Preterm Infants: Bone Health at 15-Year Follow-up
WHAT’S KNOWN ON THIS SUBJECT: Aluminum has neurotoxicity and may impair short-term bone health. We showed reduced neurodevelopmental scores in preterm infants who were previously exposed to aluminum from parenteral nutrition solutions. It is not known whether aluminum exposure has long-term health consequences.
WHAT THIS STUDY ADDS: Neonates who are exposed to parenteral aluminum may have reduced lumbar spine and hip bone mass during adolescence, potential risk factors for later osteoporosis and hip fracture. The potential adverse long-term consequences of early aluminum exposure deserve renewed attention.
abstract
OBJECTIVE:Aluminum has known neurotoxicity and may impair short-term bone health. In a randomized trial, we showed reduced neurode-velopmental scores in preterm infants who were previously exposed to aluminum from parenteral nutrition solutions. Here, in the same cohort, we test the hypothesis that neonatal aluminum exposure also adversely affects long-term bone health, as indicated by reduced bone mass.
METHODS:Bone area (BA) and bone mineral content (BMC) of lumbar spine, hip, and whole body were measured with dual radiograph ab-sorptiometry in 13- to 15-year-olds who were born preterm and ran-domly assigned standard or aluminum-depleted parenteral nutrition solutions during the neonatal period.
RESULTS:Fifty-nine children (32% of survivors) were followed. Those who were randomly assigned to standard parenteral nutrition solution had lower lumbar spine BMC, apparently explained by a concomitant decrease in bone size. In nonrandomized analyses, children who were exposed to neonatal aluminum intakes above the median (55g/kg) had lower hip BMC (by 7.6% [95% confidence interval 0.21–13.8];P⫽
0.02), independent of bone (or body) size.
CONCLUSIONS:Neonates who are exposed to parenteral aluminum may have reduced lumbar spine and hip bone mass during adoles-cence, potential risk factors for later osteoporosis and hip fracture. These findings need confirmation in larger, more detailed studies. Nev-ertheless, given our previous finding of adverse developmental out-come in these individuals and the sizeable number of contemporary infants who undergo intensive neonatal care and are still exposed to aluminum via parenteral feeding solutions, the potential adverse long-term consequences of early aluminum exposure now deserve renewed attention.Pediatrics2009;124:1372–1379
AUTHORS:Mary S. Fewtrell, MD,aNick J. Bishop, MD,b
Caroline J. Edmonds, PhD,aElizabeth B. Isaacs, PhD,aand
Alan Lucas, MDa
aMedical Research Council Childhood Nutrition Research Centre,
University College London Institute of Child Health, London, United Kingdom; andbAcademic Unit of Child Health, Sheffield
Children’s NHS Foundation Trust, Sheffield, United Kingdom
KEY WORDS
preterm infant, bone health, parenteral nutrition, aluminum
ABBREVIATIONS
PN—parenteral nutrition
DXA— dual-energy x-ray absorptiometry BMC— bone mineral content
BA— bone area
BMD— bone mineral density LS—lumbar spine WB—whole body
BMAD— bone mineral apparent density SDS—SD score
www.pediatrics.org/cgi/doi/10.1542/peds.2009-0783 doi:10.1542/peds.2009-0783
Accepted for publication Jun 9, 2009
Address correspondence to Mary S. Fewtrell, MD, MRC Childhood Nutrition Research Centre, UCL Institute of Child Health, 30 Guilford St, London WC1N 1EH, UK. E-mail: m.fewtrell@ich.ucl.ac. uk
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). Copyright © 2009 by the American Academy of Pediatrics
Aluminum is the most common metal-lic element in the earth’s crust but has no known biological role. It accumu-lates in the body when protective gastrointestinal mechanisms are by-passed, renal function is impaired, or exposure is high, all of which apply fre-quently to sick or preterm infants. Rec-ognized clinical manifestations of alu-minum toxicity, for instance from older renal dialysis solutions, included pro-gressive dementia, anemia, and bone disease.
Parenteral feeding solutions that are used for infants are contaminated with aluminum,1,2mostly from calcium
glu-conate solutions stored in glass vials, where complex-forming anions dis-solve aluminum from the glass during autoclaving. When fed parenterally, in-fants retain up to 78% of the alumi-num,3 with high serum, urine, and
tissue levels.4 Increased aluminum
concentrations have been observed postmortem in the brain of a paren-tally fed preterm infant.5
Given the known toxicity of aluminum and the increasing survival of high-risk neonates who require parenteral nu-trition (PN), we explored whether early exposure to intravenous aluminum has adverse long-term effects on health. Assigning infants to high levels of aluminum exposure would have been unethical; however, because standard PN solutions contain signifi-cant aluminum, it was ethical for us to conduct a randomized trial to compare these with corresponding solutions specially sourced for low aluminum content. Our trial, conducted in pre-term infants, showed that those who were exposed for ⬎10 days to stan-dard solutions had impaired neuro-logic development at 18 months post-term.6Bone health was not assessed
at that stage; however, in rats, pigs, dogs, and adult humans, excess alumi-num accumulates at the mineraliza-tion front and is associated with
re-duced bone formation.7 Adults with
uremia and those who are on total par-enteral nutrition have low bone forma-tion, with patchy osteomalacia.7
Sed-man et al8found that bone aluminum
concentrations were 10 times higher in preterm infants who were fed par-enterally for⬎3 weeks than in control subjects. None of these studies tested whether early aluminum exposure might influence long-term bone health and, notably, result in reduced bone mass, believed to be a key predictor of osteoporosis and fracture risk. In this study, therefore, we used our trial to test experimentally the hypothesis that neonatal exposure to aluminum in standard PN solutions results in re-duced bone mass during adolescence.
METHODS
Study subjects were adolescents who were previously randomly assigned to aluminum-depleted versus standard PN solutions during the neonatal pe-riod. Details are given elsewhere6but
summarized here.
Randomized Trial
A total of 227 preterm infants (gesta-tion⬍34 weeks, birth weight⬍1850 g)
were recruited from NICUs in Cam-bridge and Norwich, United Kingdom, between May 1988 and January 1991. Infants were eligible for the study when there was a clinical decision to initiate intravenous feeding. Infants were randomly assigned according to a multiple random permuted-block method to receive either standard (S) or aluminum-depleted (AD) PN solu-tion. Investigators and staff were blind to the assignments. The study was ap-proved by the research ethics commit-tee, and parental consent was ob-tained. PN was introduced (typically on postnatal day 2 or 3) and stopped at the discretion of NICU medical staff. The composition of the 2 solutions (Table 1) was identical except that the AD solution contained less alumi-num and more chloride, reflecting use of calcium chloride rather than calcium gluconate. Using a mixed sodium-potassium phosphate solution instead of potassium acid phosphate further reduced aluminum and mini-mized the increase in chloride.
Data were collected on the neonatal course of each infant, including de-tailed records of intravenous fluids
TABLE 1 Composition and Aluminum Content of the Standard and Aluminum-Depleted Intravenous Feeding Solutions
Component Solution
Standard Aluminum Low Aluminum Volume
(mL)
Aluminum Content ()
Volume (mL)
Aluminum Content () Vamin infanta 50.0 1.5 50.0 1.5
Intralipid 20% 15.0 0.1 15.0 0.1 Vitalipida 1.0 0.3 1.0 0.3
Solivitoa 1.0 ⬍0.1 1.0 ⬍0.1
Neotrace 1.6 1.2 1.6 1.2 Potassium acid phosphate 1.3 2.8 — — Polyfusor phosphatea — — 14.4 0.3
Calcium gluconate 8.0 38.8 — — Calcium chloride — — 2.1 0.5 Dextrose, sodium, potassium 102 ⬍0.1 102 ⬍0.1 Total aluminum intake at 180
mL/kg per day
45g/kg per d 4.0 to 4.5g/kg per d
Vamin infant contained essential amino acids without added electrolytes; 6.5 g protein/100 mL. Intralipid 20% was a fat emulsion that contained 20 g/dL fatty acids. Vitalipid contained fat-soluble vitamins, and Solivito contained water-soluble vitamins. Neotrace was an in-house preparation that contained copper and zinc only. Vamin infant, Intralipid 20%, Vitalipid, and Solivito were manufactured by Kabi Vitrum.
aNot available in the United States.
the PN solutions was measured by graphite-furnace atomic-absorption spectrometry (see Bishop et al6for
de-tails). Total aluminum exposure from PN, expressed as g/kg, was calcu-lated for each infant from the daily vol-ume of PN solution.
Follow-up Study
Subjects were invited for follow-up at ages 13 to 15 years to examine long-term effects of the intervention on (1) bone health and (2) cognitive and neu-rologic outcomes (to be reported sep-arately). Children with neurologic im-pairment or a previous Bailey score of
⬍85 were excluded. The study was ap-proved by the Great Ormond Street Hospital Research Ethics Committee. Written informed consent was ob-tained from a parent and written as-sent from the child. Weight was mea-sured by using digital scales and height by using a portable stadiom-eter. A food frequency questionnaire quantified current calcium intake (Calquest9); a simple questionnaire
de-termined hours of weight-bearing ac-tivity per week, and parents rated the child’s activity level compared with his or her peers (rated 1–5: 1⫽much less active; 5⫽much more active). A gen-eral medical and fracture history was taken, including previous and current medications.
Bone Densitometry
Dual-energy radiograph absorptiom-etry (DXA; Lunar Prodigy, GE, Wau-kesha, WI) was used to measure bone mineral content (BMC), bone area (BA) and bone mineral density (BMD) at the lumbar spine (LS; L2–L4), hips, and whole body (WB). Children wore light indoor clothing after removing metal objects. Total radiation expo-sure was below daily background lev-els (⬃7Sv/d in the United Kingdom). As recommended by the International
Society for Clinical Densitometry,10we
used “total body less head” values for WB scans.
Statistics
Groups were compared usingttest or
2 test. Some variables were
trans-formed to ensure normal distribution. The target sample size of 64 per group at follow-up would allow a difference of 0.5 SD to be detected at 80% power and 5% significance.
Bone mass was adjusted for size in 3 ways: (1) bone mineral apparent den-sity (BMAD) of the LS, calculated as BMC/BA1.5, BMADzscores, were
calcu-lated for age, gender, and ethnic group using United Kingdom machine-specific reference data11; (2) for WB
bone mass, a 2-stage procedure was used; the indices lean/height3 and
BMC/lean0.7were calculated by using
the power relationships required to remove any residual association with height determined using log-log re-gression; and (3) multiple regression was used first to examine the effect of PN solution assignment on later bone mass at skeletal sites after adjusting for age, gender, pubertal stage, and body size (weight and height) and sec-ond to adjust for potential confounding factors, including current physical ac-tivity and calcium intake. Continuous
variables were transformed to natural logarithms for regression analyses, al-lowing coefficients to be expressed as percentages (sympercents12).
Relationships between neonatal alumi-num exposure and later bone mass were also examined in a nonrandom-ized manner, by using total neonatal aluminum exposure from PN as both a continuous and a dichotomous vari-able. Multiple regression was used with backward elimination of nonsig-nificant variables (P⬎.05), adjusting for potential confounders including PN duration and factors related to neona-tal illness severity.
RESULTS
Comparison of Randomly Assigned Groups
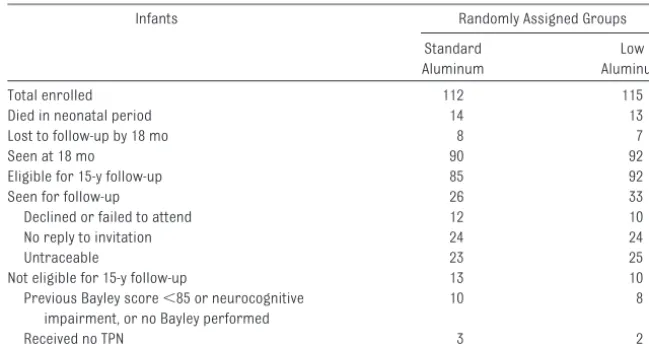
Fifty-nine children from the original co-hort (26% of those randomly assigned; 32% of survivors; 33% of those eligible for follow-up) completed the bone health protocol (Table 2). Children who were followed had significantly higher birth weight SD score (SDS) than those who were not seen, but there were no other baseline differences (Table 3).
Neonatal data for those who were fol-lowed up (Table 4) showed that the randomly assigned groups were well matched for birth weight, gestation,
Standard Aluminum
Low Aluminum Total enrolled 112 115 Died in neonatal period 14 13 Lost to follow-up by 18 mo 8 7
Seen at 18 mo 90 92
Eligible for 15-y follow-up 85 92 Seen for follow-up 26 33 Declined or failed to attend 12 10 No reply to invitation 24 24
Untraceable 23 25
Not eligible for 15-y follow-up 13 10 Previous Bayley score⬍85 or neurocognitive
impairment, or no Bayley performed
10 8
Received no TPN 3 2
days in the trial, and days of intrave-nous feeding. There were no differ-ences in neonatal peak plasma cal-cium, minimum phosphate, or maximum alkaline phosphatase (data not shown). Median (25th, 75th centile) peak alkaline phosphatase concentra-tions were 609 (502, 751) and 606 (438, 705) IU/L in groups AD and S, respec-tively, with maximum values of 982 and 1087 IU/L. Total neonatal aluminum ex-posure from PN expressed in g/kg was, by design, significantly higher in children who received standard feed-ing solutions. The proportion of breast milk in the diet did not differ between groups. All infants required ventilatory support, with no group differences in duration or time spent in⬎30% oxy-gen. Three infants (2 AD and 1 S)
devel-oped suspected necrotizing enteroco-litis; of these cases, 2 were considered equivocal and 1 (from group AD) was confirmed at surgery. Socioeconomic and educational indices did not differ between groups.
At follow-up, there were no group dif-ferences in gender distribution, puber-tal stages, age, or anthropometric variables, although there was a trend toward greater weight, weight SDS, and BMI in AD children (Table 5). Five group S children and 1 group AD child were using bronchodilators for asthma, and 1 group S child was also receiving inhaled corticosteroids. No other significant medical conditions were reported in either group, and no children were taking oral or
paren-teral steroids or any other regular medications. AD children had signifi-cantly higher LS BMC and LS BA, with a similar although nonsignificant trend in WB BMC, WB BA, WB BMD, WB BMDz
score, LS BMDzscore, hip BMC, and hip BA (Table 5).
Size-Adjusted Bone Mass
We explored whether the increase in LS BMC was attributable to a concom-itant increase in bone size in the AD group. Supporting this, we found no difference between groups in (1) LS BMC, after adjusting for height, weight, and LS BA (LS BMC 2.7% lower in group S [95% confidence interval (CI): ⫺8.9 to 3.6]), and (2) LS BMAD z scores. There were no group differences in WB BMC and hip BMC adjusted for height, weight, and BA (WB BMC 1.6% lower [95% CI:⫺4.5 to 1.4]) and hip BMC 2.5% lower [95% CI:⫺8.5 to 3.5]) in group S or in lean/height and WB BMC/lean ratios.
Neonatal Aluminum Exposure and Bone Mass: Nonrandomized Analyses
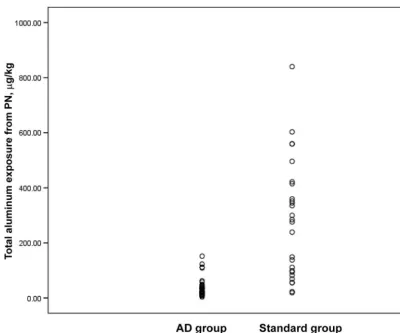
Calculated neonatal aluminum expo-sure from PN varied with both the type of solution and duration of parenteral feeding. Values for the exposure of in-fants (g/kg) by randomized group (Fig 1) showed overlap, with values for 24 infants falling into a common range. Mean (SD), median (25th, 75th cen-tiles), minimum and maximum concen-trations in the 2 groups were 3.0 (0.8), 28 (17, 46) and 4 and 152g/kg for the AD group and 21.3 (7.2), 280 (91, 417), and 19 and 840g/kg for group S (P⬍
.001 for all).
The total aluminum exposure from PN as a continuous variable was not a sig-nificant predictor of adjusted BMC at any site, after adjusting for relevant neonatal variables (birth weight, ges-tation, days of ventilation, and days of intravenous feeding) and follow-up
TABLE 3 Neonatal Data for Those Seen or not Seen at the Current Follow-up Parameter Seen at 15 y
(n⫽59)
Not Seen at 15 y (n⫽168)
P
Birth weight, mean (SD), g 1270 (295) 1204 (311) .163 Birth weight SDS, mean (SD), g ⫺0.10 (0.99) ⫺0.51 (1.20) .013a
Gestation, mean (SD), wk 28.9 (2.0) 29.0 (2.4) .758 Male,n(%) 27 (46) 91 (54) .291 Singleton,n(%) 42 (71) 125 (74) .883 Days in study, mean (SD) 41 (18) 38 (23) .355 Days of intravenous feeding, mean (SD) 15 (9) 14 (11) .552 Days to reach full enteral feeds, mean (SD) 15 (9) 14 (6) .273 Days of ventilation, median (25th, 75th centiles) 5 (3, 8) 4 (2, 9) .192
aP⬍.05.
TABLE 4 Neonatal Data for Children Seen at Follow-up According to Original Randomized Group Parameter Low Aluminum
(n⫽33)
Standard Aluminum (n⫽26)
P
Birth weight, mean (SD), g 1290 (281) 1244 (316) .556 Gestation, mean (SD), wk 29.0 (1.9) 28.8 (2.1) .619 Boys,n(%) 17 (52) 11 (42) .680 Singleton,n(%) 21 (64) 21 (81) .247 Days in trial, mean (SD) 42 (16) 41 (20) .712 Days of intravenous feeding, mean (SD) 12.5 (8.8) 13.2 (9.2) .774 Days to reach enteral full feeds, mean (SD) 14 (6) 15 (6) .572 Total aluminum exposure from PN, mean (SD),
g/kg
39.1 (35.6) 280.0 (212.8) ⬍.001 Mean aluminum exposure from PN, mean
(SD),g/kg per d
3.00 (0.83) 21.30 (7.20) ⬍.001 Received breast milk,n(%) 27 (82) 16 (62) .139 % of enteral intake as breast milk, median
(25th, 75th centile)
58 (24, 99) 55 (23, 99) .782 Days of ventilation, median (25th, 75th centile) 5 (3, 8) 5 (3, 7) .969 Days in⬎30% O2, median (25th, 75th centile) 6 (5, 27) 8 (4, 41) .496
Suspected NEC,n(%) 2 (6) 1 (4) ⬎.999
NEC indicates necrotizing enterocolitis.
variables (age, gender, weight, height, and BA); however, to look for a “thresh-old” effect, aluminum exposure was categorized as “low” and “high” by using the median exposure (55g/kg) as a cutoff. Children with high exposure had significantly lower hip BMC (by 7.6% [95% CI: 0.21–13.8];P⫽0.02). The me-dian was chosen as the cutoff to ensure equal numbers in the 2 groups, consid-ering the relatively small sample size; however, exploratory analyses by using other cutoffs (data not shown) sug-gested that there was a significant re-lationship between aluminum intake and hip BMC only once intake exceeded 45 g/kg. The largest effect size was seen by using a cutoff of 65 g/kg
(adjusted hip BMC: ⫺9.6% [95% CI:
⫺15.8 to⫺3.3] lower in group S). Above this level, the effect plateaued. This asso-ciation was not present for any other skeletal site. For example, by using the median exposure (55g/kg) as a cutoff, adjusted WB BMC was 2.7% (95% CI:⫺6.1 to 0.7) and LS BMC was 3.0% (95% CI:
⫺9.8 to 3.9) lower in group S.
Current calcium intake and physical activity did not predict size-adjusted bone mass (data not shown). Fracture rates were not influenced by (1) ran-domly assigned group or (2) whether aluminum exposure was below or above the median (24% and 23% for lower versus higher aluminum
expo-unusual fragility fractures suggestive of poor bone health.
DISCUSSION
Our study produced 2 principle find-ings suggesting that exposure to alu-minum from standard PN solutions that are used in the neonatal period may impair long-term bone mineral-ization. First, children who were born preterm and randomly assigned to an aluminum-depleted PN solution had significantly higher LS BMC and BA and higher LS BMD, WB BMC, BA, and BMD during adolescence. After adjustment for current body and bone size, these differences between groups were no longer significant, suggesting that the higher bone mass reflects greater skeletal size in the AD group. Second, in nonrandomized analyses relating neonatal aluminum exposure to later bone outcomes, we found that hip BMC was reduced in children with alu-minum exposure above the median (⬎55g/kg) than in those with lower exposure. In contrast to the effect on WB and LS bone mass seen in the ran-domized comparison, the higher hip BMC associated with lower aluminum exposure did not seem to be related to greater bone size. These findings have potential relevance for later osteopo-rosis and fracture risk.
Short-term adverse effects of alumi-num on bone health have been shown in animals and adult humans,7but no
study previously investigated whether such effects persist beyond the period of exposure; however, our work in other areas shows that neonatal influ-ences may have lasting effects on bone health indices, adding plausibility to our findings here. For example, we showed that so-called “metabolic bone disease of prematurity,” as a result of early calcium and phosphorus insuffi-ciency, is linked to stunting of linear
Parameter Low Aluminum Standard Aluminum P
Age at follow-up, y 15.29 (0.76) 15.15 (0.76) .482 Pubertal stage (breast/genital
development),n(%)
3 10 (33) 3 (12) .155
4 9 (30) 10 (39)
5 10 (33) 12 (46) Missing 1 (3) 1 (4) Reached menarche,n(%) 16 (100) 14 (93)
Weight, mean (SD), kg 63.18 (15.84) 57.38 (14.02) .147 Weight SDS 0.57 (1.29) 0.15 (1.14) .201 Height, mean (SD), cm 163.6 (8.3) 162.2 (7.4) .488 Height SDS ⫺0.40 (1.03) ⫺0.42 (0.70) .929 HC, mean (SD), cm 55.2 (5.2) 55.3 (1.8) .881 HC SDS ⫺0.48 (3.7) ⫺0.22 (1.03) .724 BMI, mean (SD), kg/m2 25.6 (13.2) 21.8 (4.2) .161
BMI SDS 1.07 (1.50) 0.50 (1.17) .114 MUAC, mean (SD), cm 26.9 (6.0) 26.6 (4.3) .837 Waist circumference, mean (SD), cm 75.0 (12.9) 73.9 (11.1) .722 Bone densitometry data, mean (SD)
WB BMC less head, g 1909 (355) 1739 (339) .068 WB BA less head, cm2 1870 (225) 1769 (215) .086
WB BMD less head, g/cm2 1.014 (0.079) 0.976 (0.083) .080
WB BMDzscore 0.26 (0.84) ⫺0.19 (0.94) .054 Hip BMC, g 32.4 (5.8) 29.7 (5.7) .080 Hip BA, cm2 31.1 (3.3) 29.8 (3.2) .144
Hip BMD, g/cm2 1.040 (0.094) 0.992 (0.130) .148
LS BMC, g 44.9 (8.8) 39.8 (6.5) .017 LS BA, cm2 40.5 (5.4) 37.8 (3.7) .031
LS BMD, g/cm2 1.102 (0.119) 1.053 (0.149) .170
LS BMDzscore ⫺0.23 (1.20) ⫺0.63 (1.28) .234 LS BMADzscore 0.046 (1.000) ⫺0.081 (1.220) .665 Fat mass, kg 18.5 (10.5) 15.5 (10.1) .286 Lean mass, kg 41.8 (9.2) 39.2 (6.9) .242 Lean/height3 9.45 (1.27) 9.14 (0.98) .245
BMC-head/lean0.7 0.20 (0.02) 0.19 (0.03) .101
growth later in childhood.13More
re-cently, we found an association be-tween greater intakes of breast milk during the neonatal period and higher WB BMC and BA in young adults who were born preterm.14
Our findings have contemporary rele-vance. In practice, despite greater rec-ognition of aluminum toxicity, little progress has been made on reducing exposure. Poole et al3 recently
con-cluded that meeting current Food and Drug Administration recommenda-tions to limit aluminum exposure to
⬍5g/kg per day is impossible in pa-tients who weigh⬍50 kg by using cur-rently available PN products, and cal-culated aluminum exposure in infants
⬍3 kg was 30.3 to 59.9 g/kg per day—indeed, somewhat higher than the calculated exposure of infants who
received standard PN solution in our trial.
The mechanism for long-term effects of aluminum on bone health is unclear. A direct toxic effect seems unlikely, be-cause bone tissue will have been re-placed more than once by age 13 to 15 years. Possibly, aluminum exposure might “program” the responsiveness of bone such that, for example, chil-dren who are exposed to more alumi-num form less bone for a given level of mechanical stimulus. This could ex-plain the apparent site-specific effects. Alternatively, aluminum might have neurotoxic effects, affecting central mechanisms that control bone mass. Indeed, bone remodeling is partly con-trolled by the central nervous system. In animals, several neuropeptides af-fect bone formation via the
hypothala-mus, with signal transmission to bone cells via the sympathetic nervous sys-tem.15 Plausibly, the effects observed
here might be another facet of early aluminum neurotoxicity rather than reflect a direct effect on bone. If so, then our study may have underesti-mated the effect of aluminum expo-sure, because, by design, our protocol excluded children with known neuro-logic impairment or with a Bayley score⬍85.
Although the effects of high aluminum exposure on LS BMC seemed to be re-lated primarily to reduced bone size (BA), effects on hip BMC seemed unre-lated to any corresponding stunting of hip bone growth. It is widely recog-nized that interventions may have dif-ferential effects at different skeletal sites. For example, exercise typically FIGURE 1
Calculated total aluminum exposure from PN during the neonatal period according to randomized group. Each symbol represents a single subject.
becular and appendicular skeleton, perhaps through differential influ-ences on trabecular and cortical bone.17Such differential effects cannot
be studied by DXA, used here, which provides no information on bone ge-ometry or structure—likely determi-nants of bone strength and fracture risk. Hence, we suggest that future ex-planatory studies require additional techniques such as hip structural analysis or pQCT.
The major limitation of our study re-lates to the inevitable cohort attrition over 15 years since study initiation. We could test only 33% of eligible children (32% of survivors), a follow-up rate typical of that reported in other recent long-term cohort studies.18 We
re-cently discussed the implications of cohort attrition for data analysis and interpretation and emphasized the im-portance of explicitly considering ef-fects on study power, bias, and gener-alizability.18With⬃60 children, we had
the power to detect a difference of 0.7 SD and might have missed smaller, al-though biologically relevant, effects. Regarding selection bias, children who were followed here tended to be those with higher birth weight SDSs; never-theless, if adverse effects of aluminum exposure were seen in these larger in-fants, then the effects on smaller, more vulnerable infants might be at least as large, if not greater.
Second, we did not quantify all possi-ble sources of parenteral aluminum, for instance from occasional albu-min infusions. This would not be ex-pected to influence the bone out-come differences seen between randomly assigned groups, but we
The long-term clinical significance of the observed effects of early aluminum exposure on bone mass at 13 to 15 years cannot currently be quantified, albeit our subjects were only 5 to 8 years from attaining peak bone mass, considered a powerful predictor of outcome. The estimated effect was siz-able: hip bone mass was 7.6% lower when aluminum exposure was above the median, and in those who were randomly assigned to standard PN so-lutions, LS BMC was 0.7 SD lower—
⬃14% of population variance, if nor-mally distributed. Of potential relevance here, we note that Hernandez19
sug-gested that the strongest predictor of osteoporosis risk is peak bone mass, estimating that a 10% increase would delay the onset of osteoporosis by 10 years.
CONCLUSIONS
Neonates who are exposed to paren-teral aluminum may have reduced LS and hip bone mass during adoles-cence, potential risk factors for later osteoporosis and hip fracture. Our ran-domized trial with long-term follow-up is, to our knowledge, the only 1 in this area. Our findings must be interpreted in the context of the relatively small sample size and multiple comparisons performed and should be confirmed on a larger sample and with additional tools to investigate bone indices, yet we recognize that such studies require many years to undertake, and reap-praisal of current practice is now needed. At 18 months of follow-up, be-fore significant cohort attrition, chil-dren from this cohort who were ex-posed to higher aluminum intakes had reduced developmental scores, with
Aluminum has no known biological purpose, and its potential hazards when given unphysiologically, by the parenteral route, are widely recog-nized in other contexts. Given our new findings, we suggest that it would be prudent, even with existing knowledge, to consider further reducing alumi-num in modern PN solutions. This is complex1and may involveⱖ1 of 3
ge-neric approaches: (1) changing (with research and product filing if re-quired) existing PN components to al-ternatives with lower aluminum, such as organic phosphorus sources (the latter are not currently available in the United States, and calcium chloride must be used judiciously to avoid pre-cipitation when attempting to provide high intakes of calcium and phos-phate); (2) use of new methods for alu-minum removal from PN products (eg, calcium salts); and (3) repackaging of PN components (eg, mineral salts) in plastic vials to reduce contamination from glass. Although these obstacles have inhibited progress, increasing safety concerns should now lead to re-evaluation of aluminum exposure in current PN, given to many thousands of preterm and high-risk infants each year.
ACKNOWLEDGMENTS
This study was supported by a re-search grant from UK Medical Re-search Council.
REFERENCES
1. Gura KM, Puder M. Recent developments in aluminum contamination of products used in parenteral nutrition.Curr Opin Clin Nutr Metab Care.2006;9(3):239 –246
2. Poole RL, Hintz SR, Mackenzie NI, Kerner JA. Aluminum exposure from pediatric paren-teral nutrition: meeting the new FDA regu-lation.JPEN J Parenter Enteral Nutr.2008; 32(3):242–246
3. McGraw M, Bishop N, Jameson R, et al. Alu-minum content of milk formulae and intra-venous fluids used in infants.Lancet.1986; 1(8473):157
4. Moreno A, Dominguez C, Ballabriga A. Alumi-num in the neonate related to parenteral nutrition.Acta Paediatr.1994;83(1):25–29 5. Bishop NJ, Robinson MJ, Lendon M, Hewitt
CD, Day JP, O’Hara M. Increased concen-tration of aluminum in the brain of a paren-terally fed preterm infant.Arch Dis Child.
1989;64(9):1316 –1317
6. Bishop NJ, Morley R, Day JP, Lucas A. Alumi-num neurotoxicity in preterm infants re-ceiving intravenous-feeding solutions.
N Engl J Med.1997;336(22):1557–1561 7. Klein G. Metabolic bone disease of total
parenteral nutrition.Nutrition.1998;14(1): 149 –152
8. Sedman AB, Klein GL, Russell MD, et al.
Evi-dence of aluminum loading in infants re-ceiving intravenous therapy.N Engl J Med.
1985;312(21):1337–1343
9. Nelson M, Hague GF, Cooper C, Bunker VW. Calcium intake in the elderly: validation of a dietary questionnaire.J Hum Nutr Diet.
1988;1(1):115–127
10. Bishop N, Braillon P, Burnham J, et al. Dual-energy X-ray absorptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions.J Clin Densitom.
2008;11(1):29 – 42
11. Crabtree NJ, Oldroyd B, Truscott JG, et al. UK Paediatric reference data (GE Lunar Prod-igy).Osteoporos Int.2004. Available at: www. springerlink.com/content/fy43tewe1x4w91x1/ fulltext.pdf. Accessed September 23, 2009 12. Cole TJ. Sympercents: symmetric
percent-age differences on the 100 log(e) scale sim-plify the presentation of log transformed data.Stat Med.2000;19(22):3109 –3125 13. Fewtrell MS, Cole TJ, Bishop NJ, Lucas A.
Neonatal factors predicting childhood height in preterm infants: evidence for a persisting effect of early metabolic bone disease?J Pediatr.2000;137(5):668 – 673 14. Fewtrell MS, Williams JE, Singhal A,
Mur-gatroyd PR, Fuller N, Lucas A. Early diet and
peak bone mass: 20 year follow-up of a ran-domized trial of early diet in infants born preterm.Bone.2009;45(1):142–149 15. Elefteriou F. Regulation of bone remodelling
by the central and peripheral nervous sys-tem.Arch Biochem Biophys. 2008;473(2): 231–236
16. Fewtrell MS. Osteoporosis: is primary pre-vention possible? In: Lucas A, Sampson HA, eds.Primary Prevention by Nutrition Inter-vention in Infancy and Childhood. Nestle Nutrition Workshop Series Pediatric Pro-gram 57. Basel, Switzerland: Karger, Vevey, Switzerland: Nestec Ltd. 2006;135–152 17. Cirmanova´ V, Beyer M, Starka L, Zajickova K.
The effect of leptin on bone: an evolving con-cept of action.Physiol Res.2008;57(suppl 1):S143–S151
18. Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and pro-spective studies?Arch Dis Child.2008;93(6): 458 – 461
19. Hernandez CJ. Theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the develop-ment of osteoporosis.Osteoporos Int.2003; 14(10):843
DOI: 10.1542/peds.2009-0783 originally published online October 26, 2009;
2009;124;1372
Pediatrics
Lucas
Mary S. Fewtrell, Nick J. Bishop, Caroline J. Edmonds, Elizabeth B. Isaacs and Alan
Services
Updated Information &
http://pediatrics.aappublications.org/content/124/5/1372
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/124/5/1372#BIBL
This article cites 17 articles, 2 of which you can access for free at:
Subspecialty Collections
skeletal_disorders_sub
http://www.aappublications.org/cgi/collection/rheumatology:musculo Rheumatology/Musculoskeletal Disorders
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2009-0783 originally published online October 26, 2009;
2009;124;1372
Pediatrics
Lucas
Mary S. Fewtrell, Nick J. Bishop, Caroline J. Edmonds, Elizabeth B. Isaacs and Alan
at 15-Year Follow-up
Aluminum Exposure From Parenteral Nutrition in Preterm Infants: Bone Health
http://pediatrics.aappublications.org/content/124/5/1372
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.