1.1
Full text
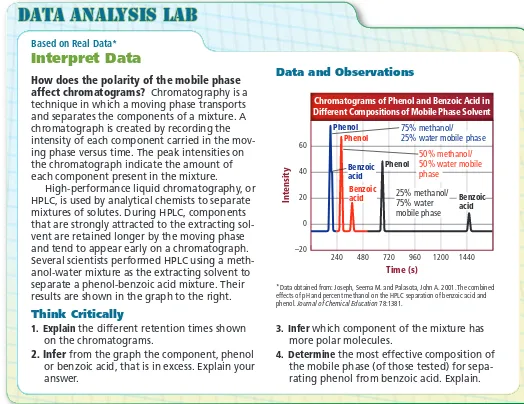
Figure



Related documents
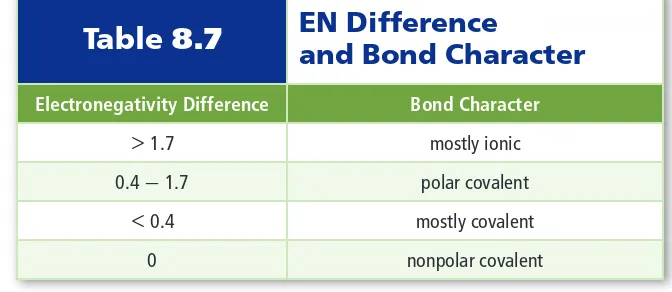
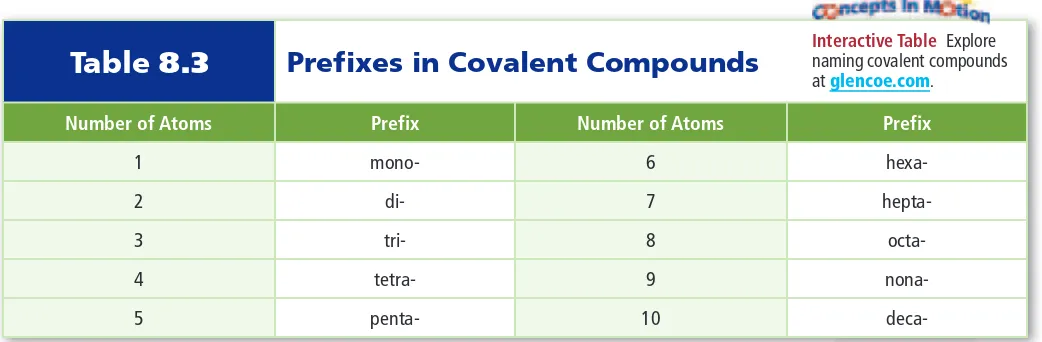
Electrons are shared in covalent bonds. By sharing electrons, each atom can have a filled outer shell to satisfy the octet rule. However, two electrons may not be shared equally in
Yes, the ban on the import of soil and growing media and host plants (including tubers but excluding seeds) from areas where the nematode is present would prevent entry into
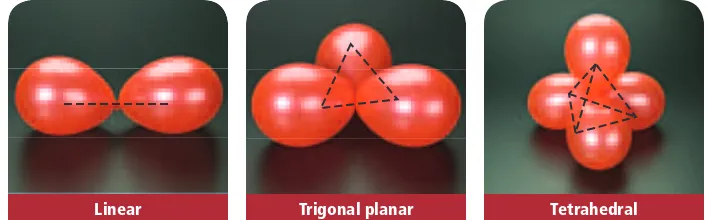
• A single covalent bond is a covalent bond in which two atoms share one pair of electrons • A double covalent bond is a covalent bond in which two atoms share two pairs of electrons
6E-8 SCHEMATIC AND ROUTING
Cash Awards will be given to the Principal and Teacher in-charge of the school depending on the total number of students registering for the Examination.. All students who register
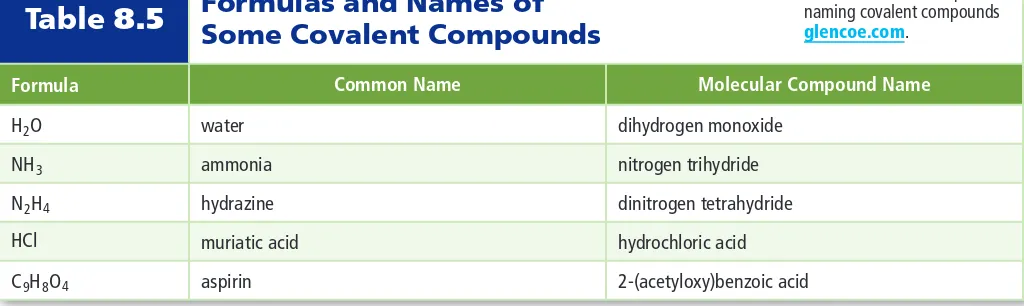
When two atoms combine by sharing their one or more valence electrons, a covalent bond is formed between them. The shared pairs of electrons present between the bonded atoms are
When oxygen and hydrogen form a covalent bond, which atom attracts the shared electrons most stronglya. neither; they share
• When the atoms are alike, as in the H-H bond of H 2 , the bonding electrons are shared equally (a nonpolar covalent bond).. • When the two atoms are of different elements,