Photocurrent Response of Bipyridine Containing Poly(p-phenylene-vinylene) Derivatives
K. S. Narayan* and K. V. Geetha
Chemistry and Physics of Materials Unit, Jawaharlal Nehru Center for AdVanced Scientific Research, Jakkur, Bangalore 560064, India
G. Nakmanovich, E. Ehrenfreund, and Y. Eichen
Solid State Institute, Technion - Israel Institute of Technology, Haifa, 32000 Israel ReceiVed: April 9, 2001
The photoinduced charge separation and subsequent transport under an external electric field is studied in the family of poly[bipyridine/(p-phenylene-vinylene)n] derivatives having n)0, 1, and 3, respectively, p-phenylene-vinylene subunits separating the bipyridylene p-phenylene-vinylene skeleton. Steady-state photocurrent of the polymers is studied in sandwich and surface configurations and correlated with transient photocurrent measurements. The results reveal the facile electric-field-induced separation of the electron-hole pair for n)1 samples relative to n)3 samples. We also estimate the energy barriers involved in the process of carrier generation and transport in these systems.
Introduction
Conjugated aromatic p-phenylene ring-containing oligomers and polymers provide model systems in the current effort to elucidate the interconnection between the chemical structure of a conjugated material, and its electronic, optical, and nonlinear optical properties. There has been sustained interest in the ordering and energies of electronic states, quantum efficiencies for charge separation and recombination processes, and their dependence on the chemical structure.1Charge generation and charge transport processes have been extensively studied in the poly(p-phenylene-vinylene) (PPV) and its derivatives.2-5In this paper, we study the effect of introducing bipyridylene vinylene, (bpv) moieties into the phenylene-vinylene skeleton (poly(5-vinylene-5′-vinylenephenylene-2,2′-bipyridine), 1, poly(p-tris-(phenylenevinylene)-2,2′-bipyridine), 2, and poly(5,5′-vinylene-2,2′-bipyridylene), 3, on the photophysical properties. An incentive to the synthesis of such polymers is their ability to exhibit reversible and tunable optical and electrooptical proper-ties, depending on their protonation state at the nitrogen atoms of the bipyridine moieties.6 The tunability of these novel polymers was clearly demonstrated to originate from a combi-nation of changes in the intrachain (“molecular”) as well as interchain (“supramolecular”) electronic properties arising from a significant change in aggregation upon protonation- depro-tonation processes.6,7Such reversible systems can be effectively utilized in sensor applications. This unique class of polymers allows the study of charge separation properties in a homologous series where the asymmetry of the charge density distribution in the repeat unit can be controlled. In this paper we focus largely on the free base form of these polymers and explore the effect of the variation in charge density distribution in the repeat unit on bulk photoconductivity properties.
On the basis of the coincidence of the onsets of the photoconductivity and absorption in MEH-PPV, it was con-cluded that photoexcitation of PPV leads to a direct generation
of mobile charges through an interbandπ-π* transition.8,9On the other hand, on the basis of a variety of complementary experiments, it is argued that the photocurrent (Iph) originates from secondary processes, where the initial intrachain excitons dissociate to free charges.10,11 The evolution of Iph upon photoexcitation and the subsequent decay is, therefore, a valuable tool to explore various processes and develop a deeper understanding of phenomena such as electroluminescence in these systems. Extrinsic mechanisms are most likely to affect the relatively long time (> nanosecond) transient photocon-ductivity measurements. This relatively slow decay process is interpreted as being extrinsic due to dissociation of polaron pairs (or interchain excitons) through interaction with oxygenated defects to create positive polarons.12The slow component has been modeled in terms of a recombination limited dispersive decay.
The spectral responses of Iphin these PPV-bipyridine systems is studied in planar or sandwich configuration, where the incident light propagation direction is perpendicular or parallel to the electric field, respectively. We use both steady-state and transient measurements to study these polymers. The depend-encies of Iphon the electric field, light intensity, and temperature are measured in order to probe the mechanisms of charge generation and transport in such polymers.
Experimental Section
Materials. Polymers poly(5-vinylene-5′ -vinylenephenylene-2,2′-bipyridine), 1, poly(p-tris(phenylenevinylene)-2,2′-bipyri-dine), 2, and poly(5,5′-vinylene-2,2′-bipyridylene), 3, were prepared as per the scheme mentioned in ref 6.
5,5-Bis(carboxyethyl)-2,2′-bipyridine, 4. This polymer was prepared according to a modified literature procedure.13
5,5′-Bis(chloromethyl)-2,2′-bipyridine dihydrochloride, 5. Sodium borohydride (6.8 g, 180 mmol) was added to an ice-cold solution of 5,5′-bis(carboxyethyl)-2,2′-bipyridine (10.4 g, 34 mmol) in 150 mL of ethanol. The solution was left to reach room temperature, then refluxed overnight under an inert * Corresponding author. Fax: 91 80 8462766. E-mail: narayan@
jncasr.ac.in.
7671
J. Phys. Chem. B 2001, 105, 7671-7677
atmosphere. The solvent was removed under reduced pressure and the residue was refluxed in acetone for 1 h, then refluxed in aqueous K2CO3for an additional hour, then dried. In view of its low solubility in most organic solvents, crude 5,5′ -bis-(hydroxymethyl)-2,2′-bipyridine, 6, (NMR: 1H (CDCl3): δ4.58 (s, 4H), 7.84 (d, 2H), 8.33 (d, 2H), 8.59 (s, 2H);13C (DMSO):
δ60.54, 119.83, 135.40, 137.83, 147.70, 153.99; mp: 155 -157°C; MS: (CI) 217, M+H+) was used “as is” for the next step. Freshly distilled thionyl chloride (120 mL) was added slowly to the grounded crude 5,5′-bis(hydroxymethyl)-2,2′-bipyridine. The solution was stirred for 2 days at 60 °C and then evaporated to dryness. Dichloromethane (100 mL) and 50 mL of water were added to the residue and the aqueous solution was neutralized using ammonia solution. The product was extracted to dichloromethane, then dried over sodium sulfate, filtered, and precipitated from solution using dry HCl gas, in the form of hydrochloric salt, yielding 3.8 g, 40%.
5,5′-Bis(formyl)-2,2′-bipyridine, 7. A solution of 5,5′-bis-(hydroxymethyl)-2,2′-bipyridine, 6, (0.81 g, 3.7 mmol) in 25 mL of dry pyridine was added dropwise to a solution of Pb-(CH3CO2)4(5.1 g, 11.5 mmol) in 25 mL of dry pyridine. The solution was stirred at 90 °C for 2 h, then the solvents were reduced under reduced pressure. Chromatography (silica, 98:2 dichloromethane/ethanol) afforded 120 mg (15% yield) of pure
7 as a colorless solid.
NMR: 1H (CDCl3): δ8.32 (dd, 2H), 8.70 (d, 2H), 9.16 (d, 2H), 10.19 (s, 2H);13C (CDCl3): δ 122.36, 131.69, 137.12, 151.55, 159.14, 190.28; mp: 228°C; MS: (CI) 212.4, M+.
5,5′-Bis(triphenyl-phosphonium-methyl)-2,2′-bipyridine di-chloride, 8. An amount of 2.5 g (7.4 mmol) of the hydrochloride salt of 5,5′-bis(chloromethyl)-2,2′-bipyridine was neutralized with NH3(aq), extracted with dichloromethane, dried with sodium sulfate, filtered, and freed from organic solvents under reduced pressure. An amount of 2 g (7 mmol) of 5,5′-bis-(chloromethyl)-2,2′-bipyridine and 4 g (15 mmol) of tri-phenylphosphine were dissolved in 60 mL of DMF and stirred overnight at 80°C. The resulting white precipitate was filtered and washed with DMF and ether yielding 4.1 g (75%) of pure 5,5′-bis(triphenyl-phosphonium-methyl)-2,2′-bipyridine di-chloride. NMR1H(CDCl3): δ5.30 (d, 4H), 7.44 (d, 2H), 7.67 -7.95 (m, 30H), 8.13 (d, 2H), 8.24 (s,2H);13C(DMSO): δ116.5, 118.2, 120.4, 125.1, 130.2, 134.0, 135.2, 139.4, 150.7, 154.0; mp: 267-270°C (dec); MS: (CI) 705.5 M+H+.
1,4-Bis(4-formylstyryl) benzene, 9. A solution of sodium methoxide (360 mg, 6.7 mmol) in 50 mL of dry ethanol was added dropwise to a solution of 1,4-bis(triphenylphosphonium)-p-xylenedichloride (2 g, 3 mmol) and terephthalaldehyde (0.8 g, 6 mmol) in 30 mL of dry ethanol. The solution was stirred overnight at room temperature, then the solvent was removed under reduced pressure and the residue chromatograph (silica: 1:2 hexane/dichloromethane and then dichloromethane). An amount of 400 mg of 6 (40%) was collected as a white solid. 1HNMR (CDCl3): δ7.20 (d, 4H), 7.55 (s, 4H), 7.64 (d, 4H), 7.86 (d, 4H), 9.98 (s, 2H); mp: 247-249°C; MS: (High Res.) 339.1, M+H+.
Poly(5-Vinylene-5′-Vinylenephenylene-2,2′-bipyridine), 1. A solution of 68 mg of sodium ethoxide (1 mmol) in 15 mL of dry ethanol was added to a solution of 318 mg (0.4 mmol) of 5,5′-bis(triphenyl-phosphonium-methyl)-2,2′-bipyridine di-chloride and 45 mg (0.4 mmol) of terephthalaldehyde in 40 mL of ethanol. The solution was stirred overnight at room temper-ature and the yellow precipitate was collected, washed with several portions of dichloromethane and THF, and dried under reduced pressure. The polymer was isolated as bright yellow
powder, 90 mg (75%), MWAv ∼5500-20000. Elemental analysis for free-base and acid-saturated (“protonated”) forms, respectively: Anal. calcd for C20H14N2: C, 85.08; H, 5.00; N, 9.92. Found: C, 79.89; H, 5.16; N, 9.05. Anal. calcd for C20H14N2.2HCl: C, 67.62; H, 4.54; N, 7.89. Found: C, 60.83; H, 4.48; N, 6.66. NMR1H(CF3COOD): δ9.92 (brs, aldehyde end groups), 9.20 (brs, 2Hpy), 8.89 (brs, 2Hpy), 8.57 (brs, 2Hpy), 7.71-7.40 (m, 8Hph+vinyl). Solid-state NMR13C: δ 153.35 (py), 149.80 (py), 137.70 (py), 135.35 (ph), 131.10 (py), 129-123 (ph+vinyl), 120.0 (py). IR (KBr): 1696, 1589, 1537, 1505, 1469, 1418, 1376, 1275, 1207, 1107, 1053, 1021, 959, 840, 803, 739, 651 cm-1. Luminescence quantum yield (Ex: 355 nm): 0.50, 0.20 (solutions of 10-7M and 10-5M in formic acid, respectively).
Poly(p-tris-(phenyleneVinylene)-2,2′-bipyridine), 2. A solution of sodium methoxide (27 mg, 0.5 mmol) in 10 mL of dry ethanol was added dropwise to a solution of 1,4-bis(4-formylstyryl)-benzene, 6, (81 mg, 0.23 mmol) and 5,5′ -bis(triphenyl-phos-phonium-methyl)-2,2′-bipyridine dichloride, 4, (178 mg, 0.23 mmol) in a mixture of DMF (25 mL) and ethanol (8 mL) at 50 °C. The reaction mixture was stirred overnight at room temperature, then the precipitate was collected, successively refluxed with several portions of dichloromethane and THF to remove short oligomers, and dried under reduced pressure, yielding 96 mg (90%) of the polymer as bright yellow powder. MWAv∼2000-5500. Elemental analysis for free-base form: Anal. calcd for C36H26N2: C, 88.86; H, 5.39; N, 5.76. Found: C, 69.78; H, 4.79; N, 4.45. NMR 1H(CF3COOD): δ 9.71 (brs, aldehyde end group), 8.71 (brs, 2Hpy), 8.53 (brs, 2Hpy), 8.37 (brs, 2Hpy), 7.54-7.08 (m, 20Hph+vinyl). Solid-state NMR13C: δ153.35 (py), 149.80 (py), 137.70 (py), 135.35 (ph), 131.10 (py), 129-123 (ph+vinyl), 120.0 (py). IR (KBr): 1696, 1589, 1537, 1505, 1469, 1418, 1376, 1275, 1207, 1107, 1053, 1021, 959, 840, 803, 739, 651 cm-1.
Poly(5,5′-Vinylene-2,2′-bipyridylene), 3. A solution of sodium
methoxide (122 mg, 1.8 mmol) in 20 mL of dry ethanol was added dropwise to a solution of 5,5′-diformyl-2,2′-bipyridine,
7, (106 mg, 0.5 mmol) and 5,5′ -bis(triphenyl-phosphonium-methyl)-2,2′-bipyridine dichloride, 4, (397 mg, 0.5 mmol) in a mixture of dichloromethane (8 mL) and ethanol (20 mL) at 50 °C. The reaction mixture was stirred overnight at room temperature, then the precipitate was collected, successively refluxed with several portions of dichloromethane, water, DMSO, ethanol, and THF to remove short oligomers, and dried under reduced pressure, yielding 113 mg (50%) of the polymer as bright yellow powder.14-17
Measurements. The polymers isolated in the form of powders
methods.18In the planar (surface) geometry, the polymer was deposited on quartz substrates. After processing it to the base form, metal electrodes, 0.1 mm apart, were deposited on the surface. Spectral response of the photocurrent was carried out using a 150 W xenon lamp along with a monochromator using the lock-in technique. Temperature-dependent measurements Iph-(T) were carried using a closed cycle refrigerator offering a wide temperature range capability of 10-500 K, with the sample mounted alongside a calibrated thermometer, on the cold head of the cryostat fitted with quartz windows. 5 mW diode lasers at 532 and 473 nm were used as the light source for the Iph(T) measurements. Transient decay measurements were performed on samples in the planar auston-strip configuration using a nanosecond pulse laser at 355 nm, 10 Hz repetition rate, and a 54520A HP digital oscilloscope. The temporal resolution for the transient measurements was∼2 ns. Iph of the protonated films was accompanied by large noise-to-signal ratio and systematic measurements were not possible.
Results and Discussion
Steady-State Photoconductivity. Figure 1 depicts the
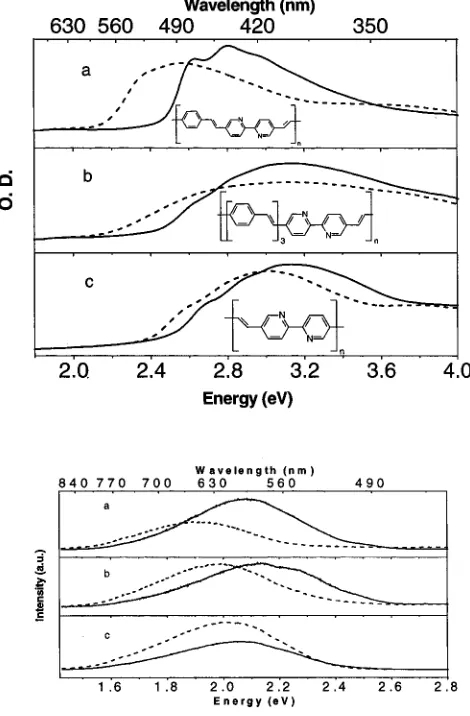
ab-sorption and luminescence spectra of thin films of the three polymers 1, 2, and 3 along with their chemical structure in their free base and protonated forms. The results of absorption and emission spectra presented in Figure 1 and in previous studies6 show the following. (i) The free base forms of these polymers have sharper absorption and luminescence profiles than their
respective protonated forms which are red shifted and broader with no vibronic features. (ii) Polymer 1 in its free base form has a more well defined absorption onset and a lesser degree of inhomogeneous broadening compared to polymers 2 and 3. Detailed studies on the changes in the absorption and PL of the films, which occur upon protonation-deprotonation processes, have been shown to originate from both molecular level and interchain (supermolecular) interactions. The increased inter-chain interaction in the accompanied protonated form by the larger charge delocalization effectively causes the red-shift. Additionally, the PL band in the case of the protonated film is broader without any vibronic features and can be attributed to the increased disorder which can also arise from increased interchain interactions.
[image:3.612.322.554.48.205.2]The spectral response of the photocurrent, Iph, in sandwich-type devices having polymers 1, 2, and 3 as active layers is shown in Figure 2. Clearly, the spectral response indicates an onset roughly around the optical absorption edge. The response Iphfor polymer 1 is an order of magnitude higher than in similar devices of polymer 2. The magnitude of Iphis 20 nA/cm2at an incident photon density of 73µW/cm2at 400 nm and applied bias of -2 V. The Iph-V response in devices of polymer 1, shown in Figure 3, is asymmetric with an open circuit voltage, Voc, of 0.2 V and short circuit current, Isc, of 0.8 nA. The asymmetry represents the photodiode type behavior of these devices with the reverse bias mode indicating the negatively biased ITO with respect to the Al electrode. The Iph-V of Figure 1. Chemical structure along with the absorption spectra of
polymers a- 1, b- 2, and c- 3 and the emission spectra (λexc )355
[image:3.612.57.292.51.409.2]nm): a- 1, b- 2, c- 3. Solid lines represent free base form, dashed lines represent acid-saturated forms.
Figure 2. Comparison of Iph(λ) in polymers 1, 2, and 3 sandwich
devices at low forward bias voltage, inset Iph(λ) at higher reverse bias
[image:3.612.323.554.249.418.2]voltage.
Figure 3. Dark current and Iphas a function of applied voltage for
polymer 2 is less asymmetric with Voc∼0.1 V and Isc∼0.6 nA/cm2at light intensity of∼50µW/cm2at 400 nm. An increase in Vocis expected with increasing photon density until reaching saturation. At higher voltages,>4 V, injection currents develop and the device exhibits the characteristic LED response.
The intensity dependence of Iphfor input power of 0.01 to 10µW/cm2is linear in the entire voltage range for all the devices in this configuration. The linear behavior of Iphas a function of photon density for the three samples indicates monomolecular kinetics; the photocurrent directly measures the efficiency of the charge carrier generation. The Iph(λ) spectral response of devices of polymer 1 depends strongly on the polarity and magnitude of induced bias as is evident from the inset in Figure 2. Clearly, a red shift of∼0.3 eV in the onset of the Iph(λ) is observed under high reverse bias. A similar, but considerably smaller, effect was observed for polymers 2 and 3. Such red-shifted bias polarity-dependent features can be qualitatively explained on the basis of exciton quenching at the Al interface and the higher hole mobility of the polymer. However, a detailed bias-dependent spectral analysis requires the knowledge of various parameters, such as exciton and/or free carrier diffusion lengths, and barrier height at the interface junctions.19
Iph(λ) in these polymers clearly consists of both extrinsic and intrinsic contributions. The Iph(λ) from intrinsic charge carrier is expected to be blue shifted relative to the absorption spectrum.20Results from the sandwich devices can be explained on this basis of bulk photoionization and interfacial exciton quenching processes. The photocarrier generation rate is clearly higher for polymer 1 based devices compared to polymer 2 and polymer 3, with Iph(λ) in polymer 1 samples strongly dependent on the bias conditions. At low bias voltage, interfacial processes for charge separation appear to be dominant as indicated by the red shift in the reverse bias Iph(λ) while at high voltage bulk photoionization takes over with the forward and reverse bias Iph(λ) appearing to be identical.
Measurements in the planar geometry were carried out in a vacuum as well as in ambient conditions. Figure 4 depicts the steady-state spectral response of Isurph(λ) in a planar geometry for the three polymers in ambient conditions under a field of 103 V/cm. Measurements in this geometry are devoid of problems related to electrode modifications and interfacial effects; however, since the electric field magnitudes are quite low the magnitude of Iph decreases proportionately. Figure 4 reveals the sharp onset of Iph(λ) at the absorption edge. The results are qualitatively similar to the response of Iph(λ) to reversed bias in a sandwich-type geometry. However, a strong
dependence of Iphon moisture conditions is observed in this planar geometry for all the samples. Iphdrops by a factor of 100 as the vapor pressure is reduced, probably due to the removal of traces of water molecules from the surface of the polymer. This effect is found to be reversible. Upon exposure to ambient atmosphere, the polymers regain their initial proper-ties, revealing the extrinsic nature of such a defect-mediated transport process in the systems. The intrinsic component of the photocurrent processes is reflected in the measurements carried out after extensive pumping at elevated temperatures. The magnitude of the spectral response Isurph(λ) after this treatment with measurements in vacuum conditions, which is scaled down by a factor of 100, was in the same range as the noise fluctuation amplitude at low light levels <1 µW/cm2. Isurph(λ) after careful analysis qualitatively resembled ambient condition responses. However, measurements using laser sources at fixed wavelengths of 473 and 532 nm were possible with large signal-to-noise ratio even in vacuum conditions.
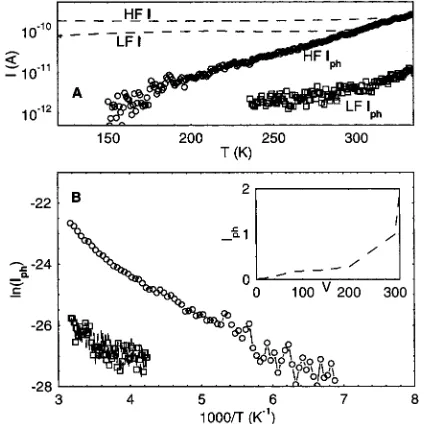
Isurphin the planar geometry varies significantly with ambient conditions, in a reversible manner, with the extrinsic processes governed by the surface-defect-mediated photocurrent in the samples. Iph(T), in the first approximation, can be analyzed as a process composed of a thermally activated barrier component for photoinduced charge generation and a complex hopping transport or a activated charge-transport component. The relatively weak temperature dependence of the dark current indicates an activation energy smaller than 0.1 eV. The much stronger Iph(T) dependence on T is indicative of a barrier limited free carrier generation mechanism in polymer 1 . This inter-pretation is supported by the strong dependence of Iph(T) on the electric field, above a threshold value (∼3× 103V/cm). The higher barrier energy of∼0.2 eV, at V>300 V (∼5× 103 V/cm), for Iph(T) along with the fact that the similar temperature dependence for Idark(T) at different voltage bias also strongly indicates the dominant charge carrier generation limited current rather than a transport limited process in this field and temperature regime.
[image:4.612.332.545.49.261.2]The temperature, T dependence of Isurphof the vacuum heated samples of polymer 1 at F<103V/cm shown in Figure 5 can Figure 4. Iph(λ) in the planar-surface geometry for polymer 1, polymer
2, and polymer 3; inset is the plot of the normalized Iphversus relative
intensity of polymer 1 in the log-log scale.
Figure 5. (A) Temperature dependence of the dark current I and the photocurrent Iphin the planar geometry of polymer 1 at high field, HF
(400 V) and low field, LF (100 V) voltage bias conditions. (B) Arrheneius plot of Iph at high and low field, inset is the voltage
[image:4.612.61.289.49.202.2]be fitted to an Arrhenius behavior with an activation energy ∼ 0.1 eV. The plot of Isurph(F) as a function of applied bias indicates a cross over to a higher nonlinear regime as F approached 5×103V/cm. This behavior is also observed in the response of Isurph(T) at F∼5×103V/cm, showing more than an order of magnitude increase in Iph, compared to the low field regime. A respective barrier of 0.2 eV is measured over the entire temperature range. The intensity dependence of the steady-state photocurrent shows a square root dependence over the entire measured range of incident photon density (10 W/cm2 to 1 mW/cm2) at 473 nm. Isurph∼ (intensity)R with R ∼0.5 (Figure 5, inset).
The observed square root dependence of Iphas a function of incident photon flux (Figure 4, inset), can be due to the bimolecular photocarrier recombination caused by their spatial restriction, often observed in this type of planar geometry.21 On the other hand, a linear relation has been observed in the sandwich configuration of these devices.21,22The transport and recombination mechanisms of the photogenerated carriers in the two geometries are clearly different with a surface mediated process in the planar geometry, and a sizable electric field/ Shotkky processes in the sandwich geometry. The results in Figure 5 indicating the Iphsur(T) and sub-linear intensity depen-dence in the coplanar geometry can also be interpreted on the basis of trap-controlled mechanisms.22A modified version of the model proposed by Rose23can possibly explain the field-dependent Iphsur(T) and also the intensity dependence. In this model the square root dependence of Iphsur(intensity) is modified by the electron traps in energy.23The density of traps in any small energy interval through which the steady-state fermi level sweeps can be typically taken to be an exponential function of the energy of traps below the band edge. The trap filling and emptying kintetics are also dependent on the electric field and may be source for the field-dependent Iphsur(T).
Transient Photoconductivity. The difference in the
photo-induced charge generation process is significantly apparent in the transient measurements. These measurements offer additional insight in the photophysics of these materials, with the results yielding a closer estimate for the efficiency of photocarrier generation and transport. The estimate involves the measurement of the total charge collected at the electrode immediately after an incident short laser pulse and the yieldφcan be expressed as φ ) J/eNp, where Npis the photon flux, J is the current density with the cross sectional area L/Rfor the carrier transport, whereR is the absorption coefficient, and L is the electrode dimension.3
Figure 6 depicts the transient decay, Iph(t) of the current after a pulsed photoexcitaion of polymer 1. The time constant for the initial fast decay, indicated in the figure, puts an upper limit on the actual decay constant in the Iph(t) response due to experimental limitations. Nevertheless, the measurements can explicitly separate the initial fast process, which are in the 0.5 -10 ns range with the slower decay kinetics in the -100 ns-1 s regime, observed in such systems. The results indicate the predominant contribution of the photocurrent from the fast decay process (<40 ns) and a relatively small contribution from longer processes (>40 ns), which constitute <5% of the overall integrated response. The field dependence of the transient response shows the following interesting features. (i) The current at t)0 shows a dramatic difference with respect to field for polymer 1 compared to polymer 2 as in Figure 6 inset (ii). There is no significant variation in the time constants involving the initial decay with respect to varying electric field. The initial
[image:5.612.330.543.48.210.2]decay in polymer 1 is fast characterized by a shorter time constant compared to polymer 2 and polymer 3 as depicted in Figure 7.
The values for the quantum yieldφare plotted as a function of field in Figure 8 for the different samples. A reasonable approach to understand the nonlinear dependence of φ(F) is the Onsager three-dimensional (3D) model for geminate re-Figure 6. Transient decay current I of polymer 1 after a pulse excitation, inset is the log(I) versus time and the initial current (at time
[image:5.612.328.549.256.391.2]t)0) as a function of electric field F.
Figure 7. Transient decay current I (on a normalized scale) of polymers 1, 2, and 3 after a pulse excitation at the same electric field. Inset is the plot on the log scale.
[image:5.612.320.558.435.630.2]combination limited charge carrier transport.24 An essential assumption of Onsager’s theory of the dissociation of short-lived charge pairs is that photon absorption creates with probabilityφ0a Coulombically bound e-h pair with initial pair distance r0. It can either recombine or dissociate with a probability in the course of a temperature- and field-assisted diffusion process. The activation energy Eaof the quantum yield, measured at low fields, is related to r0via r0)e2/(4π0Ea)), in the limit of the homogeneous medium approximation. Figure 8 also compares the experimentally obtained efficiencies with 3D Onsager model with assumptions and calculations as reported by Pai and Enck25 and the following additional assumptions for the dielectric constant, with the primary dissociation yield)1 at the high-temperature limit. The model also does not take into account any disorder effects. The 3D model yields good qualitative fits to the experimental data for polymer 2 and polymer 3 with the value of thermalization distance∼1.2 nm corresponding to an activation energy from eq 2 to be ∼0.3 eV. However, close fits to the 3D to the experimental data for polymer 1 were not possible with a reasonable wide range of values for the parameters involved in the model. It has been argued that the early stage of e-h pair dynamics should be dominated by intrachain rather than interchain processes justifying a need for a 1D model.4Attempts to fit the results of polymer 1 to an ideal 1D Onsager model were also unsuccessful. However, it is noted that the low field photocurrent measurements shown in Figure 5b (inset), which is essentially low field data<5×104V/cm, yields a better fit to a 1D model with a reasonable estimate of r0∼1.2 nm. The strong, and the sizable nonlinear dependence of the yieldφ(F) for polymer 1 in the intermediate and high field regime is indicated by the power law fit in this range withφ≈AFβwith
β≈3.5 for polymer 1 while the values ofβfor polymer 3 and polymer 2 are ≈1.5 and 1, respectively, as shown in Figure 8B. The measure ofβreveals the strong field dependence of the photocurrent for polymer 1 and is indicative of field-induced dissociation processes in this system. The observation and interpretation of the transient measurements is consistent with the observations from the bias-dependent spectral responses Iph-(λ) and the activated behavior observed in the temperature dependence of Iphat high F.
These experimental observations of the field dependence of polymer 1 can be argued on the basis that the primary ionization process is field dependent, which can be expressed accordingly;
φ0(F) )φ0+ φ1(F). The importance of this field-dependent term can be evaluated on the basis of the value of the exponent
βwhereφ1(F)/φ0)AEβ. The effective probability for charge-carrier separationφ(F)∼φ0(F)×jesc(F) whereφesc(F) is the dissociation probability for a geminate pair at an initial separation.24The ratio of the measured field-dependent photo-current and the photophoto-current expected from an initial field-independent process can then be expressed as
φesc(F) can be evaluated on the assuming a 1D Onsager model framework with an initial separation of 1.2 nm. This assumption, however, leads to a sizable uncertainty, but a lower estimate of a cubic dependence (β)3) on field is observed forφ1(F,)/φ0 in the field range (5×104)-(5×105)V/cm. The increase in the primary yield of the charge carriers can be interpreted as an increase in an oscillator strength of the band-band transition in the presence of an electric field in this range or in terms of field-induced ionization of neutral exciton states that are degenerate with band states. In the present case of polymer 1,
supramolecular factors also need to be considered, to comment on the possible origins of the field dependent primary yield.
Previous results on such family of polymers have shown that insertion of BPY units in an otherwise well conjugated polymer chain brings about structural changes that diminishπ -conjuga-tion in the polymer chain.26This was argued on the basis that the electronic coupling is reduced because the disparity in the electronic structure of the two units and the rotation about the C-C bond connecting the pyridyl rings of BPY results in nonplanar conformation between the two adjacent conjugated segments of the polymer also causing a reduced π-orbital overlap. These studies were done on dilute solutions of the polymer where interchain interactions were absent.26 It is reasonable to expect an increased interaction in the solid/ concentrated form when additional nonplanar degrees of free-dom of BPY units are present. This feature of higher charge-separation yield in the nanosecond time regime and the field dependence in polymer 1 compared to polymer 2, can be attributed to an higher interchain interaction in polymer 1, which has a higher content of BPY units. The interchain processes effectively result in electrons and holes generated on separate chains, which have a lower probability of recombination than that of intrachain charge-separation processes. In other words, increased interchain charge-transfer processes can enhance photocurrent efficiency. The nonplanar geometry introduced by insertion of BPY units in a PPV chain can facilitate the interchain interaction in the condensed phase. This qualitative picture can also explain the strong electric field dependence of the photocurrent in polymer 1, since the electric field influences the charge carrier separation across the chains. However, a systematic study of the packing arrangement with varying units of BPY is needed to conclusively establish this correlation.
In conclusion, we have studied charge generation and separation in a novel series of PPV-BPY polymers by measuring the photocurrent response at various temperatures, electric fields, and light intensity. The efficiency for photocurrent generation is clearly higher in polymer 1 (PV-BPY) than in polymer 2 ((PV)3-BPY) as observed by steady-state photo-conductivity measurements and transient photophoto-conductivity measurements. A strong electric-field dependence of the yield for photoinduced charge generation is also observed in polymer
1 compared to polymers 2 and 3. These observations can arise
from the increased interchain interaction in polymer 1. The results indicate a strong correlation of the bulk property of photoconductivity with the basic structure of the tunable repeat unit.
Acknowledgment. The authors (K.S.N. and K.V.G.) thank
the Department of Science and Technology, Govt. of India. The work at Technion was also supported by Israel Ministry of Science (2046-1-99).
References and Notes
(1) Friend, R. H.; Gymer, R. W.; Holmes, A. B.; Burroughes, J. H.; Marks, R. N.; Tallani, C.; Bradely, D. D. C.; Dos Santos, D. A.; Bredas, J. L.; Logdlund, M.; Salaneck, W. R. Nature 1999, 397, 121.
(2) Woo, H. S.; Graham, S. C.; Halliday, D. A.; Bradley, D. D. C.; Friend, R. H.; Burn, P. L.; Holmes, A. B. Phy. ReV. B 1992, 46, 7379.
(3) Gailberger, M.; Bassler, H. Phys. ReV. B 1991, 44, 8613.
(4) Lee, C. H.; Yu, G.; Heeger, A. J. Phys. ReV. B 1993, 47, 15543.
(5) Special Issue on Excited-State Phenomena in Conjugated Polymers; Bassler, H., Rothberg, L. J., Eds.; Chem. Phys. 1998, 227, and references therein.
(6) Eichen, Y.; Nakmanovich, G.; Gorelik, V.; Epeshtein, O.; Poplaw-ski, J. M.; Ehrenfreund, E. J. Am. Chem. Soc. 1998, 120, 10463.
(7) Blatchford, J. W.; Jessen, S. W.; Lin, L. B.; Gustafson, T. L.; Fu, D. K.; Wang, H. L.; Swager, T. M.; MacDiarmid, A. G.; Epstein, A. J.
φ(F)/φ0φ esc
Phys. ReV. B 1996, 54, 9180. (b) Wang, Y. Z.; Gebler, D. D.; Lin; Fu, D.
K.; Swager, T. M.; Epstein, A. J. Appl. Phys. Lett. 1997, 70, 3215. (c) Nakmanovich, G..; Epeshtein, O.; Gorelik, V.; Poplawski, J. M.; Oiknine-Schlesinger, J.; Ehrenfreund, E.; Eichen, Y. Synth. Met. 1997, 84, 883.
(8) In Primary photoexcitations in conjugated polymers: molecular
excitonsVersus semiconductor band model; Sariciftci, N. S., Ed.; World
Scientific: Singapore, 1998.
(9) Heeger, A. J. In Primary photoexcitations in conjugated
poly-mers: molecular excitonsVersus semiconductor band model; Sariciftci, N.
S., Ed.; World Scientific: Singapore, 1998; pp 20-50.
(10) Bassler, H. In Primary photoexcitations in conjugated polymers:
molecular excitonsVersus semiconductor band model; Sariciftci, N. S., Ed.;
World Scientific: Singapore, 1998; pp 51-98.
(11) Samuel, I. D. W.; Rumbles, G.; Friend, R. H. In Primary
photoexcitations in conjugated polymers: molecular excitons Versus semiconductor band model; Sariciftci, N. S., Ed.; World Scientific:
Singapore, 1998; pp 140-173.
(12) . (a) Lee, C. H.; Yu G.; Moses, D.; Sariciftci, N. S.; Wudl, F.; Heeger, A. J. Mol. Cryst. Liq. Cryst. 1994, 256, 745. (b) Lee, C. H.; Yu, G.; Moses, D.; Heeger, A. J. Phys. ReV. B 1994, 49, 2396. (c) Lee, C. H.;
Yu, G.; Heeger, A. J. Zhang, J. Synth. Met. 1995, 75, 127.
(13) a) Munavalli, S.; Gratzel, M. Chem. Ind. 1987, 722. (b) Palmer; C. R.; Sloan, L. S.; Adrian, G. C.; Cuenoud, B.; Paolella, D. N.; Schepartz, A. J. Am. Chem. Soc. 1995, 117, 8899.
(14) Depending on molecular weight selection by different purification protocols. Dissolving the polymer in formic acid and precipitating it by adding ethanol enables the selection of the MW cutoff. Molecular weights were estimated from the ration between end groups and main body groups
from NMR and IR data. See also Gagnon, D. R.; Capistran, J. D.; Karasz, F. E.; Lenz, R. W.; Antount, A. Polymer 1987, 28, 567.
(15) Elemental analysis was performed on a CHN Analyzer (Perkin-Elmer).
(16) Depending on molecular weight selection by different purification protocols. Dissolving the polymer in formic acid and precipitating it by adding ethanol enables the selection of the MW cutoff. Molecular weights were estimated from the ratio between end groups and main body groups from NMR and IR data.
(17) Microanalysis data clearly show incomplete combustion of the samples. C, H and N values are exactly 79% of the theoretical values.
(18) Narayan, K. S.; Singh, T. B. Appl. Phys. Lett. 1999, 74, 3456. (19) Harrison , M. G.; Gruner, J.; Spencer, G. C. W. Phys. ReV. B 1996, 55, 7831.
(20) Barth, S.; Bassler, H.; Rost, H.; Horlhold, H. H. Phys. ReV. B. 1997, 56, 3844.
(21) Antoniadis, H.; Rothberg, L. J.; Padimitrakopoulos, F.; Yan, M.; Galvin, M. E.; Abkowitz, M. A. Phys. ReV. B 1994, 50, 14911.
(22) Bube, R. H. Photoelectronic Properties of Semiconductors; Cam-bridge University Press: CamCam-bridge, 1992.
(23) Rose, A. Concepts in PhotoconductiVity and Allied Problems;
Interscience Publishers: New York, 1963; p 38. (b) Tabak, M. D.; Warter P. J. Phys. ReV. 1964, 148, 982.
(24) Seiferheld, U.; Reis, B.; Bassler, H. J. Phys. Chem. 1983, 16, 5189. (25) Pai, D. M.; Enck, R. Phys. ReV. B 1975, 11, 5163.