Case Report
Classical biphasic pulmonary blastoma in a young
woman: case report and review of literature
Maojiang Yang, Bing Li, Chuan Zhang, Sushant Kumar Das, Hanfeng Yang, Xiaoxue Xu
Department of Pain Management, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Received October 1, 2019; Accepted November 25, 2019; Epub December 1, 2019; Published December 15, 2019
Abstract: Pulmonary blastoma (PB) is a very rare malignant lung tumor, consisting of immature epithelium and/ or mesenchymal tissue. The two tissue components are derived from the same precursor cells. In this study, we present a case of a 29-year-old woman with classical biphasic pulmonary blastoma, who underwent right middle
lobe resection and subsequent treatment. Due to the low incidence rate and the reclassification of PB, no standard
treatment is available currently. In our case, the patient received radiotherapy and chemotherapy and is doing well at 6 months’ follow up. In retrospect, we review the pathology, clinical manifestations, imaging features and treat-ment of this disease.
Keywords: Classic biphasic pulmonary blastoma, metastasis, treatment, lung
Introduction
Primary pulmonary diphasic differentiation tumor is a very rare malignant tumor. Pulmona- ry blastoma (PB), comprising approximately 0.25%-0.5% of all lung malignancies, is consid-ered to consist of immature epithelium and/or mesenchymal tissue, similar in morphology to fetal lung tissue [1-4]. Since its first descrip-tion it was called “lung embryoma” [5, 6]. Furthermore, there is no standardized treat-ment for this disease. The prognosis of classic biphasic pulmonary blastoma is poor and the overall 5-year survival rate is around 15% [7, 8]. Herein, we describe a 29-year-old woman with classic biphasic pulmonary blastoma (CBPB), who received comprehensive treatment in our unit.
Case report
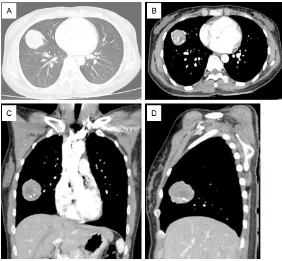
A 29-year-old woman presented with cough and phlegm accompanied by blood in sputum for three months. On chest X-ray examination at local hospital an abnormal shadow was detect-ed on the right lung field. After 2 weeks of anti-inflammatory treatment, the condition did not alleviate. She was then transferred to our hos-pital for treatment. Contrast enhanced chest CT was performed which revealed a 5.1×4.1×
4.1 cm nidus in the middle lobe of right lung (Figure 1). The boundary of the lesion was clear and smooth, the enhancement was uneven, and many small patches of necrosis could be seen in it. In addition, several large vessels were seen passing through the margin and inside the lesion. Tumor marker examination revealed gastrin-releasing peptide precursor was 116.30 pg/ml, and cytokeratin 19 frag-ment was 5.88 pg/m. A CT-guided percutane-ous needle biopsy of the lung tumor was per-formed. Results showed that the epithelial cells had adenoid and squamous cell distribution. Immunohistochemical staining showed ER (-), PR (-), TTF-1 (+), P40 (+), and CDX2 (+), mainly composed of adenocarcinoma. Some of the tumor cells expressed P40, suggesting the pos-sibility of adenosquamous carcinoma (Figure 2).
hetero-morphic spindle cells were seen. Immunohi- stochemical staining showed CK (epithelial component +), TTF-1 (adenoid region +), CgA (-), P40 (squamous differentiation component +), vimentin (spindle cell +), desmin (-), CD34 (par-tial spindle cell +), Ki67 (epithelial region 60%, spindle cell region 30%), SMA (-), and beta-catenin (+) (Figure 3). Tumors had dual differen-tiation, most of which were low-grade fetal ade-nocarcinomas, and a few (about 3%) were immature mesenchymal cells, which is consis-tent with pulmonary blastoma. Subsequently, she received radiotherapy and nedaplatin as
chemotherapy and is doing well at 6 months’ followup. Discussion
[image:2.612.89.371.70.331.2]PB is an uncommon primary lung malignancy with a fre-quency of only 0.25-0.5% of primary lung malignancies [1- 4]. It was first described by Barrett in 1945, and then Barnard named it as “embryo-ma”. Later it was renamed as “pulmonary blastomalater” [5, 6, 9]. In the past, PB was clas-sified into three subgroups: (1) classic biphasic pulmonary blastoma (CBPC), character-ized by histopathologic hetero-geneity, i.e. intermixing of epi-thelial and mesenchymal ma- lignant cells, (2) well-differen- tiated fetal adenocarcinoma (WDFA), characterized by a monophasic epithelial tumor, and (3) pleuro-pulmonary blas-toma (PPB), characterized by a monophasic mesenchymal tumor. According to the 2015 WHO classification, PB is con-sidered as a type of sarcoma-toid carcinoma and is defined as a biphasic tumor consisting of fetal adenocarcinoma (typi-cally low-grade) and primi- tive mesenchymal stroma [10]. Actual cases of PB may be less than those previously reported because many earlier reports on PB have included fetal ade-nocarcinoma and PPB [11]. Figure 1. Typical CT (computed tomography) images of our patient. Contrast
enhanced chest CT was performed which revealed a 5.1×4.1×4.1 cm nidus in the middle lobe of right lung. (A) Lung window, (B) Mediastinal window, (C, D) Coronal and sagittal CT.
Figure 2. The results of CT-guided puncture. (A) Epithelial cells had adenoid and squamous cell distribution, (B) Immunohistochemical staining showed ER (-), PR (-), TTF-1 (+), P40 (+), and CDX2 (+), mainly composed of adeno-carcinoma. Some of the tumor cells expressed P40.
[image:2.612.89.374.399.507.2]while others are often found by accident [15-17]. In our patient, cough and phlegm accom-panied by blood in sputum were the major symptoms.
Currently, there is no specific serum marker for pulmonary blastomas [18]. Serum levels of tumor markers, AFP, NSE, and CEA, were increased in our patient as reported in the previous reports. Especially, increased AFP positive tumor cells have been seen in most pulmonary blastoma cases [19, 20]. In this case study, we found abnormal increases in gastrin-releasing peptide precursors and cyto-keratin 19 fragments. On imaging, lesions often present as solitary masses in the periph-eral pulmonary zone with clear margins. Contrast enhanced CT scans often show a het-erogeneous enhancing soft tissue mass with smooth or lobulated margins and probable areas of necrosis [21]. In this patient, in addi-tion to the above findings, several large blood vessels were seen passing through the lesion and surrounding the lesion.
Due to the biphasic differentiation of tumors, general fine needle aspiration biopsy often can-not make a definite diagnosis due to lack of materials. The diagnosis depends on postop-erative pathologic analysis [11, 15]. Immuno- histochemical analysis is of great significance in the diagnosis of this disease, especially com-bining epithelial markers with mesenchymal markers [22-24]. Similar to previous literature, we found CK (epithelial component +), TTF-1 (adenoid region +), P40 (squamous differentia-tion component +), vimentin (spindle cell +), CD34 (partial spindle cell +), Ki67 (epithelial
therapy has not been well consolidated because it is still rare, though radiotherapy and chemotherapy regimens are progressing rapid-ly [25]. The prognosis of biphasic pulmonary blastoma is extremely poor as distant metasta-sis is common. As reported, the 5-year survival rate and 10-year survival rate of BPB are 16%-25% and 8% respectively [13]. The reported median survival is 26 months in patients under-going lobectomy or lobectomy, and the median survival is 9 months in patients undergoing pneumonectomy [26]. In our case, the patient underwent a right middle lobe resection and was treated with nedaplatin. After a follow-up of six months, the patient’s condition was stable and no recurrence or distant metastasis was observed.
Conclusions
Classical biphasic pulmonary blastoma is rare. Surgical resection is the main method for its treatment. In addition, radiotherapy and che-motherapy have also been reported, but so far, no consensus has been reached.
Acknowledgements
We owe thanks to the patient and her family for their willingness to provide information. The authors thank all of the staffs who contributed in this paper.
Disclosure of conflict of interest
None.
Address correspondence to: Xiaoxue Xu, Depart-
[image:3.612.91.371.72.177.2]ment of Pain Management, Affiliated Hospital of
Figure 3. Postoperative pathologic results. A. Tumors show classical bipha-sic differentiation. B. Immunohistochemical staining showed CK (epithelial component +), TTF-1 (adenoid region +), CgA (-), P40 (squamous differen-tiation component +), vimentin (spindle cell +), desmin (-), CD34 (partial spindle cell +), Ki67 (epithelial region 60%, spindle cell region 30%), SMA (-), and beta-catenin (+).
North Sichuan Medical College, Nanchong, China. E-mail: nclittlesnownc@163.com
References
[1] Weissferdt A and Moran CA. Malignant bipha-sic tumors of the lungs. Adv Anat Pathol 2011; 18: 179-189.
[2] Camerlingo R, Franco R, Tirino V, Cantile M, Rocchi M, La Rocca A, Martucci N, Botti G, Rocco G and Pirozzi G. Establishment and
phe-notypic characterization of the first human pul -monary blastoma cell line. Lung Cancer 2011; 72: 23-31.
[3] Macher-Goeppinger S, Penzel R, Roth W, Di-enemann H, Thomas M, Schnabel PA,
Schirm-acher P and Bläker H. Expression and mtation analysis of EGFR, c-KIT, and β-catenin in pul -monary blastoma. J Clin Pathol 2011; 64: 349-353.
[4] Nakayama T, Ohtsuka T, Kazama A and Wata -nabe K. Classic pulmonary blastoma: a sub-type of biphasic pulmonary biastoma. Ann Tho-rac Cardiovasc Surg 2012; 18: 125-127. [5] Barrett NR and Barnard WG. Some unusual
thoracic tumors. Br J Surg 1945; 32: 447-457. [6] BARNARD WG. Embryoma of lungs. Thorax
1952; 7: 299-301.
[7] Meng Z, Chen P, Zang F, Liu Y, Xu X, Su Y, Chen J, Lin L, Zhang L and Zhang T. A patient with classic biphasic pulmonary blastoma harbor-ing CD74-ROS1 fusion responds to crizotinib. Onco Targets Ther 2017; 11: 157-161. [8] Zhao YY, Liu L, Zhou T, Zhou NN, Yang YP, Hou
X, Li Y, Zhao HY, Huang Y and Zhang L. A retro-spective analysis of the clinicopathological and molecular characteristics of pulmonary blastoma. Onco Targets Ther 2016; 9: 6915-6920.
[9] Bosch-Barrera J, Holguin F, Baldó X, Rubio M, Porta R, Fuentes R, Teixidó C, Ramirez JL, Fer-ran N, Sebastián F and Rosell R. Neoadjuvant chemoradiotherapy treatment for a classic bi-phasic pulmonary blastoma with high PD-L1 expression. Anticancer Res 2015; 35: 4871-4875.
[10] WHO Classification of Tumors of the Lung,
Pleura, Thymus and Heart. In: Travis WD,
Brambilla E, Burke AP, Marx A and Nicholson
AG, editors. Lyon, France: ARC Press; 2015. [11] Chen CC, Yang SF, Lin PC, Luo CW, Chou SH,
Dai ZK and Yin HL. Pulmonary blastoma in chil-dren: report of a rare case and review of the literature. Int J Surg Pathol 2017; 25: 721-726.
[12] Van Loo S, Boeykens E, Stappaerts I and Rut -saert R. Classic biphasic pulmonary blastoma: a case report and review of the literature. Lung Cancer 2011; 73: 127-132.
[13] Koss MN, Hochholzer L, OL and Ool T. Pulmo-nary blastoma. Cancer 1991; 67: 2368-2381. [14] Zaidi A, Zamvar V, Macbeth F, Gibbs AR,
Kulati-lake N and Butchart EG. Pulmonary blastoma:
medium-term results from a regional center. Ann Thorac Surg 2002; 73: 1572-1575. [15] Zhao YY, Liu L, Zhou T, Zhou NN, Yang YP, Hou
X, Li Y, Zhao HY, Huang Y and Zhang L. A retro-spective analysis of the clinicopathological and molecular characteristics of pulmonary blastoma. Onco Targets Ther 2016; 9: 6915-6920.
[16] Liman ST, Altinok T, Topcu S, Tastepe AI, Uzar
A, Demircan S and Demirag F. Survival of biphasic pulmonary blastoma. Respir Med 2006; 100: 1174-1179.
[17] Adluri RK, Boddu SR, Martin-Ucar A, Duffy JP, Beggs FD and Morgan WE. Pulmonary blasto-ma-a rare tumor with variable presentation. Eur J Cardiothorac Surg 2006; 29: 236-239. [18] Force S and Patterson GA. Clinical-pathologic
conference in general thoracic surgery: pulmo-nary blastoma. J Thorac Cardiovasc Surg 2003; 126: 1247-50.
[19] Iwata T, Nishiyama N, Inoue K, Kawata Y, Izumi
N, Tsukioka T, Shinkawa K and Suehiro S. Bi -phasic pulmonary blastoma: report of a case. Ann Thorac Cardiovasc Surg 2007; 13: 40-43. [20] Nakatani Y, Kitamura H, Inayama Y, Kamijo S,
Nagashima Y, Shimoyama K, Nakamura N, Sano J, Ogawa N, Shibagaki T, Resl M and Mark EJ. Pulmonary adenocarcinomas of the
fetal lung type: a clinicopathologic study indi-cating differences in histology, epidemiology, and natural history of low-grade and high-grade forms. Am J Surg Pathol 1998; 22: 399-411.
[21] Smyth RJ, Fabre A, Dodd JD, Bartosik W, Gal -lagher CG and McKone EF. Pulmonary blasto-ma: a case report and review of the literature. BMC Res Notes 2014; 7: 294.
[22] Nakatani Y, Miyagi Y, Takemura T, Oka T, Yokoi T, Takagi M, Yokoyama S, Kashima K, Hara K, Yamada T, Nozawa A, Inayama Y, Sakamoto K,
Ogawa N, Kitamura H, Resl M, Cho SH,
Koss MN and Mark EJ. Aberrant nuclear/cyto -plasmic localization and gene mutation of be-ta-catenin in classic pulmonary blastoma: Be-ta-catenin immunostaining is useful for distinguishing between classic pulmonary blastoma and a blastomatoid variant of carci-nosarcoma. Am J Surg Pathol 2004; 28: 921-927.
[23] Hansen T, Bittinger F, Kortsik C, Wagner B and Kirkpatrick CJ. Expression of KIT (CD117) in
biphasic pulmonary blastoma. Novel data on histogenesis. Lung 2003; 181: 193-200. [24] Kilic D, Yilmaz C, Tepeoglu M, Vural C and
ed with cerebral metastasis. Turk Neurosurg
2016; 26: 169-172.
[25] Vossler JD and Abdul-Ghani A. Pulmonary blas-toma in an adult presenting with hemoptysis and hemothorax. Ann Thorac Surg 2019; 107: e345-e347.