With 9 figures Printed in Great Britain
SIZE-DEPENDENT VARIATION IN THE EVASIVE
BEHAVIOUR OF THE BIVALVE MOLLUSC
SPISULA SOLIDISSIMA
BY DAVID J. PRIOR, ANNE M. SCHNEIDERMAN* AND SHARON I. GREENEf
Physiology Group, School of Biological Sciences, University of Kentucky, Lexington, KY 40506 and Marine Biological Laboratory, Woods Hole,
MA 02543
(Received 14 March 1978)
SUMMARY
1. The evasive jump response of Spisula can be elicited by contact of the siphons with the tube feet of a starfish (Asterias forbesi).
2. The level of responsiveness varies with the size of the individual clam; small clams (2-0-5-0 cm) being very responsive, large clams
(12-0-18-0 cm) being totally unresponsive.
3. The cell bodies of touch-sensitive neurones subserving the siphons are located in the visceral ganglion. The mean area of the receptive fields of these neurones in small clams is 33-8 mm2 and in large clams is 9-7 mm2.
4. In small clams the large proportion of the total siphon surface inner-vated by single touch-sensitive neurones results in considerable overlap of receptive fields. As a result of this overlap, numerous touch-sensitive neurones are activated by a point stimulus.
5. The variation in jump responsiveness of large and small clams is correlated with the size of the receptive fields of touch-sensitive neurones.
INTRODUCTION
Evasive behaviour in sessile animals is unexpected. Bivalves, however, provide numerous dramatic examples of such behaviour. Probably the most widely known is the swimming response of the bay scallop, Aequipecten irradians (e.g. Dakin, 1910; Yonge, 1936; Mellon, 1969). Several bivalves in the superfamily, Pectinacea (in-cluding Aequipecten),' swim by repetitive adductions of their shell valves, each ad-duction ejecting a pulse of water from the mantle cavity. Sustained sequences of rapid adductions serve to propel the animal through the water. An adequate stimulus for this evasive swimming behaviour is contact with the tube feet of a starfish (e.g. Asterias forbesi). Evasive behaviour in response to contact with starfish tube feet is quite common among bivalves as well as numerous gastropod species: for example, swimming in Tritonia (Willows, 1967; Willows, Dorsett & Hoyle, 1973), jumping in
60 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE
Strombus (Berg, 1972), and rapid shell twisting in the abalone (Montgomery, 1967). Ansell has provided an extensive review of evasive behaviour in the Mollusca (1968). We have studied the evasive jumping behaviour of the surf clam, Spisula solidis-sima. Briefly, the escape jump of a Spisula lying on the substrate begins with ex-tension of the foot followed by its insertion between the lower shell valve and the substrate. One side of the foot then contracts, catapulting the animal up and through the water (see Fig. 1). Our initial observation was that small Spisula (2-0-70 cm shell length, from anterior to posterior) responded to contact with a starfish (A. forbesi) with a vigorous jump or series of jumps, whereas large Spisula (IO-O-I8-O cm) did not. Thus, the responsiveness of an individual surf clam appeared to be correlated with its size.
In this paper we describe the results of experiments designed to analyse this size-dependent variation in behavioural responsiveness.
METHODS AND MATERIALS
Spisula solidissima and Asterias forbesi were obtained from the Supply Department of the Marine Biological Laboratory, Woods Hole, Mass. All animals were collected in 5-10 ft of water in Menemsha Bay, Martha's Vineyard. Animals were kept in fresh running sea water at ambient temperatures in Woods Hole and in Instant Ocean Systems (10—15 °C) in Lexington.
Behaviour
The behavioural observations were conducted in sea tables at the M.B.L. Freshly collected animals were tested in shallow trays of sand in fresh circulating sea water (20-22 °C, in July).
Various aversive stimuli were applied to the siphons and the latencies from siphon contact with the stimulus to the first response (usually an adduction) and to the second response (if it jumped) were measured with stopwatches. The clams were tested only when the siphons were fully extended and open. Following application of a stimulus to the siphons, each clam was observed for a 1 min test period.
To study possible temperature effects, clams were placed in buckets of sea water which were kept in cold room to attain the desired temperature ( u - i 4 ° C ) . The cold clams were transferred to water at 20-22 °C and tested as soon as their siphons were extended, which usually took from 1 to 2 min. In addition, another group of animals were maintained at 5 °C in buckets of sea water in a cold room.
Various types of aversive stimuli were used to test the behavioural responsiveness of each group of animals.
Intact starfish
Glass probe
A Pasteur pipette (tip drawn out when used on small clams) was used to apply a tactile stimulus to the siphons. The stimulus (gently stroking and poking) was applied for i min or until the clam completely adducted its shell valves or ' puffed' vigorously enough to change its position. Latencies were again measured from the application of the stimulus for a i min test period.
Glass probe and starfish
The regular tactile stimulation was applied with a glass probe but in addition, a starfish was held close to the siphons (but not touching). The response latencies were measured.
Starfish homogenate
Starfish were homogenized in sea water, the homogenate centrifuged and the supernatant used as a stimulus. Various volumes (0-5-5-0 ml) of this pure"e of starfish were squirted at the siphons of clams and the type (not latency) of response recorded. In some cases starfish pure"e and tactile stimulation (with a glass probe) were applied simultaneously and the response latencies measured.
Isolated tube feet
Tube feet were removed from live starfish, held in forceps and applied to the siphons of clams. The response latencies were measured as with the other modes of stimu-lation.
Physiology
Standard intracellular and extracellular recording and stimulating methods were used to obtain electrophysiological data. Glass microelectrodes filled with potassium acetate (2-5 M) having tip resistances of from 20-60 m£2 were used to obtain intra-cellular recordings. All preparations were bathed in filtered fresh sea water or artificial sea water (MBL formula IV). The basic preparation used to map the receptive fields of TSNs was identical to that used by Mellon (1972) so that the results would be directly comparable. Preparations were made by cutting the incurrent siphon so that the siphonal apparatus could be spread open exposing the entire inner surfaces of both siphons and all siphonal tentacles (see Fig. 2). Results obtained from pre-parations in which the siphons were left intact were comparable to those obtained with the split-siphon preparations. The receptive fields of TSNs could be more accurately determined in the split-siphon preparation hence it was used for the mapping procedures. The procedure for mapping receptive fields involved recording intracellularly from single TSNs while applying tactile stimuli to the siphon surface. The receptive field of each TSN was outlined on a graph paper scale drawing of the individual siphon preparation. These drawings were cut out and weighed in a constant temperature balance room in one afternoon. Using the weight per unit area of the graph paper, the area of the receptive field of each TSN and of each siphon preparation was calculated.
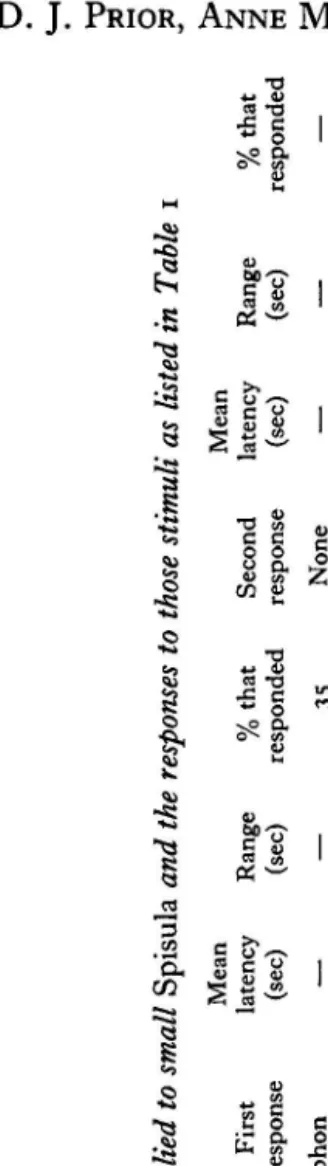
Table I. The table lists details of behavioural observations made on three size groups of Spisula following their contact with the tube feet of the starfih, Asterias forbesi, or a glass probe (The small animals responded to starfish stimulation with an initial quick adduction that resulted in a 'puff' forcing water out of the siphons. Following the 'puff response' with a variable latency, a majority of the clams jumped. In this group the animals tested at 14 OC were the most responsive, 77 % of the trials resulting in jumps. Tactile stimulation of the siphons of small clams with a glass probe resulted in a 'puff response' and sustained adduction of the shell valves which was never followed by a jump. The medium and large Spisula were found to be far less responsive than the small animals, the large clams never jumping in response to contact with starfish tube feet.)
Size Small
(N = I 12 ; 2.0-5.0 cm) Medium (N = 41 ; 5.4 = 11.5 cm) Large (N = 83 ; 12.0-18.0 cm)
Stimulus Starfish Starfish Starfish Glass
probe
Glass
probe
Starfish Starfish Glass
probe
Glass
probe
Starfish Starfish Glass
probe
Glass
probe
Temp. ("C)
22 14 5 23 9 22 I3 22 16 22 I I 22 I I
No.
of
trials 88 62 92 29 57 4
I
38 38 37 '03 57 46 72
First
response Puff Puff Puff
Puffladduct Puff/adduct Puff/adduct Puff/addcut Puff/adduct Puffladduct
Puff Puff Puff Puff Mean latency (4 6.5 3'6 3'0 4'9 3'3 10.3 4'
I
4'6 3'3 10.8 12'0 4'3 4'7
Range
%
that
(sec)
responded
Second response J~P Jump Jump None None Jump Jump None None None None None None
Range
%
that
(sec)
RESULTS
Behavioural observations
A summary of the behavioural observations is given in Table 1, in which the responses of clams of various sizes are compared.
Responses of small clams
Following contact with the tube feet of a starfish, clams usually retracted their siphons. Occasionally, however, the siphons were extended toward the tube feet. In either case after several seconds, the clams quickly adducted their shell valves, forcing a jet of sea water out of the siphons in a ' puff'. If contact was maintained between the tube feet and the siphons, several adductions often followed. Usually, however, the' puff' response was followed by extension of the foot and a jump response. Frequently there occurred a rapid series of two to seven jumps, although series of two or three were most common. Small clams could move as far as io-o-i5-o cm with each jump. Jumping did not seem to be a directed response. Occasionally the continued activity of the clams resulted in their movement back toward the starfish. An attempt was made to determine which aspect of the complex stimulus provided by a starfish was most effective in eliciting the jumping behaviour (Table 2). Tactile stimulation of the siphons of a small clam with a glass probe resulted in the ' puff' response and progressive adduction of the shell valves, but no jumping. When we tested the role of chemosensory cues by bringing a starfish close (0-5-10 cm away) to the extended siphons we never observed a jump response. In order to provide a potentially greater intensity chemosensory input, we squirted pure'e of starfish toward the extended siphons of small clams. The only response was the ' puff' and progressive adduction. As a control, sea water was squirted at the siphons and was found to elicit only siphon closure and withdrawal.
We tested the effectiveness of combinations of tactile and chemical stimuli in two ways. Initially, we positioned a starfish near the siphons of a clam while simul-taneously stimulating the siphons with a glass probe. This resulted in no more response than did stimulation with only a glass probe. We also paired application of starfish puree and tactile stimulation (glass probe). Here again we observed no more than a ' puff-adduct' response. Several more exotic stimuli were tried that included use of a glass probe that had been dipped in starfish pure'e, use of plastic suction electrodes to simulate the natural suction of tube feet and finally application of suction electrodes that had been dipped in starfish pure'e. None of these stimuli elicited jumping behaviour. We could not simulate the effectiveness of the tube feet of an intact starfish. We next removed several tube feet from starfish and used them (forceps held) to apply tactile stimulation to the siphons of small clams. A low level (10%) of jumping was elicited by contact with the tube feet.
Responses of large clams
When the tube feet of a starfish made contact with the siphons of a large clam the only response observed was a brief adduction of the shell valves resulting in a 'puff' response (Table 1). We observed no other response from large Spisula
contacted with starfish tube feet. We did, however, observe large Spisula jumping in a sea table. Hence, they were capable of jumping but did not do so in response to stimulation with a starfish.
Temperature effects
There seemed to be no uniform effect of temperature on the level of responsiveness in small or medium sized clams, although in general, animals maintained in sea water at temperatures below 15 °C were more active (Table 1).
Physiological observations
There are two major alternative explanations for the observed variation in the responsiveness of Spisula to starfish attack: (1) a size-dependent alteration in central nervous system mechanisms (e.g. increased inhibition); (2) alteration in peripheral mechanisms such as the abosolute responsiveness of primary afferent neurones.
Since Mellon (1972) had already provided a description of the physiology of tactile sensory neurones (TSNs) in large Spisula, we tested the possibility that the properties of TSNs could be correlated with the variation in behavioural responsiveness.
Clam on the half shell
By carefully removing the left shell valve of a bivalve, we exposed the entire animal. En passant recordings were made from the siphonal nerves (see Fig. 2) while applying tactile stimuli to the intact siphons. We recorded volleys of afferent and efferent activity during siphon withdrawal and valve adduction. In no case with either large or small animals were the foot movements involved in jumping observed.
[image:7.451.58.405.384.605.2]4.
66 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE A
I.S.N.
Visceral ganglior
Fig. 2. (A) is an illustration of the semi-intact siphon-visceral ganglion preparation used to map the receptive fields of the TSNs. The incurrent siphon has been split and laid open exposing the four siphonal nerves. (B) illustrates the preparation used for simultaneous recording of the afferent activity in the excurrent siphon nerves (2 and 3) and incurrent siphon nerves (1 and 4) resulting from stimulation of areas A, B, C, or D.
67
•U-U
[image:9.451.56.397.54.263.2]80 mV 40 mV 300 ms 150 ms
Fig. 3. Intracellular recordings for T S N s in semi-intact siphon preparations of small Spiiula (4-0-7-0 cm). (A) shows the response of a T S N to tactile stimulation of its receptive field. (B) illustrates the variable amplitude electrotonic potentials often seen in response to stimul-ation of the margins of the receptive field of a T S N . These potentials could be due to blockade of action potentials at some branch point of the T S N . (C) is a higher gain record illustrating the electrical coupling potentials that are often observed in response to tactile stimulation of the edges (and outside) of the receptive field of a T S N . Calibration scale is 300 ms, 80 mV for A and B; 150 ms, 40 mV for C.
44 mV 300 ms
Fig. 4. Intracellular recordings of the activity of T S N s in a semi-intact siphon preparation of a small (6-o cm) Spisula (A) and a large (15-0 cm) Spiiula (B) in response to contact of an intact starfish (Atterias forbesi) with the siphon margin. Calibration scale is 44 mV and 300 ms.
68 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE
00
90
80
70
60 50
40
30
20
10
-r-i
A.
6 7 8 9 Shell length (cm)
Hi-0-9
0-8 0-7 0-6
0-5
0-4 0-3
0-2
01
• a Si
ac
o
£
n
z
10 11 12 14-18
Fig. 5. The areas of individual TSN receptive fields and the areas of the entire siphon surface of clams are plotted as a function of shell length. The total siphon area increases progressively up to animals 7 0 cm long. There is a dramatic increase in siphon area in animals larger than 70 cm. The absolute area of TSN receptive fields is greatest in animals 6-0—7-0 cm long. The standard error of the mean is indicated in each group by a bar. Each size group includes at least five animals and in the case of the receptive fields, five TSNs.
100 90 80 70
(T 60
1 50
40 30 20 10 1 0
TSN (•)
Total siphon (•)
250 268 260
Q B
7946 7 8 Shell length (cm)
which in turn caused further activation of the TSNs (Prior, 1972 a, b). No significant degree of long term adaptation occurred during extended periods (hours) of repeated stimulation of a single TSN.
Mellon (1972) provided maps of the receptive fields of TSNs in large Spisula. We repeated this mapping procedure using animals with a wide range of shell sizes (2-0-18-0 cm). We recorded intracellularly from individual TSNs in split-siphon preparations while probing the entire surface of both siphons. Once the receptive field for a TSN was found, its boundaries were determined. The receptive field of each TSN and the total area of each preparation were drawn and measured.
As seen in Fig. 5, the increase in total siphon area of Spisula is an almost linear function of shell size over the range of 2-0-7-0 cm. In clams larger than 7-0 cm, however, there is a dramatic increase in siphon area, the mean total siphon area in the 7-0 cm group being approximately ioo-o mm2 while in the 9-0 cm group it is 250-0 mm2. In contrast to the increase in siphon area, the mean area of TSN receptive fields decreases as a function of increasing shell size. The mean area of TSN receptive fields in small Spisula is 33-8 + 20-8 mm2, whereas in large animals it is 9-7 ± 5-9 mm2 (Fig. 5). As a result of both increasing siphon area and decreasing TSN field area, a TSN in a large clam innervates only a small portion of the total siphon surface,
B 1..
3.-4 . '
C
1 . ^
I ' . •
4 . •
D
1.-2."
3.1
[image:11.451.40.398.309.581.2]Is
70 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE
A
3.-4 . •
3.^ 4 . •
% ' t , ' ' 'te=a>
c
1 1 1
2 . > • • <!..)
3 . i •!< m — l l i i \ | » H i « ' H i » » ' • ! • ! • i m ( i M i i i i | i | « l *
4 . i| ' M i » i i i • i i | i , . i |
D 1.
3. 4.
1 s
Fig. 8. The same preparation and recording arrangement as in Figure 7. This preparation was made with a large Spisula (15-0 cm) and was stimulated in the same sequence as the preparation in Fig. 5. There is some overlap of innervation of B and C particularly near the midline. There is, however, no overlap of innervation in the lateral areas. Time scale is 1 s.
whereas a TSN in a small clam innervates a large portion of the total siphon surface. A measure of the ratio of TSN area to total siphon area is presented in tig. 6. The mean TSN field area in small animals was 30-58% of the total siphon area, whereas the TSN area in large animals was only 1 % of the siphon area. A consequence of this difference is that in small animals the receptive fields of numerous TSNs overlap while in large animals there is far less overlap of TSN receptive fields. If the differ-ence in the area of siphon innervated by average TSNs in small and large animals is involved in the observed variation in behavioural responsiveness, there should be a correlation between the two as a function of shell size. We have plotted the per-centage of the trials in which contact with starfish tube feet resulted in jumping (Fig. 6; the data are from Table 1). There was a high level of responsiveness in animals in the size range 2-0-7-0 cm (with the exception of the enigmatic 4-0 cm gToup). In animals larger than 7-0 cm the behavioural responsiveness rapidly declined to zero. Likewise the ratio of TSN area to siphon area was greatest in the 5-0-7-0 cm size range, and declined in animals larger than 7-0 cm.
7
Ji,
3. 3.
4. 4.
[image:13.451.44.406.76.371.2]80 mV 40 mV
Fig. 9. Simultaneous intracellular recordings of the activity of TSN-posterior adductor moto-neurone pairs in visceral ganglion-siphon preparations in response to tactile stimulation of the primary receptive field of the impaled TSN. The records in A,_i were obtained from a pre-paration of a large clam (iS'O cm). Weak stimulation (At) results in the depolarizing-hyper-polarizing response, as does the stronger stimulation seen in A,. A stimulus sufficient to generate a high frequency burst of 21 spikes in the TSN generates an action potential in the follower cell (Ai). In a small clam (6-o cm) weak stimulation elicits an action potential in the follower cell (B,,,). In all records the TSN is the upper trace and the posterior adductor motoneurone the lower trace. Calibration 500 ms, 80 mV for upper traces and 40 mV for
lower traces.
(Fig. 2B). Fig. 5 illustrates recordings from a small clam (5-0 cm) in which tactile stimuli were applied to siphon areas A, B, C, and D respectively (see Fig. 2B). In response to stimulation of each area (sets A, B, C, and D) there was afferent activity recorded in three or four nerves, representing considerable overlap in the areas innervated by each nerve (i.e. TSNs). Stimulation of essentially any area of either siphon in a small clam resulted in afferent activity in several siphonal nerves. In contrast, the same experiment done with a large clam (15-0 cm) revealed little overlap of innervation by the siphonal nerves (Fig. 8). Except for stimulation at the border between two of the areas such as A and B (Fig. 8, set B) there was little overlap of innervation. Stimulation of an area primarily resulted in afferent activity in the siphonal nerve from that area.
72 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE
clams should result in relatively greater postsynaptic events in follower neurones in the central nervous system. In order to obtain an indication of this we recorded intracellularly from central neurones (posterior adductor motoneurones; Mellon & Prior, 1970) in visceral ganglion split-siphon preparations. The posterior adductor motoneurones respond to weak sensory input with a complex depolarizing-hyper-polarizing post-synaptic potential. A strong tactile stimulus can elicit a sufficiently large depolarizing component to result in generation of an action potential.
To test the responsiveness of the follower neurones in small and large clams tactile stimuli (single depressions of the skin) were applied to the siphons with a hand-held probe. An indication of the stimulus intensity was obtained by recording intracellularly from a TSN simultaneous with a central follower neurone and restrict-ing the application of the tactile stimulus to the primary receptive field of the impaled TSN. In this way the duration and frequency of the TSN burst were indicators of stimulus intensity.
Fig. 9 A illustrates recordings from a TSN-follower neurone pair in a large clam (15-0 cm). As can be seen from the high frequency and long duration of the TSN burst in Fig. 9A4, strong tactile stimulation was required to elicit an action potential in the follower neurone. Weaker tactile stimuli resulted in only the characteristic depolarizing-hyperpolarizing synaptic potential (Fig. gA1_3). In the small clam
(6-o cm), however, weak tactile stimuli were sufficient to activate the follower neurone (Fig. 9B2i3). Note that with increasing stimulus intensity the rise time of the pre-potential in the follower neurone increased (Fig. 9B2_4). Point tactile stimuli of this sort rarely elicited more than one action potential in the follower neurone.
DISCUSSION
The behavioural responsiveness of mature and immature individuals often varies. Our studies indicate that the neuronal basis of variability in the jump response of Spisula may involve a reduction in the receptive fields of touch-sensitive neurones. In contrast to the response variation that is often observed between developmental stages, in this case the variation is between individuals large enough to be sexually mature. Sexual maturity is here defined as the capability of producing viable gametes. Eggs isolated from Spisula as small as 2-0 cm in length can be fertilized and proceed through cleavage (L. Rebhun, personal communication). Since we do not know the exact age of individual clams we must relate our observations to the size of individuals. Small clams responded to starfish contact with a very regular evasive jump response. At 17 °C, 77% of the trials resulted in a jump response. In contrast, large Spisula never responded to starfish contact by jumping (Table 1). All attempts to elicit jumping with either tactile or chemical stimuli (or combinations) were unsuccessful. However, contact with isolated tube feet resulted in a low level jump responsivenes These results support the conclusion that the stimulation provided by the tube feet of an intact starfish is a complex combination of tactile and chemical cues to which small Spisula are particularly sensitive.
The size and shape of the shells and siphons of clams are important when considering their susceptibility to predation. The siphons of small clams extend only about 2-0 cm, thus when buried the clam is often no more than 2-0 cm below the surface of the substrate. Large Spisula, however, extend their siphons over 10 cm to reach the surface of the substrate above. Furthermore, the wedge-shaped shells of the small Spisula are slender (often less than i-o cm in breadth from valve to valve) whereas the shells of large Spisula are bulbous (6-0-7-0 cm in breadth). As a result of shallow burrowing and slender profiles, small Spisula may be more easily be removed from a buried location. Therefore, active evasive behaviour is particularly adaptive in small animals.
A similar variation in size-dependent escape behaviour has been reported in the lobster, Homarus americanus (Lang et al. 1977)- In response to threat, small lobsters escape while large lobsters respond with aggressive behaviour.
It seemed that there was no basic difference in the responsiveness of TSNs from large or small Spisula, hence no clear basis for a variation in sensory input. We did, however, find a distinct variation in the areas of the receptive fields of individual TSNs that was dependent upon the size of the animals (Fig 5). The maximum mean TSN area (over 40 mm2) was found in animals with shell lengths of 5-0-7-0 cm. In larger animals the mean TSN area was approximately 10 mm2. An increase in the number of TSNs as the animals grew larger could offset the effects of the decrease in the size of receptive fields and the increase in the total siphon area. We have no indi-cation that there are more TSNs in large clams than in small clams. Examination of serial histological sections of the visceral ganglion and of live preparations revealed no obvious difference in the number of somata in the TSN region between large and small animals. Although we have not eliminated the possibility of an increase in number of TSNs, a sizable increase is unlikely.
We are unable to provide an explanation for the reduction in size of primary receptive fields of TSNs. One alternative is that there is an actual reduction in the extent of dendritic branching in larger animals. Due to the length of the axons of TSNs (3-0-4-0 cm in large clams) anatomical techniques such as intracellular dye injection could not be used to visualize the branches of individual TSNs. An alter-native explanation that was tested, however, is that in small clams most of the action potentials generated in the periphery invade the somata of TSNs whereas in larger animals they are blocked at branch points. This would suggest that the numer of recorded electrotonic potentials (which seem to be due to blocked action potentials) should be greater in TSNs of larger animals (Figs. 3, 4). This alternative was elimi-nated by the observation that there was no measurable difference in the number or the distribution of amplitudes of electrotonic potentials recorded in TSNs from small and large clams.
74 D. J. PRIOR, ANNE M. SCHNEIDERMAN AND SHARON I. GREENE
TSN fields than there is in large clams. The large difference in the ratio between small and large clams reduces the possibility that slight errors in the estimation of the area would alter the final conclusions.
A large degree of overlap in small clams would result in numerous TSNs being activated in response to even a localized tactile stimulus. In large clams fewer sensory units would be activated by a tactile stimulus due to little TSN overlap. This hypo-thesis was supported by simultaneously recording the afferent activity in the four siphonal nerves in response to localized tactile stimulation of the siphons (Figs. 7, 8). Due to overlap of TSN fields, tactile stimulation of any area of either siphon of a small clam elicited afferent activity in three or four of the four siphonal nerves. In large clams tactile stimulation occasionally resulted in afferent activity in more than one siphonal nerve but only when the stimulus was applied to one of the borders separating two regions of the siphons (Fig. 7, trace B).
It would be desirable to extend the scope of the present study to include a com-parison of the effectiveness of the afferent inputs in small and large clams at the level of the central nervous system. This however must await identification of the inter-neurones that trigger the evasive behaviour.
In a preliminary effort to test the feasibility of an experimental design to be used with 'jumping interneurones', we examined the responses of posterior adductor motoneurones which are recruited during various complex foot movements (Trueman 1967) and are responsible for the 'puff' response preceding jumping. Although in the present case the follower neurones in small clams were activated at stimulus intensities lower than those required in large clams, variations in cell morphology and membrane properties need to be assessed when applying the procedure to inter-neurones involved in evasive behaviour.
The greatest potential for overlap of TSN fields (according to the TSN area/total siphon area ratio) is in the 5-0-7-0 cm size group. Correspondingly the highest degree of jump responsiveness was observed in animals in the same group. In animals larger than 7-0-8-0 cm there was little jump responsiveness. There is therefore a correlation between jump responsiveness and the TSN area/total siphon area ratio (i.e. degree of TSN overlap). The basis of the size-dependent variation in behavioural responsiveness of Spisula to starfish attack may, therefore, involve changes in the size of receptive fields of touch-sensitive neurones.
We thank Dr David Bentley for his comments on an early draft of this manuscript and Ms Jane Frazier for her technical assistance. A portion of the work was done during the post course session of the Experimental Invertebrate Zoology course (1976). This work was supported by grants from N.S.F. (BNS74-151217 AOi) and the Alfred P. Sloan Foundation to D.J.P. This is contribution number 107 from the Tallahassee, Sopchoppy and Gulf Coast Marine Biological Association.
REFERENCES
ANSELL, A. D. (1968). Defensive adaptations to predation in the Mollusca. Mar. biol. Atioc. India. Symposium 3 on Mollusca, 487-512.
BERG, C. J., JR. (1972). Ontogeny of the behaviour of Strombus macultus (Gastropoda: Strombidae).
DAKIN, W. J. (1910). The visceral ganglion of Pectin, with some notes on the physiology of the nervous system. Mitt. zool. Stn Neapcl 29, 1-40.
LANG, F., GOVIND, C. K., COSTELLO, W. J. & GREENE, S. I. (1977). Developmental neuroethology:
changes in escape and defensive behaviour during growth of the lobster. Science 197, 682-685. MONTGOMERY, D. H. (1967). Responses to two haliotid gastropods (Mollusca) Haiiotit assimilis and
Haliotis refescens, to the forcipulate asteriods (Echinodermata) Pycnopodia hclianthoides and Pilaster ochraceus. Veliger 9, 359-368.
MELLON, D E F . (1969). The reflex control of rhythmic motor output during swimming in the scallop.
Z. vergl. Phyriol. 6a, 318-336.
MELLON, D E F . & PRIOR, D. J. (1970). Component* of a response program involving inhibitory and excitatory reflexes in the surf clam. J. exp. Biol. 53, 711-725.
MELLON, D E F . (1972). Electrophysiology of touch sensitive neurons in a mollusc. J. comp. Phyriol. 79, 63-78.
PRIOR, D. J. (1972a). Electrophysiological Analysis of Peripheral Neurones and their possible role in the local reflexes of a Mollusc. J. exp. Biol. 157, 133-345.
PRIOR, D. J. (19726). A neural correlate of behavioural stimulus intensity discrimination in a mollusc.
J. exp. Biol. 57, 147-160.
TRUEMAN, E. (1967). The dynamics of burrowing in Emit (Bivalvia). Proc. R. Soc. B 166, 459-476. WILLOWS, A. O. D. (1967). Behavioural acts elicited by stimulation of single identifiable cells. Science
157.
57O-574-WILLOWS, A. O. D., DORSETT, D. A. & HOYLE, G. (1973). The neuronal basis of behaviour of Tritonia. I. Functional organization of the central nervous system. J. Neurobiol. 4, (3) 207-237.
YONGE, C. M. (1936). The evolution of the swimming habit in the Lamellibranchis. Mem. Mus. r. Hist.