Original Article
Evaluating MRI, CT, PET/CT in detection of lymph node
status in cervical cancer: a meta-analysis
Chaoran Wu
1*, Lina Lu
1*, Yanfang Liu
2, Ying Lu
3, Yucheng Mi
4, Wanglun Diao
11Department of Radiology, Jining No. 1 People’s Hospital, Jining 272011, China; 2Department of Cardio-Thoracic
Surgery, Jining No. 1 People’s Hospital, Jining 272011, China; 3Department of Endocrinology Surgery, Jining No. 1
People’s Hospital, Jining 272011, China; 4Department of Radiology, Taizhou Enze Medical Center (Group), Taizhou
Hospital of Zhejiang Province, Zhejiang 317000, China. *Equal contributors and co-first authors.
Received November 26, 2015; Accepted April 3, 2016; Epub June 15, 2016; Published June 30, 2016
Abstract: Objectives: We performed a meta-analysis to compare diagnostic performances of magnetic resonance imaging (MRI), computed tomography (CT) and positron emission tomography (PET) for detecting metastatic lymph nodes occurred in patients with cervical cancer. Methods: Relevant studies were searched electronically from the PubMed and Cochrane Library databases. All articles were independently reviewed and selected by two reviewers.
Chi-square test was used for heterogeneity assessment. Then, the pooled sensitivity, specificity, area under roc
curve (AUC) and partial AUC were calculated to evaluate the diagnostic value of MRI, CT and PET. Furthermore, subgroup analysis was conducted by methods, ethnicity, and metastasis position. Results: A total of 53 articles with
an overall sample size of 15,479 subjects were finally included in this meta-analysis. PET is ranked as the most
powerful imaging technique for its highest value of pooled SEN, SPE and partial AUC , followed by CT and MRI (PET:
sensitivity = 0.58, 95% CI = 0.50-0.66, specificity = 0.95, 95% CI = 0.93-0.97, partial AUC = 0.73; CT: sensitivity = 0.52, 95% CI = 0.39-0.64, specificity = 0.91, 95% CI = 0.88-0.94, partial AUC = 0.65; MRI: sensitivity = 0.51, 95% CI = 0.43-0.59, specificity = 0.91, 95% CI = 0.87-0.95, partial AUC = 0.57). Conclusion: The results from this
meta-analysis revealed that PET had slightly better diagnostic performance than CT and MRI. Nevertheless, more studies
should be carried out to confirm the diagnostic performance ranking among PET, CT and MRI.
Keywords: Cervical cancer, lymph nodes metastasis, magnetic resonance imaging, computed tomography,
posi-tron emission tomography, sensitivity, specificity
Introduction
Cervical neoplasm is considered as one of the
most life-threatening health issues in both
developing and developed countries and it
accounts for more than a quarter of one million
annual deaths over the world [1]. As suggested
by the National Cancer Institute (NCI), the
inci-dences of cervical neoplasm in Hispanic and
African American females are higher than those
in other ethnic groups, while African Americans
appear to have the highest morality of the
dis-ease (http://www.cancer.gov). Moreover, recent
research has provided evidence that the
inci-dence and mortality significantly vary with geo
-graphic regions and socioeconomic status.
There are several factors that contribute to the
development of cervical neoplasm and
persis-tent infection caused by high risk Human papil
-lomavirus (HPV) is the most critical one among
these factors [2]. The prognosis of patients with
cervical cancer is substantially associated with
the proliferation, invasion and metastasis of
the disease and lymph nodes are believed to be
the most common metastasis routes [3].
Therefore, early identification and diagnosis of
lymph nodes metastasis (LNM) could have
sig-nificant impact on the survival status of patients
with cervical neoplasm.
Popular methods for investigating the presence
and extension of LNM include physical
palpa-tion, radiologic imaging by contrast-enhanced
computed tomography (CT), magnetic resonan-
ce imaging (MRI), ultrasonography (USG),
ultra-sound (US) with fine-needle aspiration cytology
meth-ods, PET, CT and MRI are non-invasive
tech-niques that are able to precisely identify LNM of
cervical neoplasm. Compared to conventional
invasive procedures, these techniques signifi
-cantly reduced operation costs and
complica-tions [5]. Moreover, CT and MRI have been
widely accepted as conventional imaging
tech-niques for the assessment of LNM status in
patients with malignant tumors. Both CT and
MRI identify LNM through measuring the node
size and a short-axis diameter of greater than
10 mm suggests the presence of LNM [5].
Additionally, MRI has several advantages over
non-ionizing imaging techniques as it provides
more comprehensive evaluation of organ
tis-sues [6]. However, CT is not considered to be
the first choice for pregnant females who suffer
from oncologic disease due to potential hazard
of fetal radiation exposure [7].
On the other hand, PET has become
increas-ingly popular for preoperative examination and
diagnosis of gynecological cancer [8]. PET has
unparalleled sensitivity which is powerful for
detecting small difference in tumor metabolism
[9]. Apart from that, many radiotracers have
been suggested to provide robust sensitivity for
detection of tumor accumulation and
2-deoxy-2-[fluorine-18] fluoro-D-glucose (
18F-FDG) is the
most popular one associated with high
sensitiv-ity and specificsensitiv-ity [10]. Compared to CT and
MRI, PET is relatively expensive and it has
lim-ited availability since the operation of PET
can-not be repeated over a period of time due to
radiation hazard. Furthermore, PET assesses
metabolic characteristics of tumor cells which
are largely affected by tissue types, therefore,
the diagnostic accuracy of PET may be
overes-timated under certain clinical situations [11].
Since LNM staging is a key prognostic factor for
cancer patients, the purpose of this study is to
evaluate and compare the diagnostic accuracy
among PET, CT and MRI for identifying LNM of
cervical neoplasm. Besides, this study may
assist in finding the optimal imaging techniques
for cervical neoplasm preoperative
examina-tions, particularly for patients with various
clini-cal features.
Materials and methods
Search strategy
PubMed and Cochrane Library were the
elec-tron databases used for literature search. We
identified relevant articles using the keywords:
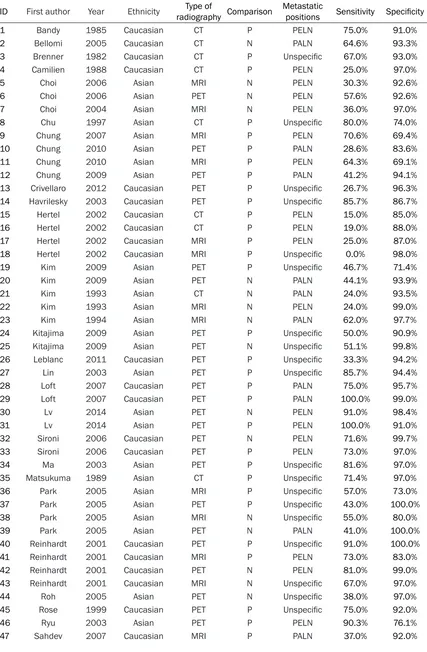
[image:2.612.89.523.72.371.2]Table 1. Main characteristics of the selected studies in the meta-analysis
ID First author Year Ethnicity radiography ComparisonType of Metastatic positions Sensitivity Specificity
1 Bandy 1985 Caucasian CT P PELN 75.0% 91.0%
2 Bellomi 2005 Caucasian CT N PALN 64.6% 93.3%
3 Brenner 1982 Caucasian CT P Unspecific 67.0% 93.0%
4 Camilien 1988 Caucasian CT P PELN 25.0% 97.0%
5 Choi 2006 Asian MRI N PELN 30.3% 92.6%
6 Choi 2006 Asian PET N PELN 57.6% 92.6%
7 Choi 2004 Asian MRI N PELN 36.0% 97.0%
8 Chu 1997 Asian CT P Unspecific 80.0% 74.0%
9 Chung 2007 Asian MRI P PELN 70.6% 69.4%
10 Chung 2010 Asian PET P PALN 28.6% 83.6%
11 Chung 2010 Asian MRI P PELN 64.3% 69.1%
12 Chung 2009 Asian PET P PALN 41.2% 94.1%
13 Crivellaro 2012 Caucasian PET P Unspecific 26.7% 96.3%
14 Havrilesky 2003 Caucasian PET P Unspecific 85.7% 86.7%
15 Hertel 2002 Caucasian CT P PELN 15.0% 85.0%
16 Hertel 2002 Caucasian CT P PELN 19.0% 88.0%
17 Hertel 2002 Caucasian MRI P PELN 25.0% 87.0%
18 Hertel 2002 Caucasian MRI P Unspecific 0.0% 98.0%
19 Kim 2009 Asian PET P Unspecific 46.7% 71.4%
20 Kim 2009 Asian PET N PALN 44.1% 93.9%
21 Kim 1993 Asian CT N PALN 24.0% 93.5%
22 Kim 1993 Asian MRI N PELN 24.0% 99.0%
23 Kim 1994 Asian MRI N PALN 62.0% 97.7%
24 Kitajima 2009 Asian PET P Unspecific 50.0% 90.9%
25 Kitajima 2009 Asian PET N Unspecific 51.1% 99.8%
26 Leblanc 2011 Caucasian PET P Unspecific 33.3% 94.2%
27 Lin 2003 Asian PET P Unspecific 85.7% 94.4%
28 Loft 2007 Caucasian PET P PALN 75.0% 95.7%
29 Loft 2007 Caucasian PET P PALN 100.0% 99.0%
30 Lv 2014 Asian PET N PELN 91.0% 98.4%
31 Lv 2014 Asian PET P PELN 100.0% 91.0%
32 Sironi 2006 Caucasian PET N PELN 71.6% 99.7%
33 Sironi 2006 Caucasian PET P PELN 73.0% 97.0%
34 Ma 2003 Asian PET P Unspecific 81.6% 97.0%
35 Matsukuma 1989 Asian CT P Unspecific 71.4% 97.0%
36 Park 2005 Asian MRI P Unspecific 57.0% 73.0%
37 Park 2005 Asian PET P Unspecific 43.0% 100.0%
38 Park 2005 Asian MRI N Unspecific 55.0% 80.0%
39 Park 2005 Asian PET N PALN 41.0% 100.0%
40 Reinhardt 2001 Caucasian PET P Unspecific 91.0% 100.0%
41 Reinhardt 2001 Caucasian MRI P PELN 73.0% 83.0%
42 Reinhardt 2001 Caucasian PET N PELN 81.0% 99.0%
43 Reinhardt 2001 Caucasian MRI N Unspecific 67.0% 97.0%
44 Roh 2005 Asian PET N Unspecific 38.0% 97.0%
45 Rose 1999 Caucasian PET P Unspecific 75.0% 92.0%
46 Ryu 2003 Asian PET P PELN 90.3% 76.1%
48 Sahdev 2007 Caucasian MRI N PALN 27.0% 99.0%
49 Sheu 2001 Asian MRI P Unspecific 82.0% 87.0%
50 Subak 1995 Caucasian MRI P PELN 62.0% 91.0%
51 Subak 1995 Caucasian CT P PELN 60.0% 91.0%
52 Vas 1985 Caucasian CT P PELN 63.0% 79.0%
53 Vas 1985 Caucasian CT P PALN 83.0% 95.0%
54 Villasanta 1983 Caucasian CT P PALN 77.0% 86.0%
55 Waggenspack 1988 Caucasian MRI P PALN 66.7% 100.0%
56 Walsh 1981 Caucasian CT P Unspecific 70.0% 78.0%
57 Yang 2000 Caucasian CT N Unspecific 64.7% 96.6%
58 Yang 2000 Caucasian MRI N Unspecific 70.6% 89.8%
59 Yeh 2002 Asian PET P Unspecific 73.3% 96.7%
60 Yildirim 2008 Asian PET P Unspecific 50.0% 73.3%
61 Heller 1990 Caucasian CT P PELN 34.4% 95.8%
62 Hawnaur 1994 Caucasian MRI P PELN 75.0% 88.0%
63 Janus 1989 Caucasian CT P PELN 33.0% 95.0%
64 Janus 1989 Caucasian MRI P PELN 75.0% 89.0%
65 Belhocine 2002 Caucasian PET N PALN 70.4% 98.4%
66 Greco 1989 Caucasian MRI P PELN 38.0% 84.0%
67 Kim 1990 Asian CT N PELN 27.0% 93.0%
68 Kim 1990 Asian MRI N PELN 20.0% 98.0%
69 Chen 2013 Asian PET P PELN 41.3% 91.8%
70 Chen 2013 Asian PET N PELN 43.5% 97.9%
71 Chen 2013 Asian PET P Unspecific 40.0% 97.5%
72 Sandvik 2011 Caucasian PET P Unspecific 20.0% 90.3%
73 Sigborelli 2011 Caucasian PET P Unspecific 32.1% 96.9%
74 Sigborelli 2011 Caucasian PET N PALN 25.0% 99.5%
75 Togashi 1989 Asian MRI P PELN 60.0% 91.2%
76 Dong 2014 Asian PET P PALN 87.5% 78.4%
77 Driscoll 2015 Caucasian PET P Unspecific 0.0% 100.0%
78 Nogami 2015 Asian PET P PELN 33.3% 92.7%
80 Nogami 2015 Asian PET N PELN 30.6% 98.9%
81 Perez-Medina 2013 Caucasian PET P PELN 77.7% 94.1%
*PET: Positron emission tomography; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; P: patient-based; N:
node-based; PELN: pelvic lymph node; PALN: para-aortic lymph node; Unspecific: no specific lymph node stated.
Metastasis” AND “Magnetic Resonance Ima-
ging or Positron-Emission Tomography or Com-
puter Tomography” AND “diagnosis or
sensitiv-ity or specificsensitiv-ity” (up to Nov. 10 2015) com
-bined with a complete set of synonyms. A
man-ual search was conducted by scanning meta-
analyses with similar research topics to identify
additional articles and to improve
complete-ness of the selection process.
Inclusion and exclusion criteria
Studies satisfying the following selection
crite-ria were included in present meta-analysis: 1)
Studies were relevant to uterine cervical
can-cer. 2) Studies should specifically evaluate the
diagnostic performance of CT, MRI or PET for
lymphatic metastasis assessment rather than
their prognostic values; 3) At least 10 patients
should be included in the study; 4) Histo-
pathological examination of lymph nodes as
the only reference standard should be carried
out to confirm lymphatic metastasis; 5)
Ade-quate diagnostic statistics including sensitivity
(SEN), specificity (SPE) true-positive (TP],
false-positive (FP), true-negative (TN), false-negative
(FN) should be presented in the study for the
derivation of 2 × 2 tables.
only diagnosis as uterine cervical cancer but
also with other disease. 2) Studies evaluate the
lymphatic metastasis by other diagnostic
meth-od. 3) Studies have not enough data to create
the 2 × 2 tables. 4) Studies were not reported
by English.
Data extraction
Three investigators were responsible for data
extraction and two of them independently
reviewed the full text of studies that were finally
[image:5.612.93.521.69.295.2]selected. When discrepancy occurred, a third
Figure 2. The forest plot of CT for sensitivity (A) and specificity (B) in detecting lymphatic metastasis in cervical cancer patients with the corresponding heterogeneity. The sensitivity and specificity of each study are presented by circle and the confidence interval (CI) is indicated by error bars. [image:5.612.94.522.355.581.2]investigator was required to resolve it. The
fol-lowing information was extracted from the
studies: first author, year of publication, ethnic
-ity (Asian, Caucasian), comparison methods
(patient-based, node-based), number of
sub-jects in the case and control group, metastasis
position (pelvic lymph nodes (PELN) and
para-aortic lymph nodes (PALN)). Diagnostic
statis-tics such as TP, FP, FN and TN were recorded.
Since different results were provided by
differ-ent comparison methods within one study, two
separate results from the same study were
recorded. Apart from that, for studies in which
pelvic and para-aortic lymph node metastases
were specifically subdivided, two separate
results were extracted from the same study.
SEN and SPE were either directly obtained from
original studies or computed from the
extract-ed data. Moreover, the corresponding authors
were contacted via email when selected
stud-ies contained missing data.
Statistical methods
Forest plots of sensitivity and specificity for the
three radiography methods (CT, MRI and PET)
were presented to visually exam heterogeneity
among selected studies and the chi-square
test was used for assessing significant statisti
-cal heterogeneity. In light of significant hetero
-geneity (P < 0.05), a random-effects mode was
used to obtain the pooled SEN and SPE along
with 95% confidence intervals (95% CIs).
Summary receiver operating characteristic
(SROC) curve was generated and areas under
the curve (AUC) along with partial AUC which
determine the overall diagnostic performance
were calculated. Furthermore, comparison me-
thod, ethnicity and metastasis position were
taken as potential sources of heterogeneity for
subgroup analysis. Finally, the publication bias
was evaluated by Deek’s funnel plot asymmetry
[image:6.612.94.524.71.409.2]A two-side P less than 0.05 was considered as
significant.
Results
Literature search
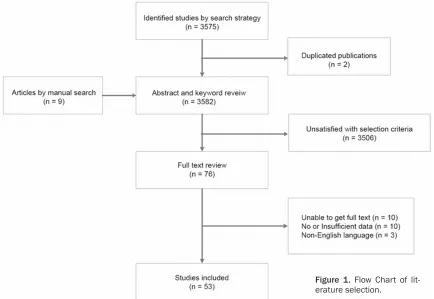
A total of 3,575 articles were identified by the
searching strategy from electronic databases.
We review the abstract and keywords of 3,573
non-duplicated article (2 duplicated
publica-tions excluded) and 9 additional manual-sear-
ched articles from meta-analysis. As a result,
3,506 articles were further excluded according
to the selection criteria listed above and only
66 of the remaining articles were subject to
data assessment. Of those 66 available
arti-cles, 53 articles were finally included in our
meta-analysis [8, 12-63] and 13 articles were
excluded due to insufficient data or non-English
language. Figure 1 summarized the whole
pro-cess of literature search beginning from article
identification to the final stage of inclusion.
[image:7.612.91.528.80.492.2]Main characteristics of the included studies
All of the included studies were published
between 1981 and 2015. The total number of
included subjects is 15,479 which incorporated
16 studies on the diagnostic performance of
CT, 19 studies on the diagnostic performance
of MRI and 18 studies on the diagnostic
perfor-mance of PET. Table 1 indicated the main
char-acteristics of selected studies.
Table 2. Summary parameters for assessment of radiography (CT&MRI&PET)
Group Sensitivity (95% CI) Specificity (95% CI) AUC Partial AUC
CT
Overall 0.52 (0.39, 0.64) 0.91 (0.88, 0.94) 0.88 0.65
Comparison method
Patient-based 0.54 (0.39, 0.68) 0.90 (0.85, 0.93) 0.87 0.61
Node-based 0.45 (0.24, 0.68) 0.94 (0.92, 0.95) 0.93 0.07
Ethnicity
Asians 0.46 (0.19, 0.76) 0.91 (0.81, 0.96) 0.85 0.60
Caucasians 0.53 (0.40, 0.66) 0.91 (0.88, 0.94) 0.90 0.64
Metastatic positions
PALN 0.62 (0.42, 0.79) 0.91 (0.84, 0.95) 0.89 0.71
PELN 0.40 (0.23, 0.58) 0.91 (0.84, 0.95) 0.84 0.48
MRI
Overall 0.51 (0.43, 0.59) 0.91 (0.87, 0.95) 0.76 0.57
Comparison method
Patient-based 0.56 (0.47, 0.65) 0.85 (0.79, 0.89) 0.75 0.53
Node-based 0.43 (0.31, 0.57) 0.96 (0.93, 0.98) 0.83 0.58
Ethnicity
Asians 0.50 (0.38, 0.62) 0.91 (0.82, 0.96) 0.74 0.56
Caucasians 0.53 (0.40, 0.64) 0.93 (0.87, 0.96) 0.80 0.52
PET
Overall 0.58 (0.50, 0.66) 0.95 (0.93, 0.97) 0.88 0.73
Comparison method
Patient-based 0.60 (0.50, 0.70) 0.91 (0.88, 0.94) 0.88 0.67
Node-based 0.55 (0.41, 0.67) 0.98 (0.97, 0.99) 0.94 0.70
Ethnicity
Asians 0.59 (0.47, 0.67) 0.94 (0.90, 0.96) 0.85 0.68
Caucasians 0.60 (0.46, 0.73) 0.97 (0.94, 0.98) 0.93 0.72
Metastatic positions
PALN 0.69 (0.53, 0.82) 0.94 (0.90, 0.96) 0.94 0.79
PELN 0.39 (0.33, 0.46) 0.95 (0.90, 0.97) 0.49 0.41
*PET: Positron emission tomography; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; P: patient-based; N:
Summary of diagnostic performance
In our meta-analysis, the chi-square test for
heterogeneity of SEN and SPE (P-value < 0.05)
together with the corresponding forest plots of
SEN and SPE were displayed in Figures 2-4
which concluded significant heterogeneity
am-ong included studies. In this case, we chose a
random-effects model to evaluate the overall
diagnostic performance of CT, MRI and PET for
detecting LNM. As suggested by Table 2, PET
was ranked as the most accurate imaging
modality because of its highest value of pooled
SEN, SPE and partial AUC, followed by CT and
MRI (PET: sensitivity = 0.58, 95% CI =
0.50-0.66, specificity = 0.95, 95% CI = 0.93-0.97,
partial AUC = 0.73; CT: sensitivity = 0.52, 95%
CI = 0.39-0.64, specificity = 0.91, 95% CI =
0.88-0.94, partial AUC = 0.65; MRI: sensitivity
= 0.51, 95% CI = 0.43-0.59, specificity = 0.91,
95% CI = 0.87-0.95, partial AUC = 0.57). Finally,
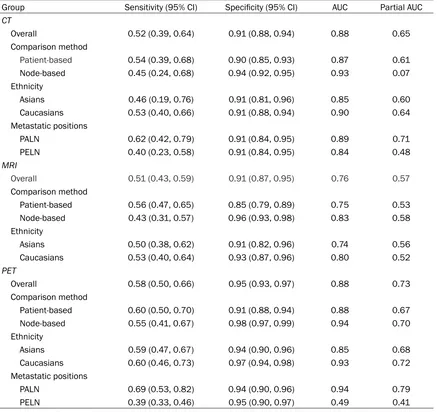
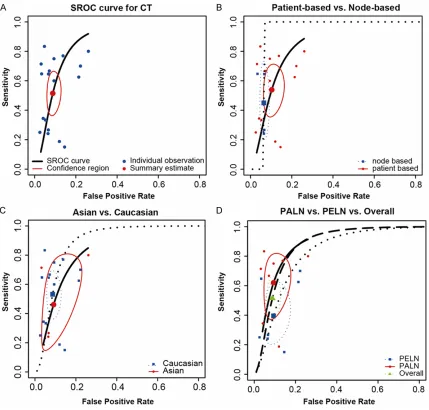
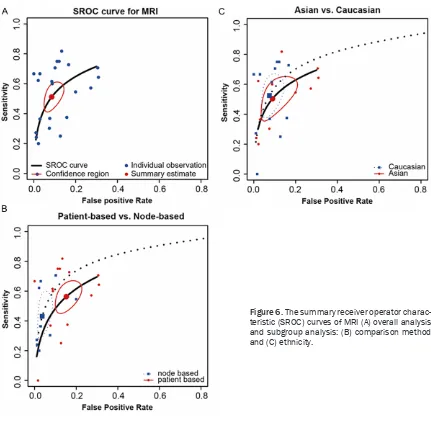
the SROC curve displayed in Figures 5A, 6A, 7A
also provided a visual indication of the overall
diagnostic performance of CT, MRI and PET.
Subgroup analysis
Three types of subgroup analysis were carried
out on the diagnostic performance of the three
radiograph techniques to investigate potential
differences in stratified populations.
Random-effects model was adopted for the subgroup
analysis due to the significant heterogeneity
(P-value < 0.05). The subgroup analysis was
carried out by ethnicity (Asian or Caucasian)
,
comparison methods (patient-based or
[image:8.612.93.522.67.477.2]based)
and metastasis position (PELN or PALN).
The statistical results were listed in Table 2.
CT for detecting LNM using patient-to-patient
comparison was more powerful than those by
node-to-node comparison (sensitivity: 0.54 >
0.45, partial AUC: 0.61 > 0.07). By contrast,
subgroup of node-to-node comparison had a
higher specificity than patient-to-patient com
-parison (specificity: 0.94 > 0.90). Additionally,
detection of lymphatic metastasis appeared to
be more accurate in Caucasians compared to
Asians (sensitivity: 0.53 > 0.46, partial AUC:
0.64 > 0.60). Finally, CT was more appropriate
in detecting PALN metastasis in comparison to
overall lymph nodes metastasis and PELN
metastasis (sensitivity: 0.62 > 0.52 >0.40,
AUC: 0.89 > 0.88 > 0.84, partial AUC: 0.71 >
0.65 > 0.48). A visual illustration of subgroup
analysis of CT for detecting lymphatic
metasta-sis was given in Figure 5B-D.
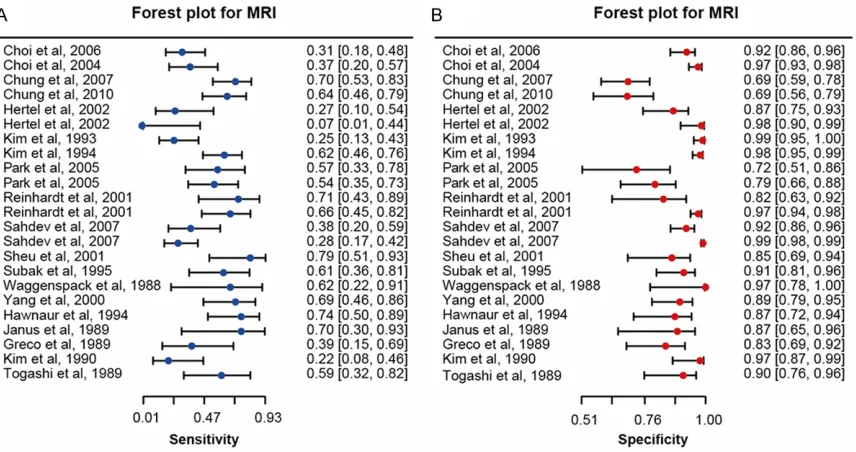
Similar to the result of CT, MRI for detecting
lymphatic metastasis using patient-to-patient
comparison was more powerful but had a lower
specificity than those using node-to-node com
-parison (sensitivity: 0.56 > 0.43, partial AUC:
0.58 > 0.53, specificity: 0.85 < 0.96). Slightly
higher sensitivity and specificity were observed
in the subgroup of Caucasians compared to the
subgroup of Asian (sensitivity: 0.53 > 0.50,
specificity: 0.93 > 0.91). Subgroup analysis by
metastasis position was not achievable since
there was only one study on MRI which
speci-fied the PALN metastasis. A visual illustration of
[image:9.612.91.522.67.500.2]subgroup analysis of MRI for detecting
lymphat-ic metastasis was given in Figure 6B, 6C.
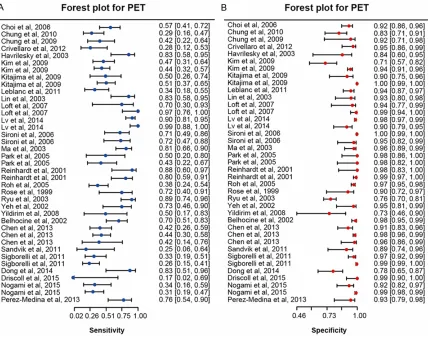
Subgroup analysis of PET by comparison
meth-od showed different results compared to those
of CT and MRI. Subgroup of patient-based
com-parison has a higher sensitivity than subgroup
of node-based comparison (sensitivity: 0.60 >
0.55). However, the estimates of specificity,
AUC and partial AUC favored the subgroup of
node-based comparison (specificity: 0.98 >
0.91, AUC: 0.91 > 0.88, partial AUC: 0.90 >
0.67). It appears that detecting lymphatic me-
tastasis by PET is more accurate in Caucasians
compared to Asians (specificity: 0.60 > 0.59,
sensitivity: 0.97 > 0.94, AUC: 0.93 > 0.85,
par-tial AUC: 0.72 > 0.68). Finally, subgroup
analy-sis by metastaanaly-sis position revealed that PET
was substantially more powerful in detecting
PALN metastasis than PELN metastasis
(sensi-tivity: 0.59 > 0.39, AUC: 0.94 > 0.49, partial
AUC: 0.79 > 0.41). A visual illustration of
sub-group analysis of MRI for detecting lymphatic
metastasis was given in Figure 7B-D.
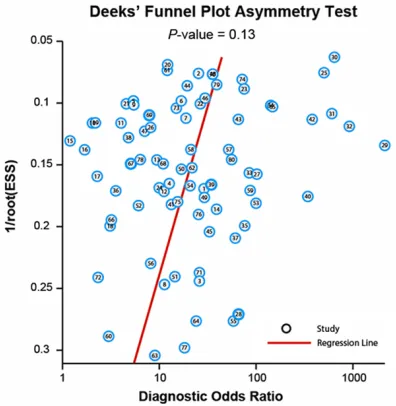
Publication bias
For publication bias, Deek’s funnel plot was
illustrated in Figure 8, which presented no
sta-tistically significant asymmetry (
P
> 0.05).
Hence, the validity of our meta-analysis was
[image:10.612.92.522.72.487.2]confirmed.
Discussion
Although lymph node metastases (LNM) is not
incorporated in the International Federation of
Gynecology and Obstetrics (FIGO) staging
clas-sification [64], in fact, the occurrence of meta
-static lymph nodes have a significant influence
on the treatment and prognosis of cervical
can-cer. For patients with cervical cancer, lymph
node status has been regarded as an
indepen-dent prognostic factor for survival [65, 66].
Compared to patients without lymphatic
metas-tasis, the 5-year survival rate for patients with
PALN and PELN metastasis is significantly
reduced from 57% to 12% and 34%,
respec-tively [67].
CT, MRI and PET which have been applied for
detecting lymphatic metastasis for the recent
two decades are the three imaging modalities
of our interest. By synthesizing evidence from
individual studies, 1645 subjects diagnosed by
CT in 16 published studies, 3686 subjects
diagnosed by MRI in 19 published studies and
10148 subjects diagnosed by PET in 28
pub-lished studies were included in our
meta-analy-sis to identify significant difference in the diag
-nostic accuracy among the three approaches.
The result indicated that PET was considered
as the most valuable imaging modality for de-
tecting lymphatic metastasis (sensitivity: 0.58
for PET, 0.52 for CT, 0.50 for MRI; specificity:
0.95 for PET, 0.90 for CT, 0.90 for MRI). Indeed,
PET displayed unexpectedly low sensitivity for
lymphatic metastasis detection which
con-firmed the conclusion of a previous meta-anal
-ysis by Kang [68]. However, results from
anoth-er previous meta-analysis by Choi et al., [69]
indicated that the sensitivity of PET
substan-tially differed between the two comparison
methods (0.82 for patient-based comparison,
0.54 for node-based comparison) and this
con-tradicted to the result from our subgroup
analy-sis (0.60 for patient-based comparison, 0.55
for node-based comparison) illustrated in Table
2 and Figure 7B
. The significant difference in
the sensitivity of PET particularly for
patient-based comparison between the two
meta-anal-yses may be explained by the fact that we
included more studies on patient-to-patient
comparison for PET than those incorporated by
Choi
et al. (25 articles vs. 12 articles). As a
result, studies with unexpectedly low sensitivity
of PET were incorporated in our meta-analysis.
Therefore, the sensitivity of PET from our
analy-sis with respect to LNM detection for cervical
cancer patients is likely to be unbiased and
representative.
Compared to PET, both MRI and CT showed
less accurate diagnostic performance possibly
due to their incompetence of accurate nodal
spread evaluation [70]. Since only enlarged
lymph nodes can be identified by MRI and CT,
metastatic lymph nodes with similar sizes to
non-metastatic lymph nodes are unlikely to be
discriminated [17]. Besides, benign lymph
nodes are likely to be enlarged by MRI and CT
which may affect the diagnostic accuracy of
these two imaging modalities.
Results from subgroup analysis by comparison
methods are consistent across the three
imag-ing modalities: subgroup of patient-based
com-parison had higher sensitivity and lower
speci-ficity. These results implied that the diagnosis
of lymphatic metastasis by the three imaging
modalities was more accurate when diagnosis
was performed based on patients instead of
nodes. By contrast, the exclusion of lymphatic
metastasis by the three imaging modalities
was more accurate when nodes were set as the
diagnostic subjects. The possible explanation
was that some studies such as the one
con-ducted by Choi [17], calculated all visible lymph
nodes in appointed regions regardless of
imag-ing findimag-ings. Thus, some lymph nodes that are
likely to be non-metastatic were not included in
[image:11.612.90.288.71.274.2]comparison appeared to be lower than that in
the patient-based comparison.
In conclusion, the highest diagnostic accuracy
of PET among three imaging modalities was
revealed by our comprehensive meta-analysis.
However, both PET and the other two imaging
modalities had limited sensitivity compensated
by their substantially high specificity for predict
-ing lymphatic metastasis. As the current gold
reference, histopathology finding is still the
most powerful approach for detecting
lymphat-ic metastasis occurred in patients with cervlymphat-ical
cancer.
Disclosure of conflict of interest
None.
Address correspondence to: Wanglun Diao, De-
partment of Radiology, Jining No. 1 People’s Hos-pital, No. 6, Jiankang Road, Jining, Shandong
272-011, China. E-mail: zhezzha@163.com
References
[1] Sagae S, Monk BJ, Pujade-Lauraine E, Gaffney DK, Narayan K, Ryu SY, McCormack M, Plante
M, Casado A, Reuss A, Chavez-Blanco A,
Kitch-ener H, Nam BH, Jhingran A, Temkin S, Milesh
-kin L, Berns E, Scholl S, Doll C, Abu-Rustum
NR, Lecuru F, Small W Jr; Gynecologic Cancer InterGroup Cervix Cancer. Advances and Con-cepts in Cervical Cancer Trials: A Road Map for the Future. Int J Gynecol Cancer 2015; 26: 199-207.
[2] Kolawole O, Ogah J, Alabi O, Suleiman M, Amu-da O and Kolawole F. Utilization of Human Pap-illomavirus DNA Detection for Cervical Cancer Screening in Women Presenting With
Abnor-mal Cytology in Lokoja, Nigeria. Jundishapur J
Microbiol 2015; 8: e22620.
[3] Yang S, Liu Y, Xia B, Deng J, Liu T, Li Q, Yang Y, Wang Y, Ning X, Zhang Y and Xiao M. DLL4 as a predictor of pelvic lymph node metastasis and
a novel prognostic biomarker in patients with
early-stage cervical cancer. Tumour Biol 2015; [Epub ahead of print].
[4] Geetha NT, Hallur N, Goudar G, Sikkerimath BC
and Gudi SS. Cervical lymph node metastasis in oral squamous carcinoma preoperative
as-sessment and histopathology after neck dis -section. J Maxillofac Oral Surg 2010; 9: 42-47. [5] Kim HJ, Cho A, Yun M, Kim YT and Kang WJ.
Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann Nucl Med 2015; 30: 104-13.
[6] de Haan J, Vandecaveye V, Han SN, Van de
Vi-jver KK and Amant F. Difficulties with diagnosis
of malignancies in pregnancy. Best Pract Res
Clin Obstet Gynaecol 2015; [Epub ahead of print].
[7] Dauer LT, Thornton RH, Miller DL, Damilakis J,
Dixon RG, Marx MV, Schueler BA, Vano E,
Ven-katesan A, Bartal G, Tsetis D, Cardella JF, Soci -ety of Interventional Radiology S, Health C; Cardiovascular and Interventional Radiology Society of Europe Standards of Practice C. Ra-diation management for interventions using
fluoroscopic or computed tomographic guid -ance during pregnancy: a joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe with Endorsement by the Ca-nadian Interventional Radiology Association. J Vasc Interv Radiol 2012; 23: 19-32.
[8] Nogami Y, Banno K, Irie H, Iida M, Kisu I,
Ma-sugi Y, Tanaka K, Tominaga E, Okuda S, Mu
-rakami K and Aoki D. The efficacy of preopera -tive positron emission tomography-computed tomography (PET-CT) for detection of lymph node metastasis in cervical and endometrial
cancer: clinical and pathological factors influ -encing it. Jpn J Clin Oncol 2015; 45: 26-34. [9] Tee SS and Keshari KR. Novel Approaches to
Imaging Tumor Metabolism. Cancer J 2015; 21: 165-173.
[10] Almuhaideb A, Papathanasiou N and Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 2011; 31: 3-13.
[11] Kidd EA, Spencer CR, Huettner PC, Siegel BA, Dehdashti F, Rader JS and Grigsby PW. Cervi-cal cancer histology and tumor differentiation
affect 18F-fluorodeoxyglucose uptake. Cancer
2009; 115: 3548-3554.
[12] Bandy LC, Clarke-Pearson DL, Silverman PM
and Creasman WT. Computed tomography in evaluation of extrapelvic lymphadenopathy in carcinoma of the cervix. Obstet Gynecol 1985; 65: 73-76.
[13] Belhocine T, Thille A, Fridman V, Albert A,
Se-idel L, Nickers P, KrSe-idelka F and Rigo P. Contri -bution of whole-body 18FDG PET imaging in the management of cervical cancer. Gynecol Oncol 2002; 87: 90-97.
[14] Bellomi M, Bonomo G, Landoni F, Villa G, Leon ME, Bocciolone L, Maggioni A and Viale G. Ac-curacy of computed tomography and magnetic resonance imaging in the detection of lymph node involvement in cervix carcinoma. Eur Ra-diol 2005; 15: 2469-2474.
[15] Brenner DE, Whitley NO, Prempree T and Villa-santa U. An evaluation of the computed tomo-graphic scanner for the staging of carcinoma of the cervix. Cancer 1982; 50: 2323-2328. [16] Camilien L, Gordon D, Fruchter RG, Maiman M
[17] Choi HJ, Roh JW, Seo SS, Lee S, Kim JY, Kim
SK, Kang KW, Lee JS, Jeong JY and Park SY.
Comparison of the accuracy of magnetic reso-nance imaging and positron emission tomog-raphy/computed tomography in the presurgi-cal detection of lymph node metastases in patients with uterine cervical carcinoma: a prospective study. Cancer 2006; 106: 914-922.
[18] Choi SH, Kim SH, Choi HJ, Park BK and Lee HJ.
Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: results of prospective study. J Comput Assist Tomogr 2004; 28: 620-627.
[19] Chu KK, Chang SD, Chen FP and Soong YK. Laparoscopic surgical staging in cervical can-cer--preliminary experience among Chinese. Gynecol Oncol 1997; 64: 49-53.
[20] Chung HH, Kang KW, Cho JY, Kim JW, Park NH,
Song YS, Kim SH, Chung JK and Kang SB. Role of magnetic resonance imaging and positron emission tomography/computed tomography in preoperative lymph node detection of uter-ine cervical cancer. Am J Obstet Gynecol 2010; 203: 156, 51-155.
[21] Chung HH, Kang SB, Cho JY, Kim JW, Park NH,
Song YS, Kim SH and Lee HP. Can preoperative MRI accurately evaluate nodal and parametrial invasion in early stage cervical cancer? Jpn J Clin Oncol 2007; 37: 370-375.
[22] Chung HH, Park NH, Kim JW, Song YS, Chung
JK and Kang SB. Role of integrated PET-CT in pelvic lymph node staging of cervical cancer before radical hysterectomy. Gynecol Obstet Invest 2009; 67: 61-66.
[23] Crivellaro C, Signorelli M, Guerra L, De Ponti E, Buda A, Dolci C, Pirovano C, Todde S, Fruscio R and Messa C. 18F-FDG PET/CT can predict nodal metastases but not recurrence in early stage uterine cervical cancer. Gynecol Oncol 2012; 127: 131-135.
[24] Dong Y, Wang X, Wang Y, Liu Y, Zhang J, Qian W
and Wu S. Validity of 18F-fluorodeoxyglucose
positron emission tomography/computed to-mography for pretreatment evaluation of pa-tients with cervical carcinoma: a retrospective pathology-matched study. Int J Gynecol Cancer 2014; 24: 1642-1647.
[25] Driscoll DO, Halpenny D, Johnston C, Sheehy N and Keogan M. 18F-FDG-PET/CT is of limited value in primary staging of early stage cervical cancer. Abdom Imaging 2015; 40: 127-133. [26] Greco A, Mason P, Leung AW, Dische S,
McIn-doe GA and Anderson MC. Staging of carcino-ma of the uterine cervix: MRI-surgical correla-tion. Clin Radiol 1989; 40: 401-405.
[27] Havrilesky LJ, Wong TZ, Secord AA, Berchuck A, Clarke-Pearson DL and Jones EL. The role of
PET scanning in the detection of recurrent
cer-vical cancer. Gynecol Oncol 2003; 90: 186-190.
[28] Hawnaur JM, Johnson RJ, Buckley CH, Tindall V
and Isherwood I. Staging, volume estimation and assessment of nodal status in carcinoma of the cervix: comparison of magnetic
reso-nance imaging with surgical findings. Clin Ra -diol 1994; 49: 443-452.
[29] Heller PB, Maletano JH, Bundy BN, Barnhill DR
and Okagaki T. Clinical-pathologic study of
stage IIB, III, and IVA carcinoma of the cervix: extended diagnostic evaluation for paraaortic node metastasis--a Gynecologic Oncology Group study. Gynecol Oncol 1990; 38: 425-430.
[30] Hertel H, Kohler C, Elhawary T, Michels W, Pos-sover M and Schneider A. Laparoscopic stag-ing compared with imagstag-ing techniques in the staging of advanced cervical cancer. Gynecol Oncol 2002; 87: 46-51.
[31] Janus CL, Mendelson DS, Moore S, Gendal ES, Dottino P and Brodman M. Staging of cervical carcinoma: accuracy of magnetic resonance imaging and computed tomography. Clin Imag-ing 1989; 13: 114-116.
[32] Kim SH, Choi BI, Han JK, Kim HD, Lee HP, Kang SB, Lee JY and Han MC. Preoperative staging of uterine cervical carcinoma: comparison of CT and MRI in 99 patients. J Comput Assist To-mogr 1993; 17: 633-640.
[33] Kim SH, Choi BI, Lee HP, Kang SB, Choi YM, Han MC and Kim CW. Uterine cervical
carcino-ma: comparison of CT and MR findings. Radiol -ogy 1990; 175: 45-51.
[34] Kim SH, Kim SC, Choi BI and Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology 1994; 190: 807-811.
[35] Kim SK, Choi HJ, Park SY, Lee HY, Seo SS, Yoo
CW, Jung DC, Kang S and Cho KS. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur J Cancer 2009; 45: 2103-2109.
[36] Kitajima K, Murakami K, Yamasaki E, Kaji Y
and Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in pa-tients with uterine cancer. Eur Radiol 2009; 19: 1529-1536.
[37] Leblanc E, Gauthier H, Querleu D, Ferron G, Zerdoud S, Morice P, Uzan C, Lumbroso S, Le-curu F, Bats AS, Ghazzar N, Bannier M, Houve-naeghel G, Brenot-Rossi I and Narducci F.
Ac-curacy of 18-fluoro-2-deoxy-D-glucose positron
cervi-cal carcinoma. Ann Surg Oncol 2011; 18: 2302-2309.
[38] Lin WC, Hung YC, Yeh LS, Kao CH, Yen RF and
Shen YY. Usefulness of (18)F-fluorodeoxyglu -cose positron emission tomography to detect para-aortic lymph nodal metastasis in ad-vanced cervical cancer with negative
comput-ed tomography findings. Gynecol Oncol 2003;
89: 73-76.
[39] Loft A, Berthelsen AK, Roed H, Ottosen C, Lun-dvall L, Knudsen J, Nedergaard L, Hojgaard L and Engelholm SA. The diagnostic value of PET/CT scanning in patients with cervical can-cer: a prospective study. Gynecol Oncol 2007; 106: 29-34.
[40] Lv K, Guo HM, Lu YJ, Wu ZX, Zhang K and Han JK. Role of 18F-FDG PET/CT in detecting pelvic lymph-node metastases in patients with early-stage uterine cervical cancer: comparison with
MRI findings. Nucl Med Commun 2014; 35:
1204-1211.
[41] Ma SY, See LC, Lai CH, Chou HH, Tsai CS, Ng KK, Hsueh S, Lin WJ, Chen JT and Yen TC. De-layed (18)F-FDG PET for detection of paraaor-tic lymph node metastases in cervical cancer patients. J Nucl Med 2003; 44: 1775-1783. [42] Matsukuma K, Tsukamoto N, Matsuyama T,
Ono M and Nakano H. Preoperative CT study of
lymph nodes in cervical cancer--its correlation
with histological findings. Gynecol Oncol 1989;
33: 168-171.
[43] Park W, Park YJ, Huh SJ, Kim BG, Bae DS, Lee
J, Kim BH, Choi JY, Ahn YC and Lim DH. The usefulness of MRI and PET imaging for the de-tection of parametrial involvement and lymph node metastasis in patients with cervical can-cer. Jpn J Clin Oncol 2005; 35: 260-264. [44] Perez-Medina T, Pereira A, Mucientes J,
Garcia-Espantaleon M, Jimenez JS, Calles L, Rodri-guez B and Iglesias E. Prospective evaluation
of 18-fluoro-2-deoxy-D-glucose positron emis -sion tomography for the discrimination of para-aortic nodal spread in patients with locally ad-vanced cervical carcinoma. Int J Gynecol Cancer 2013; 23: 170-175.
[45] Reinhardt MJ, Ehritt-Braun C, Vogelgesang D, Ihling C, Hogerle S, Mix M, Moser E and Krause TM. Metastatic lymph nodes in patients with cervical cancer: detection with MR imaging and FDG PET. Radiology 2001; 218: 776-782. [46] Roh JW, Seo SS, Lee S, Kang KW, Kim SK, Sim
JS, Kim JY, Hong EK, Cho DS, Lee JS and Park
SY. Role of positron emission tomography in pretreatment lymph node staging of uterine cervical cancer: a prospective surgicopatho-logic correlation study. Eur J Cancer 2005; 41: 2086-2092.
[47] Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW and Miraldi F. Positron
emis-sion tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopatho-logic study. J Clin Oncol 1999; 17: 41-45. [48] Ryu SY, Kim MH, Choi SC, Choi CW and Lee KH.
Detection of early recurrence with 18F-FDG PET in patients with cervical cancer. J Nucl Med 2003; 44: 347-352.
[49] Sahdev A, Sohaib SA, Wenaden AE, Shepherd
JH and Reznek RH. The performance of mag -netic resonance imaging in early cervical carci-noma: a long-term experience. Int J Gynecol Cancer 2007; 17: 629-636.
[50] Sandvik RM, Jensen PT, Hendel HW and Palle
C. Positron emission tomography-computed to-mography has a clinical impact for patients with cervical cancer. Dan Med Bull 2011; 58: A4240.
[51] Sheu MH, Chang CY, Wang JH and Yen MS. Pre-operative staging of cervical carcinoma with MR imaging: a reappraisal of diagnostic accu-racy and pitfalls. Eur Radiol 2001; 11: 1828-1833.
[52] Signorelli M, Guerra L, Montanelli L, Crivellaro
C, Buda A, Dell’Anna T, Picchio M, Milani R, Fr -uscio R and Messa C. Preoperative staging of cervical cancer: is 18-FDG-PET/CT really effec-tive in patients with early stage disease? Gyne-col OnGyne-col 2011; 123: 236-240.
[53] Sironi S, Buda A, Picchio M, Perego P, Moreni R, Pellegrino A, Colombo M, Mangioni C, Mes-sa C and Fazio F. Lymph node metastasis in patients with clinical early-stage cervical can-cer: detection with integrated FDG PET/CT. Ra-diology 2006; 238: 272-279.
[54] Subak LL, Hricak H, Powell CB, Azizi L and
Stern JL. Cervical carcinoma: computed to-mography and magnetic resonance imaging for preoperative staging. Obstet Gynecol 1995; 86: 43-50.
[55] Togashi K, Nishimura K, Sagoh T, Minami S,
Noma S, Fujisawa I, Nakano Y, Konishi J, Ozasa
H, Konishi I and et al. Carcinoma of the cervix: staging with MR imaging. Radiology 1989; 171: 245-251.
[56] Vas W, Wolverson M, Freel J, Salimi Z and Sun-daram M. Computed tomography in the pre-treatment assessment of carcinoma of the cervix. J Comput Tomogr 1985; 9: 359-368. [57] Villasanta U, Whitley NO, Haney PJ and Brenner
D. Computed tomography in invasive carcino-ma of the cervix: an appraisal. Obstet Gynecol 1983; 62: 218-224.
[58] Waggenspack GA, Amparo EG and Hannigan
EV. MR imaging of uterine cervical carcinoma. J Comput Assist Tomogr 1988; 12: 409-414. [59] Walsh JW and Goplerud DR. Prospective
[60] Yang WT, Lam WW, Yu MY, Cheung TH and Me-treweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol 2000; 175: 759-766. [61] Yeh LS, Hung YC, Shen YY, Kao CH, Lin CC and
Lee CC. Detecting para-aortic lymph nodal me-tastasis by positron emission tomography of
18F-fluorodeoxyglucose in advanced cervical
cancer with negative magnetic resonance
im-aging findings. Oncol Rep 2002; 9:
1289-1292.
[62] Yildirim Y, Sehirali S, Avci ME, Yilmaz C, Ertop-cu K, Tinar S, Duman Y and Sayhan S. Integrat-ed PET/CT for the evaluation of para-aortic nodal metastasis in locally advanced cervical cancer patients with negative conventional CT
findings. Gynecol Oncol 2008; 108: 154-159.
[63] Chen YH, Wang DB, Li YN, Jiao L and Sun XD. The diagnostic value of PET-CT for the detec-tion of lymph node metastasis in patients with early cervical cancer. Journal of China Medical University 2013: 351-354.
[64] Takeshima N, Yanoh K, Tabata T, Nagai K, Hirai
Y and Hasumi K. Assessment of the revised In-ternational Federation of Gynecology and ob-stetrics staging for early invasive squamous cervical cancer. Gynecol Oncol 1999; 74: 165-169.
[65] Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, Fujimoto T, Oikawa M,
Fujino T and Fujimoto S. Incidence and distri-bution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer 1999; 85: 1547-1554.
[66] Lai CH, Hong JH, Hsueh S, Ng KK, Chang TC, Tseng CJ, Chou HH and Huang KG. Preopera-tive prognostic variables and the impact of postoperative adjuvant therapy on the out-comes of Stage IB or II cervical carcinoma pa-tients with or without pelvic lymph node metas-tases: an analysis of 891 cases. Cancer 1999; 85: 1537-1546.
[67] Lanciano RM and Corn BW. The Role of Surgi-cal Staging for CerviSurgi-cal Cancer. Semin Radiat Oncol 1994; 4: 46-51.
[68] Kang S, Kim SK, Chung DC, Seo SS, Kim JY,
Nam BH and Park SY. Diagnostic value of (18)
F-FDG PET for evaluation of paraaortic nodal metastasis in patients with cervical carcino-ma: a metaanalysis. J Nucl Med 2010; 51: 360-367.
[69] Choi HJ, Ju W, Myung SK and Kim Y. Diagnostic performance of computer tomography, mag-netic resonance imaging, and positron emis-sion tomography or positron emisemis-sion tomog-raphy/computer tomography for detection of metastatic lymph nodes in patients with cervi-cal cancer: meta-analysis. Cancer Sci 2010; 101: 1471-1479.