inorganic papers
i96
Bobev and Bauer YbAg28Si1.72 doi:10.1107/S1600536805013103 Acta Cryst.(2005). E61, i96–i98 Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
YbAg
xSi
2x[
x
= 0.28 (1)] with the tetragonal
a
-ThSi
2structure type
Svilen Bobeva* and Eric D. Bauerb
a
Department of Chemistry and Biochemistry, 304A Drake Hall, University of Delaware, Newark, DE 19716, USA, andbMST-10 Mail Stop K764, Los Alamos National Laboratory, Los Alamos, NM 87545, USA
Correspondence e-mail: sbobev@chem.udel.edu
Key indicators
Single-crystal X-ray study T= 293 K
Mean(SiAg–Si/Ag) = 0.001 A˚ Disorder in main residue Rfactor = 0.009 wRfactor = 0.020
Data-to-parameter ratio = 13.1
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
Single crystals of the title compound, ytterbium silver silicide, were synthesized from the corresponding elements using a eutectic Ag/Si mixture as a solvent. Structure determination suggested the composition of the product to be YbAgxSi2x
[x = 0.28 (1)], i.e. a new ternary derivative of the -ThSi2
structure type, which crystallizes in the body-centered tetragonal space group I41/amd. The two atoms in the
asymmetric unit lie on special positions with Wyckoff symbols 4a(Yb), and 8e(disordered Ag and Si).
Comment
Binary rare-earth silicides and germanides are important materials, which have been extensively studied in the last two or three decades (Gschneider & Eyring, 1979). Of specific interest to us was the divalent oxidation state of Eu and the mixed valency of Yb in some silicides and germanides and their derivatives. The pronounced stability of the divalent Eu2+and Yb2+oxidation states can be explained by their half-filled and completely half-filled 4f-shells, respectively.
The purpose of the present work was to study the variations of the polyanionic network of such europium and ytterbium compounds as a function of electron count, electronegativity, and constituent size. Some results from the systematic inves-tigation of the physical properties and chemical bonding in REAlxSi2x compounds (RE is a rare earth) adopting the -ThSi2structure type (Villars & Calvert, 1991) have already
been published (Bobevet al., 2005). Those studies confirmed wide homogeneity regions in both systems, which present a significant challenge for obtaining the phases as pure products and with defined composition. We report here the synthesis and structural characterization of a new member of the family, YbAgxSi2x[x= 0.28 (1)], which also crystallizes in the
body-centered tetragonal-ThSi2structure type. Detailed physical
property studies of this new material will be reported in a forthcoming publication.
The-ThSi2type is a very common structure among such
intermetallics. As described already, many of these are indeed non-stoichiometric phases with large stoichiometry ranges. This fact, together with the rather anisotropic physical prop-erties one might expect from the crystal structure type, presents challenges for researchers in this field.
To circumvent these difficulties, we employed the flux-growth technique (Canfield & Fisk, 1992) to obtain high-quality single crystals of YbAgxSi2x [x = 0.28 (1)]. The
availability of sizeable single crystals in this and many other cases proved very important for unequivocal structure deter-mination and precise property measurements. The title compound was successfully prepared in good yield from an Ag–Si low-melting eutectic mixture.
The structure of YbAgxSi2x[x= 0.28 (1)] is a new ternary
derivative of the -ThSi2 structure type (Villars & Calvert,
1991). Notably, the binary phase YbSi2is known, although it is
also not fully stoichiometric and adopts the AlB2 structure
type (Villars & Calvert, 1991). YbAgxSi2x [x = 0.28 (1)],
therefore, is a rare example of a ternary phase, based on a structure type very common among the binary rare earth metals, but yet not realised in the Yb–Si system.
The lattice parameters a = 4.0757 (7) A˚ and c = 14.1965 (11) A˚ compare well with those for other REAgxSi2x
phases, such as CeAgxSi2x (Cordruwisch et al., 2001).
Although YbSi2(or rather YbSi2x) with the-ThSi2structure
type does not exist, an elongation of the crystal axes, especially the c axis, is clearly seen for YbAgxSi2x [x = 0.28 (1)] in
comparison with those for other unsubstituted RESi2x
compounds. Such expansion of the unit cell is typical in other solid solutions REMxSi2x (M = main-group or transition
metal), and is due to the larger atomic size of Ag compared with that of Si. Thus, all interatomic Si—Si distances are slightly longer than those found in pure binary phases (Villars & Calvert, 1991).
YbAgxSi2x[x= 0.28 (1)] and its parent structure,-ThSi2,
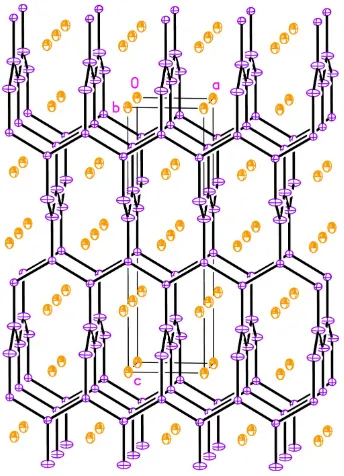
can be viewed as polar intermetallics,i.e.compounds formed by electropositive and electronegative metals and semi-metals. The structure can be considered as made up of an Si-based polyanionic subnetwork, with the rare-earth cations occupying the voids and channels within it, as shown in Fig. 1. The Yb atom is situated on a site with 4m2 symmetry, whereas the Si site has 2mm symmetry. The Si—Si contacts fall within the narrow range 2.3463 (16)–2.3664 (8) A˚ , which agrees with the
description above. The shortest Yb—Si contact is 3.1116 (3) A˚ , a distance normal for such a high coordination number (Fig. 2).
Experimental
All starting materials were used as received: Yb (Ames Laboratory, ingot, 99.99% metal basis), Ag (Alfa, foil, 99.999%) and Si (Alfa, pieces, 99.999%). A mixture of the elements in a ratio Yb:Ag:Si = 1:0.11:0.89 was loaded in an alumina crucible, which was subsequently enclosed in an evacuated fused silica jacket by flame-sealing. The reaction was carried out at a temperature of 1423 K for 2 h, followed by slow cooling (4 K h1) to 1148 K. At this point, the molten flux was removed by centrifugation. The product of the reaction were small crystals with silver metallic luster. These were later identified as YbAgxSi2x[x= 0.28 (1)]. The crystals are stable in air and moisture
over extended periods of time.
Crystal data
YbAg28Si1.72
Mr= 251.76
Tetragonal,I41=amd
a= 4.0757 (2) A˚
c= 14.1965 (11) A˚
V= 235.82 (2) A˚3
Z= 4
Dx= 7.091 Mg m
3
MoKradiation Cell parameters from 796
reflections
= 5.2–30.9 = 42.37 mm1
T= 293 (2) K Bar, grey
0.050.040.03 mm
Data collection
Bruker SMART APEX diffractometer
!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 2003)
Tmin= 0.155,Tmax= 0.280
796 measured reflections
118 independent reflections 107 reflections withI> 2(I)
Rint= 0.018
max= 30.9
h=5!5
k=5!4
l=20!19
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.009
wR(F2) = 0.020
S= 1.23 118 reflections 9 parameters
w= 1/[2(F
o2) + (0.0097P)2
+ 0.0681P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001 max= 0.45 e A˚
3 min=0.66 e A˚
3
Extinction correction:SHELXL97
Extinction coefficient: 0.0086 (6)
inorganic papers
Acta Cryst.(2005). E61, i96–i98 Bobev and Bauer YbAg28Si
[image:2.610.85.257.71.309.2]1.72
i97
Figure 2A view of the Yb coordination polyhedron in YbAgxSi2x[x= 0.28 (1)]. Displacement ellipsoids are drawn at the 90% probability level.
Figure 1
[image:2.610.363.511.74.206.2]Table 1
Selected interatomic distances (A˚ ).
Yb—Si/Agi
3.1116 (3) Si/Ag—Si/Agii 2.3463 (16) Si/Ag—Si/Agi
2.3664 (8)
Si/Ag—Ybi
3.1116 (3)
Si/Ag—Ybiii 3.1302 (6)
Symmetry codes: (i)1 2x;
1 2y;
1
2z; (ii)y 1 4;
1 4x;
3
4z; (iii)x;y1;z.
Structure solution and refinement were performed with origin choice 2 of the space groupI41/amd. The structure refinement based
on a composition ‘YbSi2’ converged at poor residuals and two
crys-tallographically unique sites (Yb and Si) exhibited unusually aniso-tropic displacement parameters. By freeing the site-occupation factor for an individual atom, while other remaining parameters were kept fixed, it became evident that the Si site is statistically occupied by Si and Ag atoms. The Si/Ag site was found to be a nearly 85:15 statistical mixture of Si and Ag, whereas the Yb site is fully occupied with deviations from full occupancy within less than 3.
Data collection:SMART(Bruker, 2002); cell refinement:SAINT
(Bruker, 2002); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2001); program(s) used to refine
structure:SHELXTL; molecular graphics:XPin SHELXTL; soft-ware used to prepare material for publication:SHELXTL.
This work was funded in part by a University of Delaware start-up grant. Work at LANL is performed under the auspices of the DOE.
References
Bobev, S., Tobash, P. H., Fritsch, V., Hundley, M. F., Thompson, J. D., Sarrao, J. L. & Fisk, Z. (2005).J. Solid State Chem.In the press.
Bruker (2002).SMARTandSAINT.Bruker AXS Inc., Madison, Wisconsin, USA.
Canfield, P. C. & Fisk, Z. (1992).Philos. Mag. B,65, 1117–1123.
Cordruwisch, E., Kaczorowski, D., Rogl, P., Saccone, A. & Ferro, R. (2001).J. Alloys Compds,320, 308–319.
Gschneider, K. A. Jr. & Eyring, L. (1979).Handbook on the Physics and Chemistry of Rare Earths. Amsterdam: North Holland.
Sheldrick, G. M. (2001).SHELXTL.Bruker AXS Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (2003).SADABS.University of Go¨ttingen, Germany. Villars, P. & Calvert, L. D. (1991).Pearson’s Handbook of Crystallographic
Data for Intermetallic Compounds, 2nd ed. Materials Park, Ohio, USA: American Society for Metals.
inorganic papers
i98
Bobev and Bauer YbAg28Sisupporting information
sup-1
Acta Cryst. (2005). E61, i96–i98
supporting information
Acta Cryst. (2005). E61, i96–i98 [https://doi.org/10.1107/S1600536805013103]
YbAg
xSi
2−x[
x
= 0.28
(1)] with the tetragonal
α
-ThSi
2structure type
Svilen Bobev and Eric D. Bauer
ytterbium silver silicide
Crystal data YbAg.28Si1.72
Mr = 251.76
Tetragonal, I41/amd
Hall symbol: -I 4bd 2 a = 4.0757 (2) Å c = 14.1965 (11) Å V = 235.82 (2) Å3
Z = 4 F(000) = 429
Dx = 7.091 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 796 reflections θ = 5.2–30.9°
µ = 42.37 mm−1
T = 293 K Bar, grey
0.05 × 0.04 × 0.03 mm
Data collection Bruker APEX SMART
diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 8.3 pixels mm-1
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 2003) Tmin = 0.155, Tmax = 0.280
796 measured reflections 118 independent reflections 107 reflections with I > 2σ(I) Rint = 0.018
θmax = 30.9°, θmin = 5.2°
h = −5→5 k = −5→4 l = −20→19
Refinement Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.009
wR(F2) = 0.020
S = 1.23 118 reflections 9 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
w = 1/[σ2(F
o2) + (0.0097P)2 + 0.0681P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.45 e Å−3
Δρmin = −0.66 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.0086 (6)
Special details
supporting information
sup-2
Acta Cryst. (2005). E61, i96–i98
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
Yb 0.0000 0.7500 0.1250 0.00700 (14)
Si 0.0000 0.2500 0.29237 (6) 0.0077 (4) 0.858 (4) Ag 0.0000 0.2500 0.29237 (6) 0.0077 (4) 0.142 (4)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Yb 0.00575 (15) 0.00575 (15) 0.00951 (17) 0.000 0.000 0.000 Si 0.0044 (5) 0.0145 (6) 0.0041 (4) 0.000 0.000 0.000 Ag 0.0044 (5) 0.0145 (6) 0.0041 (4) 0.000 0.000 0.000
Geometric parameters (Å, º)
Yb—Agi 3.1116 (3) Si—Agix 2.3463 (16)
Yb—Agii 3.1116 (3) Si—Siix 2.3463 (16)
Yb—Sii 3.1116 (3) Si—Agiv 2.3664 (8)
Yb—Siii 3.1116 (3) Si—Siiv 2.3664 (8)
Yb—Agiii 3.1116 (3) Si—Agviii 2.3664 (8)
Yb—Agiv 3.1116 (3) Si—Siviii 2.3664 (8)
Yb—Siiii 3.1116 (3) Si—Ybii 3.1116 (3)
Yb—Siiv 3.1116 (3) Si—Ybiv 3.1116 (3)
Yb—Agv 3.1116 (3) Si—Ybvii 3.1116 (3)
Yb—Agvi 3.1116 (3) Si—Ybviii 3.1116 (3)
Yb—Agvii 3.1116 (3) Si—Ybx 3.1302 (6)
Yb—Agviii 3.1116 (3)
Agi—Yb—Agii 180.00 (3) Agvi—Yb—Agviii 44.30 (3)
Agi—Yb—Sii 0.00 (3) Agvii—Yb—Agviii 135.70 (3)
Agii—Yb—Sii 180.0 Agix—Si—Agiv 120.55 (3)
Agi—Yb—Siii 180.00 (3) Siix—Si—Agiv 120.55 (3)
Agii—Yb—Siii 0.00 (3) Siix—Si—Siiv 120.55 (3)
Sii—Yb—Siii 180.00 (3) Agix—Si—Agviii 120.55 (3)
Agi—Yb—Agiii 135.70 (3) Siix—Si—Agviii 120.55 (3)
Agii—Yb—Agiii 44.30 (3) Agiv—Si—Agviii 118.90 (7)
Sii—Yb—Agiii 135.70 (3) Siiv—Si—Agviii 118.90 (7)
Siii—Yb—Agiii 44.30 (3) Agix—Si—Siviii 120.55 (3)
supporting information
sup-3
Acta Cryst. (2005). E61, i96–i98
Agii—Yb—Agiv 135.70 (3) Agiv—Si—Siviii 118.90 (7)
Sii—Yb—Agiv 44.30 (3) Siiv—Si—Siviii 118.90 (7)
Siii—Yb—Agiv 135.70 (3) Agix—Si—Ybii 67.851 (14)
Agiii—Yb—Agiv 180.0 Siix—Si—Ybii 67.851 (14)
Agi—Yb—Siiii 135.70 (3) Agiv—Si—Ybii 139.082 (6)
Agii—Yb—Siiii 44.30 (3) Siiv—Si—Ybii 139.082 (6)
Sii—Yb—Siiii 135.70 (3) Agviii—Si—Ybii 68.139 (14)
Siii—Yb—Siiii 44.30 (3) Siviii—Si—Ybii 68.139 (14)
Agiii—Yb—Siiii 0.00 (3) Agix—Si—Ybiv 67.851 (14)
Agiv—Yb—Siiii 180.0 Siix—Si—Ybiv 67.851 (14)
Agi—Yb—Siiv 44.30 (3) Agiv—Si—Ybiv 68.139 (14)
Agii—Yb—Siiv 135.70 (3) Siiv—Si—Ybiv 68.139 (14)
Sii—Yb—Siiv 44.30 (3) Agviii—Si—Ybiv 139.082 (6)
Siii—Yb—Siiv 135.70 (3) Siviii—Si—Ybiv 139.082 (6)
Agiii—Yb—Siiv 180.0 Ybii—Si—Ybiv 135.70 (3)
Agiv—Yb—Siiv 0.0 Agix—Si—Ybvii 67.851 (14)
Siiii—Yb—Siiv 180.0 Siix—Si—Ybvii 67.851 (14)
Agi—Yb—Agv 81.828 (10) Agiv—Si—Ybvii 68.139 (14)
Agii—Yb—Agv 98.172 (10) Siiv—Si—Ybvii 68.139 (14)
Sii—Yb—Agv 81.828 (10) Agviii—Si—Ybvii 139.082 (6)
Siii—Yb—Agv 98.172 (10) Siviii—Si—Ybvii 139.082 (6)
Agiii—Yb—Agv 81.828 (10) Ybii—Si—Ybvii 81.828 (10)
Agiv—Yb—Agv 98.172 (10) Ybiv—Si—Ybvii 81.828 (10)
Siiii—Yb—Agv 81.828 (10) Agix—Si—Ybviii 67.851 (14)
Siiv—Yb—Agv 98.172 (10) Siix—Si—Ybviii 67.851 (14)
Agi—Yb—Agvi 81.828 (10) Agiv—Si—Ybviii 139.082 (6)
Agii—Yb—Agvi 98.172 (10) Siiv—Si—Ybviii 139.082 (6)
Sii—Yb—Agvi 81.828 (10) Agviii—Si—Ybviii 68.139 (14)
Siii—Yb—Agvi 98.172 (10) Siviii—Si—Ybviii 68.139 (14)
Agiii—Yb—Agvi 81.828 (10) Ybii—Si—Ybviii 81.828 (10)
Agiv—Yb—Agvi 98.172 (10) Ybiv—Si—Ybviii 81.828 (10)
Siiii—Yb—Agvi 81.828 (10) Ybvii—Si—Ybviii 135.70 (3)
Siiv—Yb—Agvi 98.172 (10) Agix—Si—Ybx 139.381 (10)
Agv—Yb—Agvi 135.70 (3) Siix—Si—Ybx 139.381 (10)
Agi—Yb—Agvii 98.172 (10) Agiv—Si—Ybx 67.30 (3)
Agii—Yb—Agvii 81.828 (10) Siiv—Si—Ybx 67.30 (3)
Sii—Yb—Agvii 98.172 (10) Agviii—Si—Ybx 67.30 (3)
Siii—Yb—Agvii 81.828 (10) Siviii—Si—Ybx 67.30 (3)
Agiii—Yb—Agvii 98.172 (10) Ybii—Si—Ybx 135.443 (14)
Agiv—Yb—Agvii 81.828 (10) Ybiv—Si—Ybx 81.941 (6)
Siiii—Yb—Agvii 98.172 (10) Ybvii—Si—Ybx 135.443 (14)
Siiv—Yb—Agvii 81.828 (10) Ybviii—Si—Ybx 81.941 (6)
Agv—Yb—Agvii 44.30 (3) Agix—Si—Yb 139.381 (10)
Agvi—Yb—Agvii 180.0 Siix—Si—Yb 139.381 (10)
Agi—Yb—Agviii 98.172 (10) Agiv—Si—Yb 67.30 (3)
Agii—Yb—Agviii 81.828 (10) Siiv—Si—Yb 67.30 (3)
Sii—Yb—Agviii 98.172 (10) Agviii—Si—Yb 67.30 (3)
supporting information
sup-4
Acta Cryst. (2005). E61, i96–i98
Agiii—Yb—Agviii 98.172 (10) Ybii—Si—Yb 81.941 (6)
Agiv—Yb—Agviii 81.828 (10) Ybiv—Si—Yb 135.443 (14)
Siiii—Yb—Agviii 98.172 (10) Ybvii—Si—Yb 81.941 (6)
Siiv—Yb—Agviii 81.828 (10) Ybviii—Si—Yb 135.443 (14)
Agv—Yb—Agviii 180.00 (3) Ybx—Si—Yb 81.24 (2)