, th e control and controllability of these reactive distillation processes are exam ined. G eneral control properties of reactive batch distillation are discussed and m ethods are presented for applying linear controU abihty tools to these
non-linear process m odels. T he linear models are th en employed to d em o n strate th e
im plications for control when adopting one of th e three processes.
1.6
O u tlin e o f th is t h e s is
C h a p te r 2 presents a review of th e lite ra tu re on m odelhng and control of reactive batch distillation in b o th tra y and packed colum ns. T he m odelhng of sh o rt-p a th distillation
colum ns is also considered. It is concluded th a t little work has been und ertak en on th e control of reactive batch distiUation in tra y columns and no work has been undertaken
on th e control of reactive distillation in batch packed colum ns and sh o rt-p a th distillation
C H A P T E R 1. I N T R O D U C T I O N 23
As already noted, rigorous dynam ic models are required to enable th e stu d y of control. C h apters 3 and 4 deal w ith th e developm ent of rigorous m odels to describe reactive dis
tillation in batch tra y and packed columns. T he tra y colum n is com pared to a shghtly
simplified, m ore num erically robust column for a num ber of different op eratin g policies. As packed columns are com m only modelled using equilibrium m odels, such as th a t for
th e tra y colum n, th e tw o m odelling approaches are com pared. In order to determ ine th e equivalent tra y colum n, th e Height Equivalent to a Theoretical Plate (H E T P ) needs to be established and a m eth o d is presented for ex tra ctin g this inform ation from th e column packing.
C h a p te r 5 is concerned w ith th e control of tra y and packed b atch colum ns. In order to use linear control tools it is necessary to generate a linear ap proxim ation to th e non-linear models. T he rigorous tra y colum n m odel proves to be num erically u n stab le for this purpose
and an altern ativ e scheme is presented for generating th e necessary linear inform ation from th e simplified colum n m odel. T he effect of th e choice of colum n, packed or tray, th e effect
of reaction and th e size of th e column are investigated during th e controllability study.
C h a p te r 6 considers th e m odelling and control of reactive distillation in sh o rt-p a th distilla tion colum ns. A dynam ic sh o rt-p a th column is presented and used to investigate th e effect
of changes in o p eratio n on a complex, industrially m o tiv ated , reaction. T he com position control of this process is also investigated.
Finally, in ch ap ter 7, overall conclusions are draw n, and some possible directions for fu tu re
work are outhned.
1.7
M a in c o n t r ib u t io n s
T he m ain contributions of this thesis are th a t a rigorous, equilibrium , tra y colum n m odel
and a rigorous, rate -b a se d , packed column m odel for reactive b atch distillation are devel oped. A m ethod is presented to determ ine th e Height E quivalent to a Theoretical Plate
C H A P T E R 1. I N T R O D U C T I O N 24
linearised. T herefore, a m ethod is presented for coping w ith these difficulties by m eans
of a simplified version of th e m odel. A com parison is m ade betw een th e controllability of packed and tra y b atch colum ns. Finally, a dynam ic m odel for reactive distillation in a
C h a p te r 2
L itera tu re rev iew
In this chapter, the work that has previously been undertaken w ithin the area o f modelling and control o f reactive hatch distillation and the modelling and control o f short-path distillation is reviewed. It is concluded that, while some work on the control o f batch tray colum ns has been done, the models used have been simple. No work has been undertaken on the control o f packed colum ns or short-path columns. It is also noted that no work has been done on modelling reaction in short-path columns.
2.1
M o d e llin g o f r e a c t iv e b a tc h d is tilla tio n
2.1.1 I n tr o d u c tio n
Reactive distillation can be op erated in b o th continuous and b atch m odes of operation. As is generally th e case, continuous operation is best suited for large production volumes
b u t, since th e continuous design is tailored to a particu lar re a c tio n /se p a ra tio n system , it lacks th e flexibility afforded by batch operation. C ontrol of b o th m odes is complex but
th e b atch m ode offers additional challenges. In the continuous m ode, control is generally required about a desired ste a d y -sta te when n ot considering s ta rt-u p or shut-dow n. Batch
control, on th e o th er hand, is inherently dynam ic which adds an e x tra dim ension to its
C H A P T E R 2. L I T E R A T U R E R E V I E W 26
com plexity. T he process is not controlled to a desired stead y s ta te b u t is controlled to a desired o p eratin g policy which may, and often does, change w ith tim e. T he process itself
changes w ith tim e, th e volume in th e reboiler is decreasing and th e com position profile
in th e colum n changes. Consequently, th e response of th e process and its control system to disturbances wiU change w ith tim e. Efficient control is im p o rta n t, as m inim ising the
im pact of d isturbances reduces batch inconsistencies and hence w astage. Good set-point tracking of an o ptim al o p erating policy ensures m ore economic o p eratio n . In this thesis,
the focus is therefore on investigating th e dynam ic behaviour and th e controllability of these processes as a m ore detailed u n derstanding will lead to b e tte r control. In this
section, th e lite ra tu re th a t has been published on reactive b atch distillation (sum m arised in Tables 2.1 and 2.2) is exam ined to identify w hat work has been und ertak en on the m odelling and control of these processes.
2 . 1 . 2 R e a c t i v e b a t c h d i s t i l l a t i o n l i t e r a t u r e
Egly et al. (1979) developed a m ethod for optim ising th e o p eratin g policies of b atch distilla
tion operations. T h e sim ple m odel used consisted of com ponent m ass and energy balances and a vapour-liquid equilibrium equation. R eaction could be considered th ro u g h o u t th e
liquid phase in th e colum n and th e still. T he holdup of liquid in th e tra y s and condenser
were assum ed to be c o n stan t and no pressure dynam ics were considered. O ptim isations were perform ed using a modified conjugated gradient m ethod, m inim ising th e b atch tim e
while m ain tain in g p ro d u ct specifications in term s of yield and com position. O ptim isations
considered were c o n sta n t reflux ratio, tim e variable reflux ra tio and tim e variable reflux ratio w ith feed o f a re a c ta n t. This was applied to a theo retical reactive case stu d y w ith reaction confined to th e still. T he optim al, sh o rtest batch tim e, was found for th e tim e
varying reflux ra tio policy w ith feed of re a c ta n t which was 40% sh o rte r th a n th e constant
reflux ratio policy. T he au th o rs also developed a non-linear, m ulti-variable control algo rith m th a t d eterm ined th e required reflux ratio at any in sta n t from tem p e ra tu re s in th e
C H A P T E R 2. L I T E R A T U R E R E V I E W 27
Cuille and Reklaitis (1986) considered th e sim ulation of b a tc h distillation w ith and w ith out liquid phase chemical reaction in th e reboiler. T he m odel was a system of differential
and algebraic equations and th e au th o rs discussed th e num erical problem s associated w ith
these system s and strateg ies for tackling these. T he system was initialised at ste a d y -sta te w ith to ta l reflux and no reaction and integ rated using G e a r’s m ethod. C o n stan t volum et ric holdup on th e tra y s was assum ed and tra y efficiencies were included. T he reaction
considered was an equilibrium estérification of 1-propanol and acetic acid. T he ra te of reaction was simplified by assum ing no te m p e ra tu re dependence. However, th e reaction
is not practical for reactive batch distillation since 1-propanol, a re a c ta n t, is th e m ost volatile com ponent in th e system and is hence rem oved preferentially. T he au th o rs com pared th e non-reactive separation of cyclohexane and toluene w ith experim ental d a ta . T he agreem ent was good for th e distillate com position profiles for a num ber of constant reflux ratio sim ulations. However, there were no com parisons m ade for reactive b atch distillation.
R euter et al. (1989) considered th e m odelling of reactive distillation w ith control system s. T h e m odel considered non-ideal stages and variable pressure. A ssum ptions included: con
sta n t liquid hold-up on th e tray s and in th e condenser and reaction only in th e liquid phase. T hey considered an equilibrium transestérification reaction, alth o u g h no details were given
on either th e com ponents or the reaction. T he m odel was s ta rte d from steady s ta te , to ta l reflux w ith no reactio n and th e steady s ta te profile was calculated using N ew ton-R aphson
procedures. T h e dynam ic sim ulation was perform ed using a m odified relaxation m ethod.
T he control schem e employed consisted of th re e controllers: C ondenser cooling was used to control th e condenser outlet tem p e ra tu re , reboiler steam flow for colum n pressure drop
and distillate r a te for th e te m p e ra tu re at th e top of th e colum n. T he a u th o rs com pared b o th controlled and uncontrolled operation of th e colum n b u t did n ot evaluate th e two
C H A P T E R 2. L I T E R A T U R E R E V I E W 28
th e reflux ratio set by th e controller in th e sim ulation was always less th a n in th e exper im ent when m aintaining a constant distillate com position. This suggest th a t th e m odel
agreem ent is not very good, although this is diflficult to verify from th e results.
Sprensen and Skogestad (1994) investigated control strategies for reactive batch distil
lation. T hey indicated th a t m ost au th o rs had considered o ptim al control in order to m axim ise profit or minimise batch tim e. However, th ey argued t h a t, in some cases, it may
be a m ore im p o rta n t control objective to m aintain p ro d u ct consistency betw een batches. T hey considered th e modelling of an in d u strial esteriflcation process w here th e reaction
was lim ited to th e reboiler. T he reaction produced a se p ara te polym er phase and w ater, th e m ost volatile com ponent in th e reaction m ixture. M odelling assum ptions included: perfect m ixing and equilibrium betw een th e liquid and vapour phases, constant pressure,
negligible vapour holdup, constant liquid enthalpies, linear tra y hydraulics, to ta l condensa tion w ith o u t sub-cooling in th e condenser, R a o u lt’s law for th e liquid vapour equilibrium ,
perfectly controlled vapour holdup and im m ediate h e a t in p u t. T he a u th o rs highlighted the differences betw een non-reactive and reactive distillation by applying typical non-reactive
o p e ra tin g policies to th e reactive exam ple. T he open loop policies: co n stan t reflux ra tio, R, and tim e varying reflux ratio , R (t), were found to be in ap p ro p riate, although
th e re was acceptable separation, as th e reacto r te m p e ra tu re varied to o g reatly under dis tu rb a n c es, resulting in varying polym er com position betw een batches. Only a constant
p ro d u ct com position pohcy, im plem ented using feedback control, m aintained th e reactor
w ithin perm issible te m p e ra tu re lim its. T he au th o rs considered th e controU abihty of the process by generating hnear models at different tim es during th e b atch . T he linerised
m odels were used to investigate th e controllability by m eans of step responses and RGA analysis. T hey identified th e reacto r te m p e ra tu re and th e distillate com position as being
th e m ost im p o rta n t variables to control. T hey also concluded t h a t th e sam e am ount of side p ro d u ct being form ed should be rem oved in th e distillate a t any tim e, th a t th e sy ste m ’s
C H A P T E R 2. L I T E R A T U R E R E V I E W 29
non-linear. T he a u th o rs evaluated a series of control schemes: one point b o tto m control
(reacto r te m p e ra tu re directly), two point control (controlling b o th distillate com position
and reacto r te m p e ra tu re ) and one point column control (controlling th e te m p e ra tu re on a
tra y in th e colum n). It was concluded th a t th e la tte r offered good control of th e process w ith disturbances and did not have th e disadvantages of th e interactio n s encountered with
two point control.
S0rensen et al. (1996) addressed th e issues of optim al control of th e sam e case stu d y as
S0rensen and Skogestad (1994). They developed optim al profiles for th e o p erating vari
ables, assessed th e controllability properties at optim al conditions, designed controllers to im plem ent th e op tim al profiles and verified th e stab ility and control perform ance. A series of optim isations were perform ed for m axim um profit w ith and w ith o u t raw m aterial costs and for m inim um batch tim e. T he im plem entation was perform ed using th e tem p e ra tu re on a colum n tra y to control reflux while m aintaining th e h eat to th e reboiler a t th e optim um value. The tra y selected was th e one t h a t gave th e largest response,
identified in th e controllability analysis. T he stab ility of th e controlled and uncontrolled
model were assessed by introducing disturbances in th e reboiler heat supply and in the reaction p aram eters which were used to indicate u n c e rtain ty in those p aram eters. The
uncontrolled case gave significant variations in th e reboiler te m p e ra tu re and b reakthrough of th e volatile re a c ta n t into th e distillate. In th e controlled case, th e controllers were
tu n ed using a linerised m odel ab o u t a series of o p eratin g points and a polynom ial was
used to describe th e te m p e ra tu re set point profile. T his yielded significant deviations in reboiler te m p e ra tu re only tow ards th e end of th e b atch. T he m odel was also interfaced
to an in d u strial real-tim e control system and th e controllers were im plem ented using the system s facilities. R a th e r th an a polynom ial description of th e set points, a series of set
points were used. T his yielded good perform ance, sim ilar to th e continuous controller.
M u jta b a and M acchietto (1997) considered a co m putationally efficient m ethod for optim is
C H A P T E R 2. L I T E R A T U R E R E V I E W 30
this o p erational condition is strongly dependent on th e cost p a ra m ete rs which in tu rn are
dependent on m arket forces. T hey pointed out th a t to perform a rigorous optim isation procedure, involving in teg ratio n of th e m odel equations such as th a t used by Sqrensen
et al. (1996), would be too com putationally expensive to perform every tim e m arket con
ditions change. T h e a u th o rs proposed a less com putationally expensive m ethod th a t used polynom ial approxim ations to estim ate th e optim al o perating policy from previously p er form ed optim isations. T heir exam ple was th e esteriflcation of eth an o l and ethanoic acid
to produce ethyl a c e ta te and w ater and th ey used simple m odels for th e VLE and did not consider azeotropes such as th a t form ed betw een ethyl e th a n o a te and ethanol. However,
they indicated th a t th e m ethodology is general and could be applied to m ore complex sys tem s such as azeotropic m ixtures by employing m ore rigorous V apour-Liquid equilibrium models. T he m odel includes reaction th ro u g h o u t the whole colum n in th e liquid phase and assum es co n stan t m olar holdup on th e plates and condenser. T he energy balance is
algebraic and hence assum es no change in liquid enthalpies. T his is sim pler th a n th a t used by Sprensen and Skogestad (1994) and Sprensen et al. (1996) as th e tra y holdup is
assum ed to be c o n sta n t which is reasonable as they are not considering dynam ics and th e colum n would rem ain in pseudo ste a d y -sta te during a large p o rtio n of th e batch. The a u th o rs asserted t h a t in reactive distillation w here one of th e p ro d u cts, desired or unde
sired, is th e m ost volatile com ponent, finding th e m axim um yield is equivalent to finding
th e m axim um pro d u ctio n of distillate. This, th ey indicated from previous w ork, is equiv alent to th e m axim um profit for fixed batch tim e. A series of optim um co n stan t reflux
ratios were calculated for m axim um product yield for a series of fixed b atch tim es and for
two p ro d u ct specifications using rigorous non-linear program m ing techniques (N L P). T he results to these o p tim isatio n s were used to develop polynom ial descriptions of m axim um conversion, optim um distillate, optim um reflux ratio and to ta l reboiler heat load. These
were all functions of b a tc h tim e. These were th en used in th e form ulation of a m axim um profit o p tim isatio n which was an algebraic optim isation in only one variable, tim e. O pti
m isations perform ed using this m ethod were approxim ately 200 tim es faster th a n using th e
C H A P T E R 2. L I T E R A T U R E R E V I E W 31
due to th e accuracy of th e regression of th e polynom ials to th e initial optim isation d a ta
which behaved well, justifying th eir use of low order polynom ials.
W ajge et al. (1997) exam ined th e accuracy and speed of num erical m ethods for sim ulating b o th reactive b atch distillation and non-reactive distillation in packed colum ns. They in
dicated th a t packed columns models differ from tra y m odel colum ns in th a t m ass transfer
effects need to be considered. T hey considered th e finite difference technique which in volves converting th e differential equations to algebraic equations of sm all intervals. This
m eth o d was used for th e sim ulation of batch distillation and it was concluded th a t th e finite difference m ethod is very com putationally expensive. T hey considered an orthogonal collocation m ethod where th e equations are approxim ated to polynom ials. T hey concluded th a t this was m ore efficient but the co m putational advantages over finite elem ent m ethods were lost as the need for g reater accuracy required th e use of higher order polynom ials.
T hey proposed a hybrid m ethod called collocation on finite elem ents which perm its high accuracy while retaining th e use of low order polynom ials and th e ir sh o rter solution tim es.
T hey also identified th a t com putation also took longer if th e com position profiles becam e widely sep arated in th e column. T hey indicated th a t th e use of sparse m a trix techniques
in th e solution also yielded im proved solution tim es.
W ilson and M artinez (1997a) investigate m ethods for th e estim atio n of s ta te variables such
as com position from te m p e ra tu re m easurem ents in reactive b a tc h distillation. T he m oti
vation for this was th a t good com position control is essential in reactive b atch distillation b u t th e cost of on-line com position m easurem ent is usually prohibitively expensive. T he a u th o rs considered two types of s ta te estim ato r. F irstly an E x tended Luenberger Observer
(ELO ) th a t derives its estim ates from a linearised model of th e process and secondly an E xtended K alm an Filter (E K F ) which, although also based on a linear m odel, produces
its estim ates based on th e sta tistic a l characteristics of th e prevailing random process dis
C H A P T E R 2. L I T E R A T U R E R E V I E W 32
and a slightly m ore complex m ulticom ponent distillation m odel. T he simple non-hnear
m odel was used to generate th e Hnear models for th e s ta te observers as th e m ore complex,
m ulti-com ponent m odel was too com putationaU y expensive for this purpose. T em pera tu re m easurem ents were taken from th e m ore detailed m odel and m easurem ent noise was
added. T he authors d em onstrated th a t th e E K F e stim ato r produced b e tte r estim ates th a t th e ELO , which had stability problem s. They concluded th a t th e process m ism atch
betw een th e models did result in reduced estim ato r accuracy and fu rth e r refinem ent was
required. However, they concluded th a t this accuracy was sufficient basis for com position control.
W ilson and M artinez (1997b) considered th e au to m atio n of b atch processes using fuzzy m odelling and reinforcem ent learning and applied their techniques to reactive b atch dis tillation. They s ta te d th a t, in general, the operation of b atch processes rehed heavily on
th e skills of operators to achieve th e desired p roduct due to th e difficulties of developing effective au to m atic control system s. They suggested th a t th e abilities of th e o p e ra to r can
be im ita ted , and even im proved on, by th e use of em bedded autonom ous agents. T he
agent “perceives” th e s ta te of th e process at each tim e step, executes an action and re ceives a rew ard or payoff in retu rn . T he a g e n t’s task is to react continuously to th e process
s ta te , influenced by disturbances and events, by determ ining a sequence of actions which m axim ises some cum ulative m easure of rew ards, driving th e process tow ards its “goal
s ta te ” . W ilson and M artinez (1997b) concluded th a t the m ost com m on algorithm for this
reinforcem ent learning, Q-Learning, had significant stabiHty problem s and com putational expense when appHed to b atch processes w here states vary w ith tim e. T hey presented a
hybrid approach w here Q -Learning was combined w ith fuzzy rules relating process states to control actions. This hybrid approach was term ed fu zz y Q-Learning. This technique was successfully dem o n strated for th e control of th e in d u strial reactive distiUation case stu d y presented in W ilson and M artinez (1997a).
C H A P T E R 2. L I T E R A T U R E R E V I E W 33
optim isation variables in an upper level optim isation w ith reflux ratio profile optim isation
as a lower level decision problem . To simplify th e procedure, th ey in tro d u ced th e concept of
D istillation C haracteristics which, is th e com position profile developed a t to ta l reflux. They deduced th a t for reversible reactions where holdup in th e colum n is neghgible, two m ixtures
have th e same distillation characteristic if th e p roducts and re a c ta n ts are in th e sam e stoichiom etric proportions. T hus th e optim al operational policy for th e column would be
th e sam e in b o th cases. They considered th e recycling of off-cuts, m ixed w ith fresh feed, which have th e sam e distillation characteristic as th e feed charge. T he a u th o rs illu strated th eir m ethodology w ith th e esteriflcation of ethanol, using a packed colum n based on th e
model rep orted by W ajge et al. (1997). They concluded th a t th e distillation characteristic
is relevant in th e design of cam paign stru ctu res which m inim ise off-cut frag m en tatio n and reduce operational com plexity by minimising th e num ber of distinct distillation tasks. In addition to a b e tte r reprocessing strategy, it offers insight into th e trade-off betw een
production ra te , rea c ta n t utiUsation and w aste generation. T he a u th o rs noted th a t the featu re of distillation characteristics breaks down if th e reaction is irreversible and if th e holdup w ithin th e column is not negligible. This would Hmit its appHcability to real processes.
Bollyn and W right (1998) considered th e use of experim ental d a ta in developing and
refining a dynam ic m odel describing a fed-batch reactive distillation colum n. T he reaction considered was th e synthesis of ethyl esters of pentenoic acid by su b stitu tio n of aUyl
alcohol for eth an o l on trie th y l o rth o a c eta te . T he reaction was assum ed to occur in th e
reboiler only. T hey simphfled th e reaction scheme by ignoring reaction steps th a t occurred sufficiently fast to be considered in stan tan eo u s, but were stiU left w ith 3 equilibrium
reactions and 4 ehm inations, yielding a to ta l of 10 reactions. T heir objective was to establish an o ptim al operating policy, th ro u g h sim ulation, such th a t a high conversion
of trieth y l a c e ta te and a high selectivity for th e desired ethyl ester was achieved while m inim ising excess alcohol. T hey also noted th a t in previous w ork, su b sta n tia l tim e was
C H A P T E R 2. L I T E R A T U R E R E V I E W 34
was spent on th e details of th e reaction kinetics. T hey in d icated th a t m ore a tte n tio n
should be paid to th e kinetics as th ey are m ore te m p e ra tu re dependent th a n m ost other
physical properties. Few details of th e modeUing assum ptions were given except th a t th e
process was sim ulated in B atchC A D which uses a rigorous dynam ic m ass tra n sfe r based distillation model. E xperim ental d a ta was collected and analysed in order to refine th e m odels. This was carried out a t several levels, sta rtin g w ith a lab b atch investigation into
th e kinetics moving th ro u g h to pilot plant. T he refined m odels were used at each stage to enable effective targ etin g of fu rth e r experim ents to enhance th e m odel. This led to th e
developm ent of an optim al o p erating policy which was successfully im plem ented on th e
pilot plant resulting in an im provem ent in selectivity from ab o u t 50% to over 98%.
Li et al. (1998) considered th e optim isation of a sem i-batch distillation process and used an industrial process to validate th eir model. T he reaction considered was an industrial transestérification reaction w here an alcohol pro d u ct is th e m ost volatile rea c ta n t. A
sem i-detailed m odel was developed including constant holdup, co n stan t tra y efficiencies and co nstant pressure profile. T he pressure on each tra y was in te rp o la te d from th e top
and b o tto m pressures in th e in d u strial column and th e M urphree tra y efficiency was de duced by trial and error th ro u g h com parison to th e experim ental results. T he sim ulation results were sufficiently close to th e experim ental to ju stify th e use of th e m odel for op
tim isatio n . T he optim isation was perform ed for m inim um b a tc h tim e em ploying control
vector p aram eterisatio n (C V P ) and sequential q u a d ra tic program m ing. T hey optim ised
th e feed how rate of th e alcohol, th e reflux ratio and th e sw itching tim e betw een th e m ain and off-cuts. Tw o scenarios were considered: firstly th e o p tim isatio n of th e process under
present requirem ents, where a 30% tim e saving was achieved, and th e optim isation of th e
process under slightly lower p ro d u ct p u rity requirem ents. T he a u th o rs acknowledged th a t although th eir solutions were feasible, it is hkely th a t su b-optim al solutions were found due to th e nonconvexity and com plexity of th e problem .
C H A P T E R 2. L I T E R A T U R E R E V I E W 35
reactive distillation colum n. In their m odel, th ey considered reaction in b o th liquid and
vapour phases and indicated th a t this was im p o rta n t for p h oto reactions because differ ent kinetic behaviours occur in th e two phases. Their m odel was a staged equilibrium
m odel w here b o th liquid and vapour phases are considered to be a t equilibrium in each
co m p artm en t. T hey included co nstant volum etric holdup of each phase, co nstant pres sure and assum ed perfect mixing. T he reaction considered was th e chlorination of toluene
where selectivity for th e desired benzyl chloride is enhanced by reactive distillation as the pro d u ct is distilled away from th e reaction zone. The presence of UV light also enhances the selectivity as it prom otes th e desired chlorination of th e m ethyl group and not th e
chlorination of th e benzene ring. They perform ed sim ulations for a series of th ree different
colum n configurations and com pared it w ith experim ental d a ta . T he com parison w ith experim ental d a ta was poor and it was concluded th a t th e sim ulation only agreed with th e experim ental d a ta in term s of trends. It was only as th e real system approached ideal op eratin g conditions described by th e m odel th a t th e best perform ance could be achieved.
V enim adhavan et al. (1999) considered th e synthesis of a reactive b a tc h distillation pro
cess for th e m an u fa ctu re of butyl acetate, an im p o rta n t in d u stria l solvent. T he reaction is an esteriflcation of b u tan o l and acetic acid to form butyl a c e ta te and w ater. T hey de veloped a very sim ple m odel to cap tu re th e essence of th e process and to provide insight
for exploring process alternatives. A ssum ptions included: liquid-phase reaction confined to th e reboiler, co n sta n t m olar overflow, o p erating reflux sufficiently large to be approx
im ated as to ta l reflux for th e purposes of calculating colum n profiles, and tra y holdups
negligible com pared w ith th a t of th e still. T hey considered a reflux policy such th a t the
instan tan eo u s D am kohler num ber rem ained approxim ately co n sta n t which is equivalent to th e ra te of p ro d u c t rem oval being kept equal to the ra te of pro d u ctio n . T he reaction
kinetic p a ra m ete rs were regressed from earher published w ork. T he phase equilibrium is quite complex w ith organic and aqueous phases being form ed. T he au th o rs indicated th a t th ere was a deb ate w ithin lite ra tu re as to which of two azeotropes was th e lightest
C H A P T E R 2. L I T E R A T U R E R E V I E W 36
Using topological argum ents for th e n a tu re of singular points on th e residue curve m ap for th e non-reacting te rn a ry system w ith acetic acid absent, th ey concluded th a t th e lightest
was th e te rn a ry azeotrope. T hey indicated th a t despite th e tw o azeotropes having close
boiling tem p e ra tu re s, th e detection of th e correct azeotrope had large im plications for th e
process design. In this case, th e aqueous phase, containing a sm all am ount of butanol, was rem oved as distillate and th e organic phase was retu rn ed as reflux. This results in th e exclusion of w ater and th e accum ulation of butyl ac eta te in th e still which can be purifled
th ro u g h non-reactive distillation at th e end of th e reaction. A second m odel was proposed which included constant holdup on th e tray s. This model was com pared favourably w ith the simplifled model although th e inclusion of traydynam ics resulted in a slower response.
M onroy-Lopereba and Alvarez-Ram irez (2000) com m ented th a t o p tim isatio n approaches, such as th a t presented by M u jta b a and M acchietto (1997) have an im p o rta n t draw back in th a t th e optim al solution depends strongly on th e m odel and m odel p a ra m ete rs and th e re fore feedback control is essential in order to m aintain optim um profitability in th e wake
of uncertainty. T he a u th o r’s objective was to obtain an outp u t-feed b ack controller w ith
guaran teed tracking properties, despite uncertainties in th e dynam ics of th e RED process. They also w anted to d em o n strate th a t th e resulting reflux ratio policy approaches th a t
obtained via optim isation techniques. T he controller design is based on an approxim ate m odel of th e com position dynam ics and m akes use of a reduced order observer to estim ate
th e m odelhng error. T he resulting controUer is shown to have th e sam e s tru c tu re as a PID
controUer w ith anti-reset windup. T he controller perform ance was te ste d on th e column
m odel as presented by M u jta b a and M acchietto (1997) and d e m o n stra ted th a t th e resu lt ing reflux ratio pohcy approached th e optim al.
Schneider et al. (2001) developed a ra te based m odel for reactive distiUation in a packed distiUation colum n. T he heterogeniously catalysed reaction was assum ed to be pseudo-
hom ogenious. T he column m odel contained dynam ic m ass and energy balances. T he
C H A P T E R 2. L I T E R A T U R E R E V I E W 37
stru c tu re d packing. T he liquid holdup in th e packing was determ ined using an experi
m entally derived correlation, specific to th e packing. T he au th o rs neglected vapour phase
holdup due to th e low pressure (< 1.2 B ar). No details on th e discretisation m ethod em ployed were given. T he m odel was validated w ith experim ental results from th e synthesis
of m ethyl a c e ta te in a sem i-batch column. Following a sensitivity analysis it was concluded th a t th e reaction kinetics and m odels of th e column periphery have a significant influence
on th e sim ulation results. It was indicated th a t, for th e colum n to be used for control
A u th o r s R e a c tio n lo c a tio n
M o d e l L iquid D y n a m ic s
P r e s s u r e D y n a m ic s
W o rk
Egly et al. (1979) liquid
phase
equilibrium constant
m olar holdup
constant optim isation of reflux ratio
policies
Cuille and Reklaitis (1986) liquid
phase
equilibrium + efficiency
constant volume holdup
constant sim ulation only
R euter et al. (1989) liquid
phase
equilibrium + efficiency
constant volume holdup
variable pressure and te m p e ra tu re control
Sprensen and Skogestad (1994) reboiler
only
equilibrium linear constant controllability and control strategies
Sprensen et al. (1996) reboiler
only
equilibrium linear constant optim isation and im plem entation
of optim al policies M u jta b a and M acchietto (1997) liquid
phase
equilibrium co nstant
m olar holdup
constant m ethods for online optim isation
W ajge et al. (1997) bquid
phase
rate-based packed
variable from correlations
variable from correlations
accuracy and efficiency of sp atial discretisation techniques
W ilson and M artinez (1997a) reboiler
only
equilibrium constant
m olar holdup
constant s ta te estim ation of com position
from colum n tem p e ra tu re s
W ilson and M artinez (1997b) reboiler
only
equilibrium unknow n unknow n application of neuro-netw orks
to control
g
I
toI
HI
g
Table 2.1; Sum m ary of papers on reactive batch distillation 1979 to present day
CO