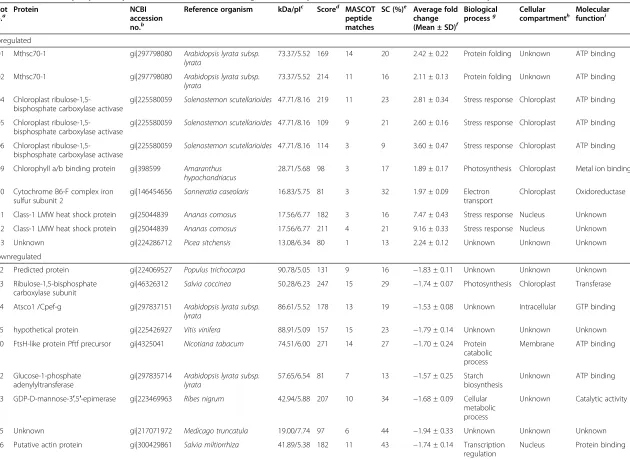
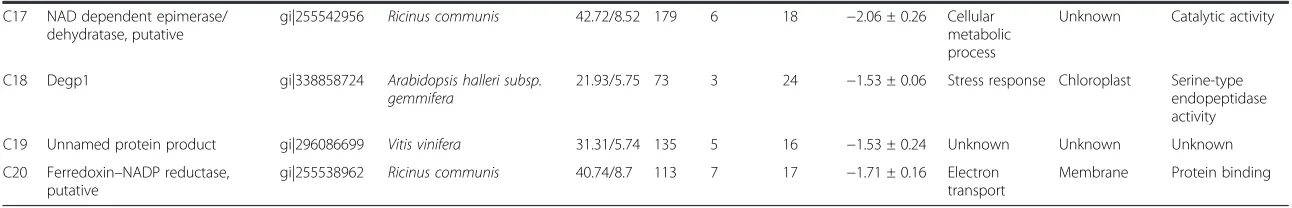
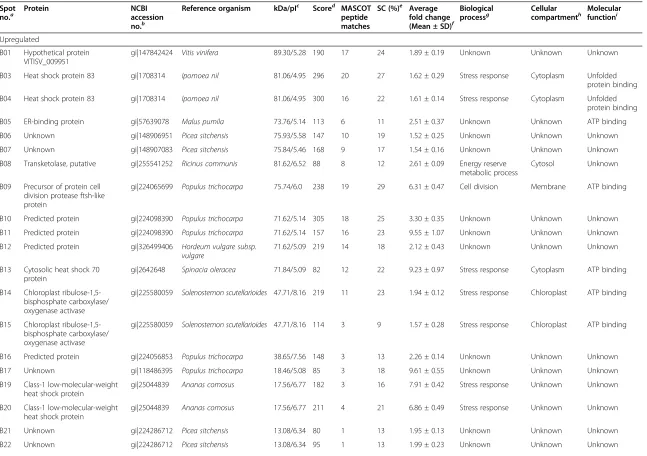
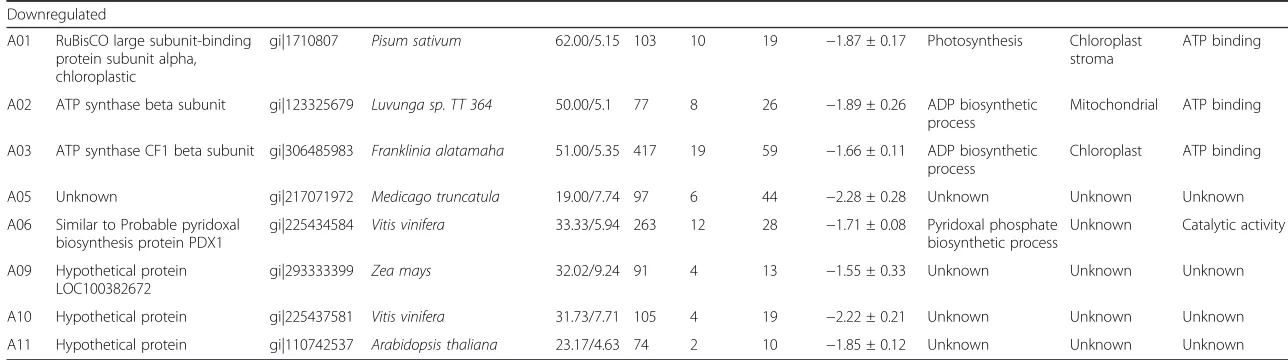
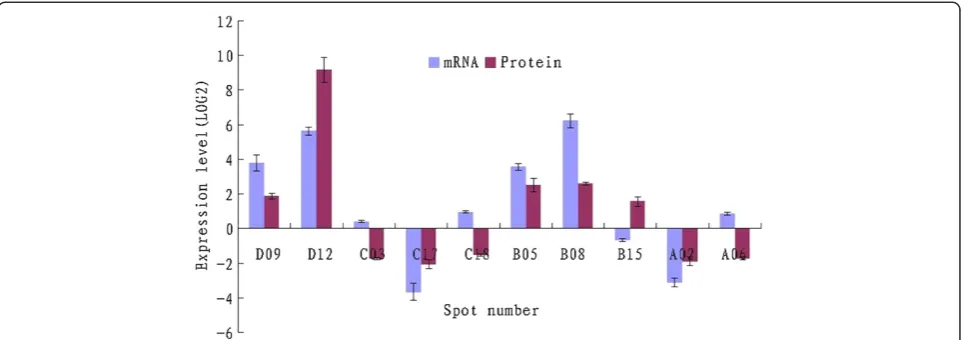
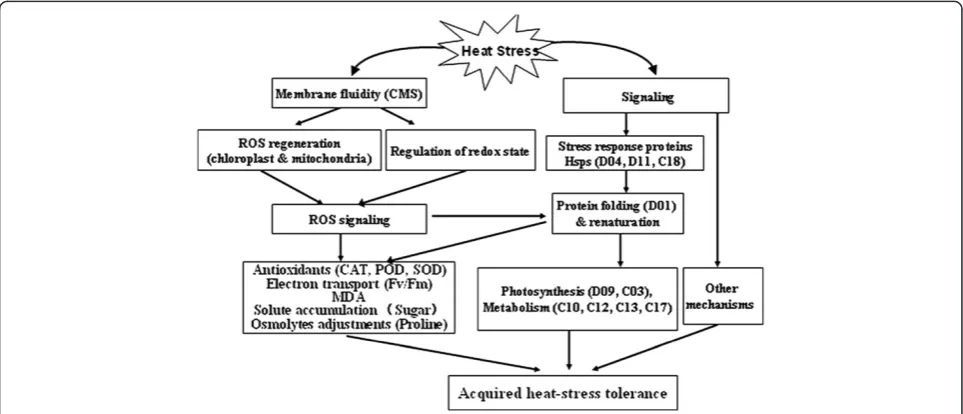
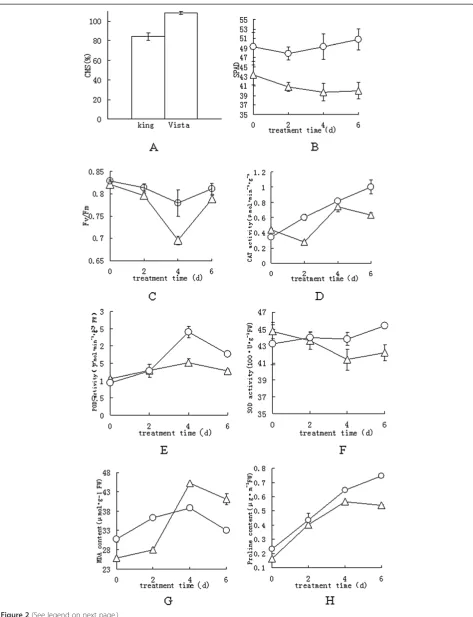
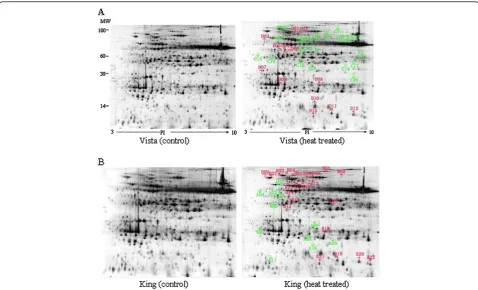
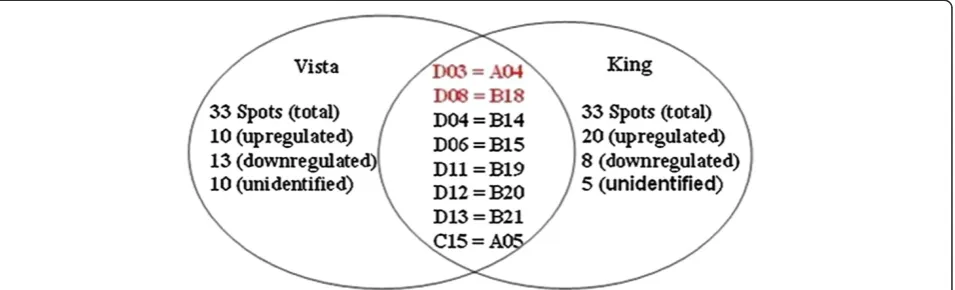
Heat stress-induced response of the proteomes of leaves from Salvia splendens Vista and King
Full text
Figure

Related documents
The corona radiata consists of one or more layers of follicular cells that surround the zona pellucida, the polar body, and the secondary oocyte.. The corona radiata is dispersed
innovation in payment systems, in particular the infrastructure used to operate payment systems, in the interests of service-users 3.. to ensure that payment systems
The projected gains over the years 2000 to 2040 in life and active life expectancies, and expected years of dependency at age 65for males and females, for alternatives I, II, and
This essay asserts that to effectively degrade and ultimately destroy the Islamic State of Iraq and Syria (ISIS), and to topple the Bashar al-Assad’s regime, the international
However, the GCR rate in pif1-m2 rrm3Δ cells was ~8 times higher in the presence of the G4 motifs compared to the pif1-m2 rrm3Δ strains containing no insert or non-G4 ScPif1
19% serve a county. Fourteen per cent of the centers provide service for adjoining states in addition to the states in which they are located; usually these adjoining states have
It was decided that with the presence of such significant red flag signs that she should undergo advanced imaging, in this case an MRI, that revealed an underlying malignancy, which
In Afghanistan, this is a qualification through either the Institute of Health Sciences or the Community Midwifery Education programme.. Skilled