www.wjpr.net Vol 7, Issue 8, 2018. 234
SYNTHESIS, CHARACTERISATION AND ANTIMICROBIAL
STUDIES OF MANGANESE(II), COBALT(II) AND CADMIUM(II)
COMPLEXES CONTAINING OXINE AND 2 – PICOLINE
R. Bharathiand Dr. M. Nagarathinam*
PG & Research Department of Chemistry, D.K. M. College for Women (Autonomous),
Vellore District, Tamil Nadu.
ABSTRACT
The complexes of transition metal ions Mn(II), Co(II) and Cd(II) with
oxine (8-hydroxy quinoline) and mixed ligand complexes with oxine
and 2-picoline have been synthesized. The structural, spectroscopic
and thermal properties have been studied on the basis of Infrared
spectra, TGA-DTA analysis, X-ray diffraction analysis and Molar
conductivity measurements. From the results obtained the following
general formula has been given for the complexes [M(oxine)2] and
[M(oxine)2(2-pic)2]. The oxine complexes exhibit antiseptic,
disinfectant and pesticide properties. So the metal complexes have
been tested against microorganisms using Agar well diffusion method
in order to assess their antimicrobial properties. The metal complexes
were screened for antibacterial activity against gram negative (Escherichia coli) and gram
positive bacteria (Bacillus subtilis) and the fungal activity against Aspergillus niger and
Penicillium chrysogenum.
KEYWORDS: Transition metal complexes, oxine, 2-picoline, Thermal Analysis,
Antimicrobial studies.
INTRODUCTION
Transition metals have varying utility and interesting chemistry. Coordination compounds are
important due to their role in biological and chemical systems in various ways. It has been
observed that metal complexes with appropriate ligands are chemically more significant and
specific than the metal ions in original. The mixed ligand complexes are biologically active
Volume 7, Issue 8, 234-242. Conference Article ISSN 2277–7105
*Corresponding Author
Dr. M. Nagarathinam
PG & Research Department of Chemistry, D.K. M. College for Women (Autonomous), Vellore District, Tamil Nadu. Article Received on 05 March 2018,
Revised on 25 March 2018, Accepted on 15 April 2018
www.wjpr.net Vol 7, Issue 8, 2018. 235 against pathogenic microorganisms. Oxine is a monoprotic bidentate chelating agent. Oxine
and its metalloquinolates have attracted great interest because of their higher thermal
stability. Oxine is the only one, among seven isomeric monohydroxyl quinolines, capable of
forming complexes with divalent metal ions through chelation.[1] Most bioactivities of oxine
and its derivatives originate from their chelating ability.[2] 2-Picoline was the
first pyridine compound reported to be isolated in pure form. The complexation of metallic
elements with biologically inactive compounds renders them active and in case the compound
is already active, it makes them more active. In this paper, we have investigated the
preparation, properties and antimicrobial studies of some metal ions with oxine and
2-picoline.
MATERIALS AND METHODS
Reagents and solvents: All the chemicals (MnSO4, CoCO3 and CdSO4) and reagents used for
the preparation of complexes were commercial products (E Merck Ltd, India) and used
without further purification. DMF and DMSO were purchased from SRL chem.
Melting points of the synthesised compounds were measured using a melting point apparatus
obtained from Guna Enterprises, Chennai.
The IR spectra of the synthesised compounds were recorded using SHIMADZU
spectrophotometer in 4000 – 400 cm-1 range, using KBr pellet.
The X – ray diffraction patterns of the samples were tested by an X – ray scattering
SHIMADUZ XD – DI diffractometer using in filter Cu Kα radiation source (LAMDA =
0.154 nm ), set a scan rate = 10 / min, using a voltage of 40 Kv and a current of 30 Ma.
The complexes were tested in a SDT Q600 V8.0 build 95 instrument at Anna University,
Chennai. The temperature range was varied from room temperature to 800 °C with heating
rate of 20 °C/min in oxygen atmosphere.
The molar conductivities were measured with the conductivity meter of model DCM 900
using the freshly prepared solution of the complexes (10-3 M) in DMSO.
The effect of various metal complexes on the several bacterial strains and fungal strains were
www.wjpr.net Vol 7, Issue 8, 2018. 236
Preparation of metal complexes
Complexes of the type [M(oxine)2]
5 ml of DMF was added to an aqueous solution of the metal salt in 25 ml water. 5.8 g of
oxine was dissolved in 5 ml of DMF, 10 ml of ethanol and 20 ml of 2 M NaOH. Both the
solutions were mixed well and heated in a boiling water bath for about three and a half hour.
The precipitate formed was filtered using Whatmann no.1 filter paper in hot condition,
washed with ether and dried.
Complexes of the type [M(oxine)2(2-pic)2]
5.8 g of oxine was dissolved in 10 ml ethanol and 20 ml of 2 M NaOH. The mixture was
added to an aqueous solution of metal salt and stirred for half an hour using a magnetic
stirrer. A dense yellow precipitate was formed. To this solution 2 ml of 2-picoline was added
slowly and heated in a boiling water bath for half an hour. The precipitate formed was filtered
with Whatmann no.1 filter paper, washed with ether and dried.
RESULTS AND DISCUSSION
The metal complexes obtained were solid and coloured. They were insoluble in water and
[image:3.595.77.525.463.584.2]soluble in solvents like dimethyl sulphoxide and dimethyl formamide.
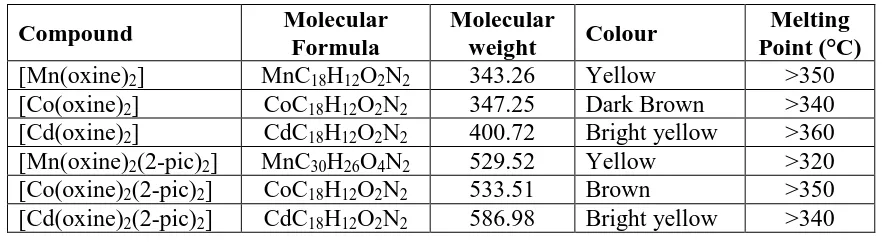
Table 1: Analytical data of synthesized transition metal complexes.
Compound Molecular
Formula
Molecular
weight Colour
Melting Point (°C)
[Mn(oxine)2] MnC18H12O2N2 343.26 Yellow >350
[Co(oxine)2] CoC18H12O2N2 347.25 Dark Brown >340
[Cd(oxine)2] CdC18H12O2N2 400.72 Bright yellow >360
[Mn(oxine)2(2-pic)2] MnC30H26O4N2 529.52 Yellow >320
[Co(oxine)2(2-pic)2] CoC18H12O2N2 533.51 Brown >350
[Cd(oxine)2(2-pic)2] CdC18H12O2N2 586.98 Bright yellow >340
1. Infrared spectra
The IR spectra provide valuable information regarding the nature of the functional groups
attached to the metal ion. The transition metal complexes show characterized functional
bands such as C-O, C-N, O-H, and C=N groups. Formation of nitrogen and
metal-oxygen bonds were further confirmed by the presence of the stretching vibration of ʋ(M-N)
www.wjpr.net Vol 7, Issue 8, 2018. 237 500 1000 1500 2000 3000 4000 1/cm 40 60 80 100 %T 3 1 2 4 .6 8 3 0 6 6 .8 2 3 0 4 7 .5 3 3 0 2 6 .3 12 9 3 1 .8 0 2 8 5 4 .6 5 1 5 7 7 .7 7 1 5 0 0 .6
214
7 3 .6 2 1 4 3 3 .1 1 1 4 0 9 .9 6 1 3 8 1 .0 3 1 2 8 6 .5 2 1 2 7 4 .9 5 1 2 2 2 .8 7 1 2 0 5 .5
111
6 5 .0 0 1 1 3 8 .0 0 1 0 9 3 .6 4 8 1 7 .8 2 7 8 1 .1 7 7 4 0 .6 7 7 0 9 .8 0 6 3 6 .5 1 L1 500 1000 1500 2000 3000 4000 1/cm 40 60 80 100 %T 3 4 6 4 .1 5 3 4 1 4 .0
0 31
6 3 .2 6 3 0 5 9 .1 0 1 6 2 0 .2 1 1 5 7 3 .9 1 1 4 9 8 .6 9 1 4 6 5 .9 0 1 3 8 6 .8 2 1 3 2 3 .1 7 1 2 8 0 .7
3 12
3 8 .3 0 1 1 3 6 .0 7 1 1 0 7 .1 4 1 0 6 8 .5 6 1 0 3 3 .8
5 90
4 .6 1 8 5 6 .3 9 8 1 9 .7 5 7 9 0 .8 1 7 4 2 .5 9 6 4 4 .2 2 4 9 9 .5 6 B1
[image:4.595.109.492.74.272.2]Wavenumber (cm-1) Wavenumber (cm-1)
Fig.1: IR Spectrum of oxine Fig. 2: IR spectrum of [Mn(oxine)2]
The IR spectrum of the ligand is shown in Fig.1. The spectrum shows the characteristic
frequencies of υ(O-H) at 3157.47 cm-1, υ(C-O) at 1278.81 cm-1
and υ(C-N) at 1101.35 cm-1.
The IR spectrum of [Mn(oxine)2] exhibited a strong band at 1107.14 cm-1 and this could be
due to υ (C-O) where as other strong bands at 3464.15 cm-1
, 1323.17 cm-1, 819.75 cm-1,
742.59 cm-1 and 1165.00 cm-1 are due to υ(O-H), υ(C-N) respectively. Metal-nitrogen and
metal-oxygen bands are further confirmed by the presence of stretching vibration of υ(Mn-N)
and υ(Mn-O) are at 499.56 cm-1
and 644.22 cm-1 respectively.
The IR spectrum of 2-picoline and its complexes
500 1000 1500 2000 3000 4000 1/cm 45 60 75 90 %T 3 4 4 6 .7 9 3 1 3 4 .3 3 3 0 8 8 .0 3 3 0 1 4 .7
4 29
2 6 .0 1 2 8 5 6 .5 8 1 5 9 5 .1 3 1 5 7 7 .7 7 1 4 7 7 .4 7 1 4 0 0 .3 2 1 2 9 6 .1 6 1 2 3 8 .3 0 1 1 4 9 .5 7 1 0 5 1 .2
0 99
9 .1 3 7 4 8 .3 8 7 3 1 .0 2 6 3 0 .7 2 5 4 7 .7 8 4 7 0 .6 3 4 0 1 .1 9 L2 500 1000 1500 2000 3000 4000 1/cm 0 25 50 75 100 %T 3 3 8 3 .1 4 3 1 6 1 .3 3 3 1 4 3 .9 7 3 0 6 4 .8 9 2 9 2 7 .9 4 2 8 5 2 .7 2 2 3 1 4 .5 8 1 6 3 5 .6 4 1 5 7 5 .8 4 1 4 9 8 .6 9 1 4 6 5 .9 0 1 3 8 8 .7 5 1 3 7 3 .3 2 1 3 2 5 .1 0 1 2 8 2 .6 6 1 1 5 3 .4 3 1 1 0 9 .0 7 1 0 6 8 .5 6 8 2 1 .6 8 7 4 4 .5 2 B5
[image:4.595.97.504.478.701.2]Wavenumber (cm-1) Wavenumber (cm-1)
Fig. 3: IR Spectrum of 2-picoline Fig. 4: IR spectrum of [Co(oxine)2(2-pic)2]
The band appeared at1587.42 cm-1 in Fig.3 indicate the presence of C=N linkage. Bands
www.wjpr.net Vol 7, Issue 8, 2018. 238 spectrum of the sample is shown in Fig.4. The spectra exhibited strong bands at 1109.7 cm-1
and 1282.66 cm-1 and this could be due to υ(C-O). Vibrations due to aromatic ring appear at
3064.89 cm-1, 1575.84 cm-1 and 1465.90 cm-1. Metal-nitrogen and metal-oxygen bands were
further confirmed by the presence of stretching vibration of υ(Co-N) and υ(Co-O) at 503.14
cm-1 and 642.30 cm-1 respectively.[4]
2. X-Ray Diffraction Analysis
XRD analysis is used to determine the percentage of crystallinity present in the material. The
[image:5.595.114.477.269.574.2]crystal size has been calculated using Debye-Scherrer’s equation.[5]
Table 2:X-Ray Diffraction of the Complexes.
Complexes FWHM (deg) 2θ (deg) Crystal size (nm)
[Mn(oxine)2] 0.3053 8.654 26.0987
[Co(oxine)2] 0.4594 8.713 17.3449
[Cd(oxine)2] 0.2593 8.529 30.7261
Mn(oxine)2(2-pic)2] 0.3748 8.653 21.2591
[Co(oxine)2(2-pic)2] 0.3354 23.471 24.1943
[Cd(oxine)2(2-pic)2] 0.2959 22.558 27.3796
Fig. 5: X-Ray Diffraction of [Co(oxine)2] Fig. 6: X-Ray Diffraction of [Cd(oxine)2(2-pic)2]
3. Thermal Data
The thermal properties for metal oxine complexes and metal oxine and 2-picoline complexes
have been investigated. All the stages of decomposition follow one after the other without
any stable compound in between.[6] The exothermic peaks are due to oxidative degradation of
www.wjpr.net Vol 7, Issue 8, 2018. 239
14.38% (0.6489mg)
48.82°C
70.71°C 63.28°C
7.833% (0.3534mg) 143.66°C
170.34°C 158.84°C
11.77% (0.5311mg) 439.87°C
465.93°C 455.44°C
335.54°C368.90°C 5.920% (0.2671mg) 499.24°C
524.20°C
514.95°C 602.39°C
741.23°C
34.47% (1.555mg)
592.24°C
676.26°C Residue: 11.32% (0.5106mg)
0.0 0.1 0.2 0.3 0.4 0.5
Deriv. Weight
(%/°
C)
0 10 20 30 40 50 60 70 80 90 100 110 120
Weight (%)
0 100 200 300 400 500 600 700 800
Temperature (°C)
Sample: Govin B2 Jan 27 2014 Size: 4.5120 mg Method: Test Comment: Test
DSC-TGA File: C:...\govin B2 Jan 27 2012.001Operator: TA Run Date: 2014-01-27 08:58 Instrument: SDT Q600 V8.0 Build 95
Universal V4.1D TA Instruments
10.90% (0.2579mg) 119.14°C
138.11°C 130.89°C
28.29% (0.6694mg)
400.40°C
462.64°C 444.63°C
17.67% (0.4182mg) 677.96°C
740.23°C 713.28°C
Residue: 30.10% (0.7123mg)
258.01°C
0.0 0.2 0.4 0.6
Deriv. Weight
(%/°
C)
10 20 30 40 50 60 70 80 90 100 110 120
Weight (%)
0 100 200 300 400 500 600 700 800
Temperature (°C)
Sample: Govin B4 Jan 23 2014 Size: 2.3660 mg Method: Test Comment: Test
DSC-TGA File: C:...\Govin B4 Jan 23 2012.001Operator: TA Run Date: 2014-01-23 08:55 Instrument: SDT Q600 V8.0 Build 95
[image:6.595.106.497.83.287.2]Universal V4.1D TA Instruments
Fig. 5: Thermal data of [Co(oxine)2] Fig. 5: Thermal data of [Mn(oxine)2(2-pic)2]
4. Molar Conductance
The synthesized transition metal complexes were dissolved in DMSO and the molar
conductivities of 10-3 M solution at 25±2 °C were measured. The molar conductivity values
of the complexes are given in table-3.
Table 3: Molar Conductance data of the complexes.
Compound Specific Conductance
ohm-1 cm2
Molar Conductance
ohm-1 cm-2 mol-1
[Mn(oxine)2] 0.04 × 10-6 0.04
[Co(oxine)2] 0.04 × 10-6 0.04
[Cd(oxine)2] 0.04 × 10-6 0.04
[Mn(oxine)2(2-pic)2] 0.04 × 10-6 0.04
[Co(oxine)2(2-pic)2] 0.04 × 10-6 0.04
[Cd(oxine)2(2-pic)2] 0.04 × 10-6 0.04
The measured molar conductance of all synthesized transition metal complexes were of 0.04
ohm-1 cm-2mol-1. The lower molar conductivity values showed the non-electrolytic nature of
the complexes.[7]
5. Antimicrobial Studies
Bioeffiency of the transition metal complexes were tested against bacteria such as Bacillus
species, E. coli and fungi like, Aspergillus niger and Pencillium chrysogenum using well
www.wjpr.net Vol 7, Issue 8, 2018. 240
Antibacterial Activity of Metal Complexes
The antibacterial activities of complexes were studied using Agar well diffusion method. The
bacterial species used in the screening were Bacillus species (gram positive) and E. coli
(gram negative).
The diameter of zone of inhibition was calculated in millimeters. The well diameter was
detected from the zone diameter to get the actual zone of inhibition and the values have been
tabulated. The results indicate that most of the test compounds exhibit very good antibacterial
and antifungal activity.[8]
IMAGES OF ANTIBACTERIAL ACTIVITY
ESCHERICHIA COLI BACILLUS SPECIES
[M(oxine)2] [M(oxine)2(2-pic)2] [M(oxine)2] [M(oxine)2(2-pic)2]
Table 4: Antifungal Activity of Metal Complexes.
Complexes Concentration
Zone of inhibition( in diameter)
E. coli Bacillus
species
Ampicilln (standard)
[Mn(oxine)2] 1 mg/ml 13 17 16
[Co(oxine)2] 1 mg/ml 15 14 16
[Cd(oxine)2] 1 mg/ml 13 15 16
[Mn(oxine)2(2-pic)2] 1 mg/ml 19 18 16
[Co(oxine)2(2-pic)2] 1 mg/ml 10 14 16
[Cd(oxine)2(2-pic)2] 1 mg/ml 12 24 16
Antifungal Activity of Metal Complexes
The Antifungal activity of standard fungicide (polymyxin) and complexes were tested for
their effects on the growth of microbial cultures and studied for their interaction with
Aspergillus niger and Penicillium chrysogenum. The zone of inhibition measured for standard
www.wjpr.net Vol 7, Issue 8, 2018. 241
IMAGES OF ANTIFUNGAL ACTIVITY
ASPERGILLUS NIGER PENICILLIUM CHRYSOGENUM
[M(oxine)2] [M(oxine)2(2-pic)2] [M(oxine)2] [M(oxine)2(2-pic)2]
Table 5: Antifungal Activity of the Metal Complexes.
Complexes Concentration
Zone of inhibition(in diameter)
Aspergillusniger Penicilliumchrysogenum polymyxin
(standard)
[Mn(oxine)2] 1 mg/ml 12 31 11
[Co(oxine)2] 1 mg/ml - 16 11
[Cd(oxine)2] 1 mg/ml 15 24 11
[Mn(oxine)2(2-pic)2] 1 mg/ml 5 27 11
[Co(oxine)2(2-pic)2] 1 mg/ml 5 20 11
[Cd(oxine)2(2-pic)2] 1 mg/ml 16 23 11
The results are corroborated with the findings of other researchers (3). The proposed structure
of the complexes is given below.
where M = Mn2+, Co2+ and Cd2+.
CONCLUSIONS
The complexes of Mn(II), Co(II) and Cd(II) were synthesised using oxine as ligand and also
with mixed ligands oxine and 2-picoline. The complexes were coloured and they are
insoluble in water and soluble in DMF and DMSO. The Complexes were characterized by
www.wjpr.net Vol 7, Issue 8, 2018. 242 The metal oxine complexes and metal oxine, 2-picoline complexes exhibit good activity
aganist bacterial strains Gram positive (Bacillus species) and Gram negative(Escherichia
coli), fungal strains such as (Aspergillus niger, Penicillium chrysogenum) compared with the
standard drugs. The increase in antimicrobial activity may be due to metal chelation.
Manganese and cadmium chelates are highly active due to the combined effect of Mn(II) and
Cd(II) and its functional groups present in the ligands.
The lower conductivity values indicated the non-electrolytic nature of the complexes. The
melting points of metal chelates are higher which suggests their thermal stability. The thermal
degradation steps were identified and are mainly due to the direct decomposition of oxine and
2-picoline from metal complex leaving the corresponding metal oxide. The exothermic peaks
are due to oxidative degradation of the complexes. The XRD of the complexes were studied.
The complexes [Co(oxine)2(2-pic)2] and [Cd(oxine)2(2-pic)2] are found to be crystalline and
the other complexes are found to be semicrystalline. The structure assignment cannot be
considered final in the absence of X-ray crystal studies.
REFERENCES
1. Abeer Abdul Jaleel Ibrahim. Synthesis and Characterization of Mixed Ligand Complexes
of Some Metal Chlorides with 8-Hydroxyquinoline and Amino Acid (L-Lysine). Diyala
Journal for Pure Sciences, 2011; 7: No. 4.
2. Patel KB, Kharadi GJ and Nimavat KS. Synthesis and Description of Transition Metal
Complexes ond Antimicrobial Studies. www.jocpr.com, 2012; 5: 2422- 2428.
3. Alia Salman Kindeel, Ibtisam Jameel Dawood, Manhel Remon Aziz. J. Baghdad for Sci,
2013; 10: 396 – 403.
4. Gopalan R, Subramanian PS, Rengarajan K. Elements of Analytical Chemistry.
5. Mishra A. J. Phy. Conference Series, 2012; 012010, 365.
6. Mackenzie RC. Thermochim. Acta, 1979; 28: 1.
7. Robert K Bogges. J. Chem. Edu, 1975; 52: 649-665.
![Fig. 3: IR Spectrum of 2-picoline Fig. 4: IR spectrum of [Co(oxine)2(2-pic)2]](https://thumb-us.123doks.com/thumbv2/123dok_us/857865.596930/4.595.97.504.478.701/fig-ir-spectrum-picoline-fig-ir-spectrum-oxine.webp)

![Fig. 5: Thermal data of [Co(oxine)2] Fig. 5: Thermal data of [Mn(oxine)2(2-pic)2]](https://thumb-us.123doks.com/thumbv2/123dok_us/857865.596930/6.595.106.497.83.287/fig-thermal-data-oxine-fig-thermal-data-oxine.webp)