Cardiac Biomarkers and Acute Kidney
Injury After Cardiac Surgery
Emily M. Bucholz, MPHa,b, Richard P. Whitlock, MDc, Michael Zappitelli, MD, MScd, Prasad Devarajan, MDe, John Eikelboom, MD, MBBSc,f, Amit X. Garg, MD, PhD, MA, FACPg,h, Heather Thiessen Philbrook, MMath, AStatg,
Philip J. Devereaux, MD, PhDi, Catherine D. Krawczeski, MDj, Peter Kavsak, PhDk, Colleen Shorttk, Chirag R. Parikh, MD, PhDl,m, for the TRIBE-AKI Consortium
abstract
OBJECTIVES:To examine the relationship of cardiac biomarkers with postoperative acute kidneyinjury (AKI) among pediatric patients undergoing cardiac surgery.
METHODS:Data from TRIBE-AKI, a prospective study of children undergoing cardiac surgery,
were used to examine the association of cardiac biomarkers (N-type pro–B-type natriuretic
peptide, creatine kinase-MB [CK-MB], heart-type fatty acid binding protein [h-FABP], and troponins I and T) with the development of postoperative AKI. Cardiac biomarkers were
collected before and 0 to 6 hours after surgery. AKI was defined as a$50% or 0.3 mg/dL
increase in serum creatinine, within 7 days of surgery.
RESULTS:Of the 106 patients included in this study, 55 (52%) developed AKI after cardiac surgery. Patients who developed AKI had higher median levels of pre- and postoperative cardiac
biomarkers compared with patients without AKI (allP,.01). Preoperatively, higher levels of
CK-MB and h-FABP were associated with increased odds of developing AKI (CK-MB: adjusted odds
ratio 4.58, 95% confidence interval [CI] 1.56–13.41; h-FABP: adjusted odds ratio 2.76, 95% CI
1.27–6.03). When combined with clinical models, both preoperative CK-MB and h-FABP provided
good discrimination (area under the curve 0.77, 95% CI 0.68–0.87, and 0.78, 95% CI 0.68–0.87,
respectively) and improved reclassification indices. Cardiac biomarkers collected postoperatively
did not significantly improve the prediction of AKI beyond clinical models.
CONCLUSIONS:Preoperative CK-MB and h-FABP are associated with increased risk of postoperative AKI and provide good discrimination of patients who develop AKI. These biomarkers may be useful for risk stratifying patients undergoing cardiac surgery.
WHAT’S KNOWN ON THIS SUBJECT:Acute kidney injury (AKI) occurs in up to 50% of children after cardiopulmonary bypass and is associated with adverse outcomes. Renal biomarkers have been shown to predict postoperative AKI, but few studies have examined cardiac biomarkers for risk classification.
WHAT THIS STUDY ADDS:Preoperative levels of creatine kinase-MB and heart-type fatty acid binding protein are strongly associated with the development of postoperative AKI after pediatric cardiac surgery and can be used to improve preoperative clinical risk prediction.
lDepartment of Internal Medicine,aSchool of Medicine, andbDepartment of Chronic Disease Epidemiology, School of
Public Health, Yale University, New Haven, Connecticut;cDivision of Cardiac Surgery, Population Health Research Institute,
andiDepartments of Clinical Epidemiology and Biostatistics,fMedicine, andkPathology and Molecular Medicine,
McMaster University, Hamilton, Ontario, Canada;dDivision of Nephrology, Department of Pediatrics, Montreal Children’s Hospital, McGill University Health Centre, Montreal, Quebec, Canada;eDepartment of Nephrology, Cincinnati Children’s
Hospital Medical Center, Cincinnati, Ohio;gDivision of Nephrology, Department of Medicine, andhDepartment of Epidemiology and Biostatistics, University of Western Ontario, London, Canada;jDivision of Pediatric Cardiology, Lucile
Packard Children’s Hospital, Stanford University School of Medicine, Palo Alto, California; andmClinical Epidemiology
Research Center, Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut
Acute kidney injury (AKI) occurs in up to 50% of children after
cardiopulmonary bypass (CPB),1,2
and is independently associated with adverse outcomes, including longer lengths of stay (LOSs), prolonged mechanical ventilation, and higher mortality.3,4Early identification of
patients at risk for developing AKI may allow implementation of strategies to reduce related morbidity and mortality.5,6
Preliminary evidence suggests that biomarkers can help to identify patients at risk for postoperative AKI.2In both pediatric and adult
populations, renal biomarkers, such as serum cystatin C and neutrophil gelatinase-associated lipocalin (NGAL), have been shown to predict postoperative AKI and improve risk
classification when combined with
other clinical models.7–11In addition,
studies in adults have shown that pre- and postoperative levels of cardiac biomarkers, such as B-type natriuretic peptide (BNP), strongly predict postoperative AKI risk.12Only
2 studies in children have examined the prognostic value of BNP on
postoperative AKI.13,14 Both found
that although BNP was not an independent predictor of AKI, it was associated with longer intubation times and higher postoperative mortality. To our knowledge, no studies have examined other cardiac biomarkers, such as troponins, creatine kinase-MB (CK-MB), or heart-type fatty acid binding protein (h-FABP), in children or adults. Preoperative elevations in cardiac biomarkers may reflect the severity of the underlying heart disease,15
whereas postoperative levels of cardiac biomarkers reflect the complexity of the surgery and the degree of intraoperative cardiac
damage, which can increase patients’
risk of postoperative complications, such as AKI.
The purpose of this study was twofold. First we evaluated the prognostic utility of 5 cardiac
biomarkers (N-type [NT] pro-BNP, CK-MB, h-FABP, high-sensitivity cardiac troponin T [hs-cTnT], and cardiac troponin I [cTnI]), measured preoperatively and immediately after surgery in children, to predict postoperative AKI risk and LOS. We then determined whether these biomarkers provided additional
benefit for predicting AKI above that
afforded by clinical models.
METHODS
Data from the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury consortium study (TRIBE-AKI) were used for these analyses.16,17In brief, this
translational study was designed to validate novel kidney injury biomarkers by using a prospective cohort design with serial sample collections on 3 consecutive days. Participants included children aged 1 month to 18 years undergoing cardiac surgery at 3 academic hospitals. To increase the number of children at risk for AKI, we oversampled children undergoing risk adjustment of congenital heart
surgery-1 (RACHS-1) category$2
surgeries. Children were recruited preoperatively and followed postoperatively until discharge (n= 319). Institutional review board approval was obtained at each participating center, and all patients provided written informed consent. To capture cardiac biomarker kinetics around the time of cardiac surgery, only patients with a full set of pre- and postoperative samples were included (n= 106). Subject selection was not based on any clinical criteria.
Data Collection
Data on patient demographics and medical history were recorded before surgery. Details about the surgical procedure (eg, type of cardiac abnormality, procedure, bypass time, elective or urgent, and severity) were obtained from the medical record by using standardized definitions of the Society of Thoracic Surgeons data
collection tool. Severity of condition and surgical risk were evaluated
using the RACHS-1 method.18,19
Venous blood samples were collected preoperatively, within 6 hours after surgery, and on postoperative days 2 and 3. Blood was collected in EDTA tubes and centrifuged to separate plasma, divided into bar-coded 0.5-mL cryovials, and stored at–80°C. One vial from each time-point was used for biomarker measurements with a single freeze-thaw. Biomarkers were measured with a Roche automated analyzer (Roche Elecsys 2010; Roche Diagnostics, Basel, Switzerland) for NT pro-BNP (pmol/L) (coefficient of variation [CV] range
3.6%–7.7%) and hs-cTnT (ng/L)
(CV range 2.5%–10.5%), the Beckman
Coulter Access II instrument (Beckman Coulter, Brea, CA) for the
AccuTnI assay cTnI (µg/L) (CV range
5.4%–20%) and CK-MB (µg/L)
(CV range 2.7%–8.2%), and the
Evidence Investigator Cytokine Custom Array (Randox Crumlin,
United Kingdom) for h-FABP (µg/L)
(CV 17%). Preoperative serum creatinine (SCr) was measured as part of routine clinical care using modified Jaffe or enzymatic assays, and preoperative glomerular
filtration rates (GFRs) were estimated
by using the Schwartz equation.
Outcome Definition
The primary outcome in this study was development of AKI, which was
defined as rise in SCr of$50% or
0.3 mg/dL from preoperative baseline within thefirst 7 days after surgery.
Severe AKI was defined as either
a doubling of creatinine or dialysis requirement.20–22Secondary
outcomes included in-hospital mortality, hospital and intensive care unit (ICU) LOS, and time to
extubation.
Statistical Analyses
or Kruskal-Wallis tests for continuous variables andx2or Fisher’s exact test for categorical variables. Median biomarker values were plotted and compared across AKI groups by using Kruskal-Wallis tests for NT pro-BNP, cTnI, hs-cTnT, CK-MB, and h-FABP. Colinearity between biomarkers was assessed by using scatterplot and correlation matrices.
We evaluated unadjusted associations between cardiac biomarkers and the development of AKI by using logistic regression. Because 95% of AKI cases
occurred in thefirst 2 postoperative
days, we focused on cardiac biomarkers collected preoperatively and immediately postoperatively (within 6 hours of surgery). Biomarker levels were introduced into the models as log
transformations to normalize the distributions of these values. Analyses were repeated adjusting for
demographic and preoperative characteristics, including patient age, preoperative estimated GFR (eGFR) percentile, hospital site, RACHS-1
category$3, and CPB time.120
minutes. Covariates for the models were selected by using a combination of previously reported AKI predictors in this cohort16,23and significance
testing (P,.1).
We calculated the area under the receiver operating characteristic (ROC) curve (AUC) for each biomarker alone to determine its ability to discriminate between patients who did and did not develop AKI. Contingency tables were used to determine the optimal cutpoint for each biomarker. We used 2
approaches to assess the added value of biomarkers in the clinical
prediction models. First, we evaluated incremental changes in the AUC when individual biomarkers were added to the clinical model. This approach, however, is often an insensitive measure of the ability of a new marker to add value to a preexisting model because the c-statistic
minimally moves after a few powerful
risk factors are already in the model. Accordingly, we also calculated the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices. The NRI indicates how much more frequently appropriate
reclassification of AKI risk occurs than inappropriate reclassification with use of the model containing the biomarker, whereas the IDI measures how far individuals move on average along the continuum of predictive risk. These measures have advantages over the AUC because they directly quantify the appropriateness and
amount of reclassification when
biomarker values are added to the clinical prediction models.24–26Both
the NRI and IDI have been used extensively in cardiovascular outcomes and nephrology research.27–32
We then evaluated the association between these biomarkers with ICU
and hospital LOS by using logistic regression. ICU and hospital stays were dichotomized by using the median value of each (3 days for ICU stay and 7 days for hospital stay) to quantify the relation between elevations in cardiac biomarkers and risk of prolonged hospitalization by using risk measures. Models were adjusted for patient and clinical characteristics as well as AKI status to determine whether AKI explained the longer LOS in patients with high cardiac biomarker values.
RESULTS
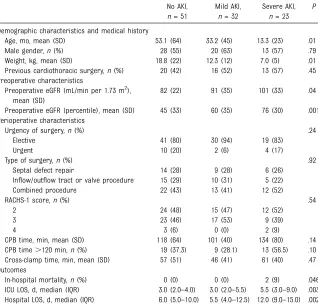
Of the 106 pediatric patients enrolled in this study, 23 (21.7%) developed severe AKI and 32 (30.2%) developed mild AKI after cardiac surgery (Table 1). Patients who developed AKI tended to be younger and to have lower preoperative weights. Patients who developed severe AKI had longer hospital and ICU LOS than patients
TABLE 1 Patient Characteristics AKI Status
No AKI,
n= 51
Mild AKI,
n= 32
Severe AKI,
n= 23
P
Demographic characteristics and medical history
Age, mo, mean (SD) 53.1 (64) 33.2 (45) 13.3 (23) .01
Male gender,n(%) 28 (55) 20 (63) 13 (57) .79
Weight, kg, mean (SD) 18.8 (22) 12.3 (12) 7.0 (5) .01
Previous cardiothoracic surgery,n(%) 20 (42) 16 (52) 13 (57) .45 Preoperative characteristics
Preoperative eGFR (mL/min per 1.73 m2), mean (SD)
82 (22) 91 (35) 101 (33) .04
Preoperative eGFR (percentile), mean (SD) 45 (33) 60 (35) 76 (30) .001 Perioperative characteristics
Urgency of surgery,n(%) .24
Elective 41 (80) 30 (94) 19 (83)
Urgent 10 (20) 2 (6) 4 (17)
Type of surgery,n(%) .92
Septal defect repair 14 (28) 9 (28) 6 (26)
Inflow/outflow tract or valve procedure 15 (29) 10 (31) 5 (22)
Combined procedure 22 (43) 13 (41) 12 (52)
RACHS-1 score,n(%) .54
2 24 (48) 15 (47) 12 (52)
3 23 (46) 17 (53) 9 (39)
4 3 (6) 0 (0) 2 (9)
CPB time, min, mean (SD) 118 (64) 101 (40) 134 (80) .14
CPB time.120 min,n(%) 19 (37.3) 9 (28.1) 13 (56.5) .10
Cross-clamp time, min, mean (SD) 57 (51) 46 (41) 61 (40) .47
Outcomes
In-hospital mortality,n(%) 0 (0) 0 (0) 2 (9) .046
ICU LOS, d, median (IQR) 3.0 (2.0–4.0) 3.0 (2.0–5.5) 5.5 (3.0–9.0) .003 Hospital LOS, d, median (IQR) 6.0 (5.0–10.0) 5.5 (4.0–12.5) 12.0 (9.0–15.0) .002
with mild or no AKI. Only 2 deaths occurred in-hospital, both among patients with severe AKI.
The trajectories of the 5 cardiac
biomarkers during thefirst 3 days of
hospitalization stratified by AKI status are displayed in Fig 1. Markers of cardiac injury (CK-MB, h-FABP, cTnI, and hs-cTnT) peaked within 6 hours after surgery, whereas markers of cardiac function (NT pro-BNP) peaked on day 2. Across all 4 time-points, median levels of cardiac biomarkers were higher among patients who developed severe AKI compared with mild or no AKI.
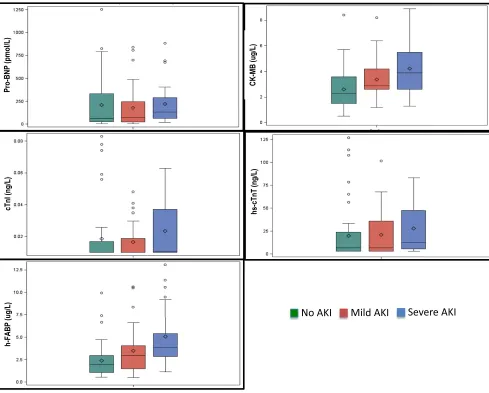
Preoperative Cardiac Biomarkers
Fig 2 shows the distributions of the
5 preoperative biomarkers stratified
by AKI status. There were significant
differences across AKI groups in preoperative CK-MB, hs-cTnT, and
h-FABP levels (P= .001,P= .048,
and P,.001, respectively), but
preoperative levels of NT pro-BNP and cTnI were comparable across groups (P= .2 andP= .3, respectively).
Higher preoperative levels of CK-MB and h-FABP were associated with higher crude odds of developing AKI (Table 2). After adjustment for patient characteristics, the association of preoperative CK-MB and h-FABP with development of postoperative AKI remained significant. A 1-unit increase in log
CK-MB was associated with afivefold
increase in the odds of developing
AKI after surgery. Similarly, a 1-unit increase in log h-FABP was associated with a threefold increase in the odds of developing AKI. No other
preoperative biomarkers were associated with the development of AKI after adjustment for patient characteristics (allP..1).
ROC analyses revealed preoperative CK-MB and h-FABP to be candidate biomarkers with high discriminatory value for predicting AKI (Fig 3 A and B). When used alone, preoperative CK-MB and h-FABP had relatively high AUCs (CK-MB: AUC 0.70, 95% confidence interval [CI] 0.60–0.81;
h-FABP: AUC 0.70, 95% CI 0.60–0.81)
(Table 3). Adding preoperative CK-MB or h-FABP to the clinical model did
not significantly improve the AUC of
FIGURE 1
the clinical model (P= .60 andP= .51, respectively); however, both
biomarkers were associated with significant NRI and IDI values (Table 4). Given that changes in the AUC are insensitive when comparing nested models, it is not surprising that preoperative CK-MB and h-FABP added significant discriminative value to the clinical model when assessed by NRI and IDI, but increased the AUC only slightly in ROC analyses. The optimal cutoff for detecting
postoperative AKI was 2.9µg/L
(sensitivity 64.2%, specificity 64.6%) for preoperative CK-MB and 2.6 pg/mL (sensitivity 68%, specificity 68.8%) for preoperative h-FABP (Table 5).
Secondary analyses showed that higher preoperative hs-cTnT, CK-MB, and h-FABP were associated with longer ICU and hospital stays independent of AKI status (Table 6). Preoperative NT pro-BNP and cTnI were associated with longer ICU but not hospital stays. Of the 5
preoperative biomarkers tested, CK-MB and h-FABP exhibited the strongest association with LOS.
Postoperative Day 1 Cardiac Biomarkers
Biomarker distributions on
postoperative day 1 are presented in Fig 4. Median levels of biomarkers differed significantly across AKI
groups for all biomarkers except
CK-MB (P,.05 for NT pro-BNP, cTnI,
hs-cTnT, and h-FABP). In unadjusted analyses, only postoperative hs-cTnT was borderline associated with the development of AKI, but adjustment for patient and operative
characteristics attenuated this relationships (Table 2). All 5
biomarkers were nonsignificant in
multivariable analyses.
Postoperative hs-cTnT yielded the highest AUC for predicting AKI in both unadjusted and adjusted ROC analyses (Fig 3C). When used alone, postoperative hs-cTnT had a moderately high AUC (0.62, 95% CI 0.51–0.73), but addition of hs-cTnT
FIGURE 2
to the clinical model did not significantly improve the AUC of the clinical model (P= .74) (Table 3). Similarly, neither the NRI nor IDI proved significant for hs-cTnT or any other postoperative
biomarkers (allP..05) (Table 4).
In secondary analyses, higher postoperative levels of NT pro-BNP predicted longer ICU and hospital LOS, which persisted even after adjustment for AKI status (Table 6). Postoperative hs-cTnT and h-FABP
also were associated with increased ICU but not hospital stay.
DISCUSSION
In this multicenter, prospective study of pediatric patients undergoing cardiac surgery for congenital conditions, we found that preoperative CK-MB and h-FABP were strong and independent predictors of postoperative AKI. A 1-unit increase in preoperative log
CK-MB was associated with afivefold
increase in the odds of AKI, and a 1-unit elevation in preoperative log h-FABP increased the odds of developing AKI by 3 times. We found that a single measurement of each of these biomarkers provided good discrimination between patients who developed AKI and those who did not when used alone and in combination with clinical models. Previous studies examining the association of preoperative renal biomarkers with postoperative AKI have yielded AUC values ranging 0.44 to 0.72 for NGAL, cystatin C, and kidney injury molecule-1.33–36 TABLE 2 Logistic Regression Models for Prediction Development of AKI by Using Pre- and
Postoperative Biomarker Values
Unadjusted Adjusteda
OR (95% CI) P OR (95% CI) P
Preoperative biomarkers
NT Pro-BNP,n= 105 1.08 (0.84–1.38) .55 0.94 (0.68–1.30) .71 cTnI,n= 100b 1.01 (0.68–1.49) .97 092 (0.56–1.49) .73 hs-cTnT,n= 106c 1.22 (0.92–1.62) .17 1.09 (0.75–1.57) .65 CK-MB,n= 100 4.19 (1.85–9.48) ,.001 5.09 (1.64–15.82) .005 h-FABP,n= 98 2.72 (1.48–5.01) .001 3.02 (1.35–6.75) .01 First postoperative biomarkers
NT Pro-BNP,n= 103 1.20 (0.94–1.55) .15 1.15 (0.81–1.64) .43
cTnI,n= 101 1.29 (0.95–1.77) .11 1.14 (0.73–1.80) .56
hs-cTnT,n= 106 1.38 (0.99–1.94) .06 1.26 (0.75–2.11) .38 CK-MB,n= 100 1.41 (0.93–2.14) .11 1.04 (0.58–1.88) .90 h-FABP,n= 105 1.29 (0.86–1.92) .21 1.18 (0.69–2.05) .54
OR, odds ratio.
All values of biomarkers have been log transformed.
aPreoperative biomarker models adjusted for age (years), site, preoperative eGFR percentile, and RACHS-1 category$3. Postoperative biomarker models adjusted for age (years), site, CPB time.120 minutes, preoperative eGFR, and RACHS-1 category$3.
bOf the 100 patients with available cTnI values, only 45 had detectable values (.0.01mg/L). Sensitivity analyses using only patients with detectable cTnI values showed similar results to those in the table (unadjusted OR 0.89, 95% CI 0.53–1.50; adjusted OR 0.94, 95% CI 0.49–1.80).
cOf the 106 patients with available hs-cTnT values, only 68 had detectable values (.3 ng/L). Sensitivity analyses using only patients the detectable hs-cTnT values showed similar results to those in the table (unadjusted OR 1.33, 95% CI 0.86–2.04; adjusted OR 1.26, 95% CI 0.76–2.10).
FIGURE 3
Additionally, most clinical risk models for the prediction of postoperative AKI have produced AUCs in the range of 0.65 to 0.83, depending on the
definition of AKI and the number of
clinical parameters included.11,37–40
When placed in the context of
previous studies, ourfindings suggest
that CK-MB and h-FABP may be useful biomarkers for assessing preoperative risk of AKI in children undergoing cardiac surgery. However, larger follow-up studies are needed to verify the results of this study and confirm the utility of these
biomarkers as risk stratification tools for AKI.
This is thefirst prospective study analyzing the use of multiple cardiac
biomarkers to identify patients at high risk of postoperative AKI in children. Although several studies have evaluated the utility of
biomarkers for AKI risk stratification after cardiac surgery in children, most of these studies have focused on renal
biomarkers.41–45To our knowledge,
only 1 study in adults and 2 studies in children have examined cardiac biomarkers for the prediction of AKI after cardiac surgery.12–14However,
in all 3 studies, the authors examined only BNP and observed only modest associations between biomarker levels and postoperative AKI. In the pediatric studies, Cantinotti et al13
found that pre- and postoperative BNP were associated with AKI in
unadjusted analyses but lost their predictive value once urinary NGAL and other conventional risk factors were added to the model. Similarly, Hornik et al14found that
preoperative BNP was not associated with increased risk of postoperative AKI. Our results for NT pro-BNP are consistent with those reported previously.
Our study expands the current literature by examining additional cardiac biomarkers in the prediction of AKI, including markers of cardiac function (NT pro-BNP) and cardiac injury (cTnI, hs-cTnT, CK-MB, and h-FABP). In our study, preoperative CK-MB and h-FABP outperformed NT pro-BNP in predicting postoperative AKI as assessed by larger odds ratios and higher AUCs. We also found that higher preoperative levels of CK-MB and h-FABP were associated with extended ICU and hospital LOS in addition to AKI, which is consistent with previous studies.46–49However,
addition of AKI to these models did not attenuate the risk ratios, suggesting that the relationship between these biomarkers and extended LOS is mediated by factors
other than AKI. Thesefindings
suggest that CK-MB and h-FABP may be superior to BNP in evaluating risk of postoperative AKI in children.
Several mechanisms may explain the relationship between preoperative CK-MB and h-FABP with
postoperative AKI. In children with congenital heart disease, elevations in CK-MB and h-FABP are most likely released from damaged myocardium, suggesting that patients with higher preoperative levels may have more
severe underlying cardiac disease.50
These patients may have reduced cardiac reserve at baseline and may be at higher risk of hemodynamic instability in the perioperative setting, which may place them at greater risk of postsurgical complications, including AKI. Additionally, patients with more severe baseline disease may require TABLE 3 ROC Analysis: AUC for Prediction of AKI
Unadjusted AUC (95% CI) Adjusted AUC (95% CI)
Biomarker Only Biomarker + Clinical Model
Preoperative biomarkers
Clinical modela — 0.75 (0.66–0.85)
NT pro-BNP 0.53 (0.42–0.65) 0.77 (0.67–0.86)
cTnI 0.47 (0.37–0.58) 0.76 (0.66–0.85)
hs-cTnT 0.57 (0.46–0.48) 0.76 (0.67–0.85)
CK-MB 0.70 (0.60–0.81) 0.79 (0.70–0.88)
h-FABP 0.70 (0.60–0.81) 0.80 (0.71–0.89)
Postoperative biomarkers
Clinical modelb — 0.76 (0.67–0.85)
NT-pro BNP 0.57 (0.46–0.68) 0.77 (0.68–0.86)
cTnI 0.61 (0.50–0.72) 0.77 (0.67–0.86)
hs-cTnT 0.62 (0.51–0.73) 0.78 (0.69–0.87)
CK-MB 0.61 (0.50–0.72) 0.74 (0.65–0.84)
h-FABP 0.56 (0.45–0.67) 0.76 (0.67–0.85)
aPreoperative clinical models adjusted for age (years), site, preoperative eGFR percentile, and RACHS-1 category$3. bPostoperative clinical models adjusted for age (years), site, CPB time.120min, preoperative eGFR percentile, and RACHS-1 category$3.
TABLE 4 Comparison of Models With Cardiac Biomarkers to Clinical Model by Using Reclassification Indices
NRI IDI
Estimate (SE) P Estimate (SE) P
Preoperative biomarkers
NT pro-BNP 20.13 (0.20) .53 ,0.01 (0.01) .73
cTnI 0.09 (0.20) .67 ,0.01 (0.01) .98
hs-cTnT 0.11 (0.20) .56 0.01 (0.01) .40
CK-MB 0.46 (0.20) .02 0.05 (0.02) .03
h-FABP 0.40 (0.20) .04 0.08 (0.03) .01
Postoperative biomarkers
NT pro-BNP 20.25 (0.20) .21 0.01 (0.01) .27
cTnI 0.31 (0.20) .13 0.03 (0.02) .08
hs-cTnT 0.37 (0.20) .06 0.03 (0.02) .08
CK-MB 0.30 (0.20) .13 0.01 (0.01) .47
longer cross-clamping and CPB times, which can independently increase the risk of postoperative AKI.16
Although the proposed mechanisms are plausible, our data showed mixed results for several of these
explanations. For example, neither cross-clamping nor CPB duration was associated with postoperative AKI in
our sample. Additionally, it is unclear why only 2 markers of cardiac injury (CK-MB and h-FABP) predicted AKI but not preoperative cardiac troponins. One potential explanation is the large number of children with undetectable levels of preoperative
cTnI (n= 55) and hs-cTnT (n= 38).
Even with low levels of cardiac
damage, the range of cardiac troponin values may not have been wide enough to detect subtle differences between children who developed postoperative AKI and those who did not. In fact, previous studies also have shown that cardiac troponin levels are often undetectable in children with congenital heart abnormalities or other cardiac diseases such as Kawasaki,51–53but CK-MB levels are
frequently elevated and vary by severity and type of cardiac defect.50,54Thus, it is possible that
the kinetics of the cardiac troponins, CK-MB, and h-FABP differ in children.
The high performance of CK-MB and h-FABP in our study may be partly explained by the fact that we limited our sample to only higher-risk patients undergoing complex cardiac surgeries. Larger studies are needed to replicate ourfindings in patients with varying levels of surgical risk and to determine the cost-effectiveness of monitoring CK-MB and h-FABP preoperatively. Recently, the concept of a renal angina index has been proposed to direct
biomarker assessment in noncardiac ICU patients who meet a certain level of risk and demonstrate signs of evolving AKI.55,56This concept has
arisen due to concerns that widespread diagnostic testing may yield high numbers of false-positive results.57In the cardiac surgery
setting, a similar parallel would be clinical risk scores. In TRIBE-AKI, we found that using a simple baseline clinical risk model, including age, gender, RACHS-1 category, preoperative eGFR percentile, CPB time, and study site, predicted postoperative AKI with AUC 0.77 and was improved by adding biomarkers
to this model.36Similar risk models
may help to increase the specificity and positive predictive value of
biomarker screening tests.58
Before the use of these biomarkers can be incorporated into routine clinical practice, however, AKI prevention strategies must be TABLE 5 Biomarker Cutoff Values for Predicting AKI
Biomarker Value Sensitivity Specificity PPV NPV
Preoperative CK-MB (mg/L)
90% Sensitivity 1.6 92.5 27.1 58.3 76.5
Optimal 2.9 64.2 64.6 66.7 62.0
90% Specificity 4.5 30.2 93.8 84.2 54.9
Preoperative FABP (mg/L)
90% Sensitivity 1.3 90.0 29.2 57.0 73.7
Optimal 2.6 68.0 68.8 69.4 67.4
90% Specificity 4.8 26.0 91.7 76.5 54.3
Postoperative hs-cTnT (ng/L)
90% Sensitivity 344 90.9 9.8 52.1 50.0
Optimal 2668 58.2 58.8 60.4 56.6
90% Specificity 7737 18.2 90.2 66.7 50.6
NPV, negative predictive value; PPV, positive predictive value.
TABLE 6 Logistic Regression Models for ICU LOS.3 Days and hospital LOS.7 Days (Median LOS)
Unadjusted Adjusted Adjusted for AKI
OR (95% CI) P OR (95% CI) P OR (95% CI) P
Preoperative valuesa ICU LOS
NT Pro-BNP 1.47 (1.12–1.93) .004 1.46 (1.07–1.98) .02 1.50 (1.09–2.06) .01 cTnI 2.25 (1.28–3.97) .005 2.16 (1.24–3.76) .007 2.24 (1.27–3.95) .005 hs-cTnT 1.70 (1.23–2.34) .001 1.72 (1.17–2.51) .005 1.73 (1.18–2.54) .005 CK-MB 2.60 (1.24–5.42) .01 2.97 (1.12–7.89) .03 2.50 (0.92–6.85) .07 h-FABP 2.76 (1.47–5.15) .002 3.20 (1.52–6.77) .002 2.96 (1.37–6.40) .006 Hospital LOS
NT Pro-BNP 1.28 (0.99–1.66) .06 1.29 (0.94–1.77) .11 1.32 (0.96–1.83) .09 cTnI 1.41 (0.92–2.16) .11 1.45 (0.92–2.29) .11 1.48 (0.93–2.33) .10 hs-cTnT 1.44 (1.07–1.93) .02 1.46 (1.01–2.10) .04 1.45 (1.01–2.10) .05 CK-MB 3.11 (1.45–6.70) .004 3.47 (1.27–9.52) .02 3.27 (1.16–9.28) .03 h-FABP 2.38 (1.32–4.30) .004 2.42 (1.17–5.04) .02 2.26 (1.06–4.82) .03 Postoperative valuesb
ICU LOS
NT Pro-BNP 1.81 (1.33–2.46) ,.001 1.84 (1.26–2.69) .002 1.84 (1.25–2.71) .002 cTnI 1.42 (1.03–1.96) .03 1.33 (0.85–2.07) .21 1.31 (0.84–2.04) .24 hs-cTnT 1.67 (1.17–2.39) .005 1.69 (1.00–2.85) .05 1.64 (0.98–2.76) .06 CK-MB 1.36 (0.89–2.07) .15 1.11 (0.63–1.94) .73 1.11 (0.62–1.96) .73 h-FABP 2.00 (1.28–3.12) .002 2.10 (1.18–3.75) .01 2.11 (1.17–3.82) .01 Hospital LOS
NT Pro-BNP 1.39 (1.07–1.81) .01 1.53 (1.06–2.19) .02 1.52 (1.06–2.18) .02 cTnI 1.13 (0.83–1.54) .43 0.96–0.61–1.51) .87 0.95 (0.60–1.50) .81 hs-cTnT 1.44 (1.07–1.93) .02 1.25 (0.76–2.06) .37 1.23 (0.75–2.03) .41 CK-MB 1.14 (0.75–1.71) .54 0.92 (0.51–1.66) .68 0.92 (0.51–1.67) .79 h-FABP 1.47 (0.98–2.22) .07 1.49 (0.86–2.58) .16 1.47 (0.85–2.55) .17
OR, odds ratio.
All biomarkers have been log transformed.
developed and shown to be efficacious in patients with elevated biomarkers. If replicated, our study has important implications for decisions regarding timing of surgery and for identifying patients at risk for
AKI before surgery that may benefit
from certain interventions. Although there are no established preventive measures or early treatments for AKI in children undergoing cardiac surgery, risk stratification may help to avoid AKI by minimizing CPB time, avoiding nephrotoxic medications, and optimizing hemodynamics
throughfluid management and
inotropic support. In addition, it may be worth considering postponing the
surgery in children at high risk
undergoing elective procedures.2
This study has a few limitations. First, despite being a multicenter
international study, we examined only patients with RACHS-1 category 2 to 4, which may limit the
generalizability of our results. Although our results are compelling, they need to be validated by larger prospective studies that include children with varying levels of surgical risk at baseline. Second, we included only patients for whom both pre- and postoperative samples had been collected. This approach may have been subject to sampling bias if biomarker collection was associated
with disease severity; however, the distribution of RACHS-1 scores in our sample was similar to that in the overall pediatric cohort. Third, there is no true gold standard for AKI.
Therefore, we based the definition of
AKI on elevations in SCr, which may
be a potentiallyflawed outcome
variable for evaluating the
performance of novel biomarkers. It is possible that this study may have yielded different results if a different
definition had been used. Fourth,
TRIBE does not contain information on a few potentially important variables, including preoperative mechanical ventilation, inotropic support, and diuretic use, which may
FIGURE 4
have confounded the relationship between cardiac biomarkers and postoperative AKI. Nevertheless, we adjusted for RACHS-1 category as an overall assessment of preoperative illness severity. Fifth, neonates were excluded from this study because previous studies have shown that using SCr to diagnose AKI in neonates
can be problematic.59Neonates have
higher baseline levels of SCr at birth, which gradually decline during the
first few weeks of life due to ongoing
renal functional development.60,61
These changes, combined with the lower muscle mass in neonates, make it difficult to interpret changes in SCr
with AKI. Thus, ourfindings are not
generalizable to children,1 year of
age, and future multicenter studies should be performed in that age group. Finally, the number of patients experiencing dialysis or death in our study was low, limiting our ability to evaluate the association of
biomarkers with these outcomes.
CONCLUSIONS
In summary, preoperative levels of CK-MB and h-FABP were highly associated with the development of AKI and prolonged LOS and
mechanical ventilation after pediatric cardiac surgery. Both biomarkers improved clinical risk prediction of
postoperative AKI; however, additional studies are needed to confirm thesefindings in patients with varying levels of surgical risk and to evaluate the cost-effectiveness of obtaining these biomarkers for risk-stratification purposes. The ability to predict postoperative sequelae using preoperative clinical data has important implications for identifying patients who may benefit from earlier interventions for AKI. If replicated by future studies, thesefindings suggest that preoperative measurement of CK-MB and h-FABP may be useful for risk stratifying patients before surgery.
www.pediatrics.org/cgi/doi/10.1542/peds.2014-2949
DOI:10.1542/peds.2014-2949 Accepted for publication Jan 7, 2015
Address correspondence to Chirag R. Parikh, MD, PhD, Department of Internal Medicine, Yale University School of Medicine, 60 Temple Street, Suite 6C, New Haven, CT 06510. E-mail: chirag.parikh@yale.edu
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:Drs Devarajan and Devereaux are co-inventors on patents submitted for the use of neutrophil gelatinase-associated lipocalin as a biomarker of kidney disease. Dr Devereaux has received research grants from Abbott Diagnostics and Roche Diagnostics. Dr Kavsak has received honorarium and research grants from Abbott Laboratories, Beckman Coulter, Ortho Clinical Diagnostics, Randox Laboratories, and Roche Diagnostics; he has consulted for Abbott
Laboratories and Roche Diagnostics. The other authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:Supported by the National Institutes of Health (NIH) (R01HL085757 to Dr Parikh) to fund the TRIBE-AKI Consortium to study novel biomarkers of acute kidney injury in cardiac surgery and the O’Brien Center Award from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK079310). Dr Parikh is also supported by NIH (K24DK090203). Drs Garg and Parikh are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute
Kidney Injury Consortium (U01DK082185). Biomarker measurements were supported by Roche Diagnostics, Beckman Coulter, and Randox Laboratories. The granting agencies and Roche Diagnostics, Beckman Coulter, and Randox Laboratories did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST:Drs Devarajan is a co-inventor on patents submitted for the use of neutrophil gelatinase-associated lipocalin as a biomarker of kidney disease. Dr Devereaux has received research grants from Abbott Diagnostics and Roche Diagnostics. Dr Kavsak has received honorarium and research
grants from Abbott Laboratories, Beckman Coulter, Ortho Clinical Diagnostics, Randox Laboratories, and Roche Diagnostics; he has consulted for Abbott Laboratories and Roche Diagnostics. The other authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
1. Aydin SI, Seiden HS, Blaufox AD, et al. Acute kidney injury after surgery for congenital heart disease.Ann Thorac Surg. 2012;94(5):1589–1595
2. Pedersen K. Acute kidney injury in children undergoing surgery for congenital heart disease.Eur J Pediatr Surg. 2012;22(6):426–433
3. Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects
of acute kidney injury on outcomes.J Thorac Cardiovasc Surg. 2012;143(2):368–374
4. Pedersen KR, Povlsen JV, Christensen S, et al. Risk factors for acute renal failure requiring dialysis after surgery for congenital heart disease in children.Acta Anaesthesiol Scand. 2007;51(10):1344–1349
5. Molitoris BA. Transitioning to therapy in ischemic acute renal failure.J Am Soc Nephrol. 2003;14(1):265–267
6. Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy.
J Clin Invest. 2004;114(1):5–14
7. Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification.Circulation. 1997;95(4): 878–884
treatment of acute kidney injury.Int Anesthesiol Clin. 2009;47(4):89–105
9. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery.
Lancet. 2005;365(9466):1231–1238
10. Parolari A, Pesce LL, Pacini D, et al; Monzino Research Group on Cardiac Surgery Outcomes. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of
perioperative management.Ann Thorac Surg. 2012;93(2):584–591
11. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery.
J Am Soc Nephrol. 2005;16(1):162–168
12. Patel UD, Garg AX, Krumholz HM, et al; Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium. Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery.Circulation. 2012; 125(11):1347–1355
13. Cantinotti M, Storti S, Lorenzoni V, et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery.Clin Chem Lab Med. 2012;50(11): 2009–2017
14. Hornik CP, Krawczeski CD, Zappitelli M, et al; TRIBE-AKI Consortium. Serum brain natriuretic peptide and risk of acute kidney injury after cardiac operations in children.Ann Thorac Surg. 2014;97(6): 2142–2147
15. Hernández-Leiva E, Dennis R, Isaza D, Umaña JP. Hemoglobin and B-type natriuretic peptide preoperative values but not inflammatory markers, are associated with postoperative morbidity in cardiac surgery: a prospective cohort analytic study.J Cardiothorac Surg. 2013;8:170
16. Li S, Krawczeski CD, Zappitelli M, et al; TRIBE-AKI Consortium. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study.Crit Care Med. 2011;39(6):1493–1499
17. Parikh CR, Devarajan P, Zappitelli M, et al; TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric
cardiac surgery.J Am Soc Nephrol. 2011; 22(9):1737–1747
18. Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method.Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–184
19. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease.J Thorac Cardiovasc Surg. 2002;123(1):110–118
20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure —definition, outcome measures, animal models,fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group.Crit Care. 2004;8(4):R204–R212
21. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury.
Crit Care. 2007;11(2):R31
22. Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics.Pediatr Crit Care Med. 2011;12(3):339–347
23. Zappitelli M. Preoperative prediction of acute kidney injury—from clinical scores to biomarkers.Pediatr Nephrol. 2013;28(8):1173–1182
24. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction.Circulation. 2007;115(7): 928–935
25. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to
reclassification and beyond.Stat Med. 2008;27(2):157–172, discussion 207–212
26. Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers.Clin J Am Soc Nephrol. 2012;7(8):1355–1364
27. Fox CS, Gona P, Larson MG, et al. A multi-marker approach to predict incident CKD and microalbuminuria.J Am Soc Nephrol. 2010;21(12):2143–2149
28. Haase-Fielitz A, Mertens PR, Plass M, et al. Urine hepcidin has additive value in ruling out cardiopulmonary bypass-associated acute kidney injury: an
observational cohort study.Crit Care. 2011;15(4):R186
29. Hall IE, Coca SG, Perazella MA, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis.Clin J Am Soc Nephrol. 2011; 6(12):2740–2749
30. Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study.J Am Coll Cardiol. 2012;59(3):246–255
31. Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults.J Am Soc Nephrol. 2009;20(8):1823–1832
32. Tzoulaki I, Liberopoulos G, Ioannidis JP. Use of reclassification for assessment of improved prediction: an empirical evaluation.Int J Epidemiol. 2011;40(4): 1094–1105
33. Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery.Clin J Am Soc Nephrol. 2009;4(5):873–882
34. Hassinger AB, Backer CL, Lane JC, Haymond S, Wang D, Wald EL. Predictive power of serum cystatin C to detect acute kidney injury and pediatric-modified RIFLE class in children undergoing cardiac surgery.Pediatr Crit Care Med. 2012;13(4):435–440
35. Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury.Clin J Am Soc Nephrol. 2010;5(12):2154–2165
36. Zappitelli M, Krawczeski CD, Devarajan P, et al; TRIBE-AKI consortium. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery.Kidney Int. 2011;80(6):655–662
37. Kiers HD, van den Boogaard M, Schoenmakers MC, et al. Comparison and clinical suitability of eight prediction models for cardiac surgery-related acute kidney injury.Nephrol Dial Transplant. 2013;28(2):345–351
Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery.Circulation. 2006;114(21):2208–2216, quiz 2208
39. Rahmanian PB, Kwiecien G, Langebartels G, Madershahian N, Wittwer T, Wahlers T. Logistic risk model predicting
postoperative renal failure requiring dialysis in cardiac surgery patients.Eur J Cardiothorac Surg. 2011;40(3):701–707
40. Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery.JAMA. 2007;297(16):1801–1809
41. Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study.Crit Care. 2007;11(6):R127
42. Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children.Am J Kidney Dis. 2010;56(4): 632–642
43. Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass.J Am Coll Cardiol. 2011;58(22):2301–2309
44. Krawczeski CD, Vandevoorde RG, Kathman T, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric
cardiopulmonary bypass.Clin J Am Soc Nephrol. 2010;5(9):1552–1557
45. Peco-Antic A, Ivanisevic I, Vulicevic I, et al. Biomarkers of acute kidney injury in
pediatric cardiac surgery.Clin Biochem. 2013;46(13–14):1244–1251
46. Bresolin N, Bianchini AP, Haas CA. Pediatric acute kidney injury assessed by pRIFLE as a prognostic factor in the intensive care unit.Pediatr Nephrol. 2013;28(3):485–492
47. dos Santos El Halal MG, Carvalho PR. Acute kidney injury according to pediatric RIFLE criteria is associated with negative outcomes after heart surgery in children.Pediatr Nephrol. 2013;28(8):1307–1314
48. Gil-Ruiz Gil-Esparza MA, Alcaraz Romero AJ, Romero Otero A, et al. Prognostic relevance of early AKI according to pRIFLE criteria in children undergoing cardiac surgery.Pediatr Nephrol. 2014; 29(7):1265–1272
49. Soler YA, Nieves-Plaza M, Prieto M, García-De Jesús R, Suárez-Rivera M. Pediatric Risk, Injury, Failure, Loss, End-Stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study.Pediatr Crit Care Med. 2013;14(4):e189–e195
50. Boucek RJ Jr, Kasselberg AG, Boerth RC, Parrish MD, Graham TP Jr. Myocardial injury in infants with congenital heart disease: evaluation by creatine kinase MB isoenzyme analysis.Am J Cardiol. 1982;50(1):129–135
51. Checchia PA, Borensztajn J, Shulman ST. Circulating cardiac troponin I levels in Kawasaki disease.Pediatr Cardiol. 2001; 22(2):102–106
52. Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury.Circulation. 1997;96(8):
2641–2648
53. Sato YZ, Molkara DP, Daniels LB, et al. Cardiovascular biomarkers in acute Kawasaki disease.Int J Cardiol. 2013; 164(1):58–63
54. Britton CV, Hernandez A, Roberts R. Plasma creatine kinase isoenzyme determinations in infants and children. Characterization in normal patients and after cardiac catheterization and surgery.Chest. 1980;77(6):758–760
55. Basu RK, Chawla LS, Wheeler DS, Goldstein SL. Renal angina: an emerging paradigm to identify children at risk for acute kidney injury.Pediatr Nephrol. 2012;27(7):1067–1078
56. Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children.Kidney Int. 2014;85(3):659–667
57. Johnson XD, Liu KD. Acute renal syndrome/renal angina: a new paradigm for studies of acute kidney injury?Clin J Am Soc Nephrol. 2010;5(5):753–755
58. Goldstein SL. Acute kidney injury biomarkers: renal angina and the need for a renal troponin I.BMC Med. 2011;9: 135
59. Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn?Pediatr Nephrol. 2009; 24(2):265–274
60. Abrahamson DR. Glomerulogenesis in the developing kidney.Semin Nephrol. 1991;11(4):375–389
61. Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm neonates with gestational age,or = 32 weeks.
DOI: 10.1542/peds.2014-2949 originally published online March 9, 2015;
2015;135;e945
Pediatrics
TRIBE-AKI Consortium
Catherine D. Krawczeski, Peter Kavsak, Colleen Shortt, Chirag R. Parikh and for the
Eikelboom, Amit X. Garg, Heather Thiessen Philbrook, Philip J. Devereaux,
Emily M. Bucholz, Richard P. Whitlock, Michael Zappitelli, Prasad Devarajan, John
Cardiac Biomarkers and Acute Kidney Injury After Cardiac Surgery
Services
Updated Information &
http://pediatrics.aappublications.org/content/135/4/e945 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/135/4/e945#BIBL This article cites 61 articles, 16 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/cardiac_surgery_sub Cardiac Surgery
http://www.aappublications.org/cgi/collection/cardiology_sub Cardiology
http://www.aappublications.org/cgi/collection/nephrology_sub Nephrology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2014-2949 originally published online March 9, 2015;
2015;135;e945
Pediatrics
TRIBE-AKI Consortium
Catherine D. Krawczeski, Peter Kavsak, Colleen Shortt, Chirag R. Parikh and for the
Eikelboom, Amit X. Garg, Heather Thiessen Philbrook, Philip J. Devereaux,
Emily M. Bucholz, Richard P. Whitlock, Michael Zappitelli, Prasad Devarajan, John
Cardiac Biomarkers and Acute Kidney Injury After Cardiac Surgery
http://pediatrics.aappublications.org/content/135/4/e945
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.