Early-Onset Neutropenia in
Small-for-Gestational-Age Infants
Robert D. Christensen, MDa,b,c, Bradley A. Yoder, MDa,c, Vickie L. Baer, RNa, Gregory L. Snow, PhDd, Allison Butler, MSd
abstract
BACKGROUND:Early neutropenia is more common in small for gestational age (SGA) neonates (birth
weight,10th percentile) than in appropriately grown neonates. However, several aspects of this variety of neutropenia are unknown, including the duration, kinetic mechanism, and outcomes.
METHODS:Using 10 years of multihospital records, we studied SGA neonates who, during thefirst
week after birth, had neutrophil counts,1000/mL.
RESULTS:This degree of neutropenia was more common in SGA neonates (6%, 207/3650) than in
non-SGA matched controls (1%, 46/3650;P,.001). Neutrophil counts stayed below the lower reference interval for 7 days. Ratios of immature to total neutrophils were within the reference interval, suggesting reduced neutrophil production, not accelerated neutrophil use or destruction. Increased nucleated red cells at birth correlated with decreased neutrophils (P,.001). Neutropenia was not independently associated with maternal hypertensive disorders, over and above the effect of SGA. Of 201 neutropenic SGA neonates, 129 (64%) also had thrombocytopenia. Sixteen percent of neutropenic neonates were treated with recombinant granulocyte colony-stimulating factor (rG-CSF) or intravenous immunoglobulin (IVIG), with no reduction in late-onset sepsis or necrotizing enterocolitis (NEC). Regression analysis showed that neutropenia (but not thrombocytopenia in the absence of neutropenia) was independently associated with increased odds of developing necrotizing enterocolitis (odds ratio 4.01, 90% confidence interval 2.08 to 7.35,P,.001).
CONCLUSIONS:Neutropenia of SGA is a condition of 1-week duration. It is more closely associated
with SGA than maternal hypertension (likely owing to neutrophil hypoproduction associated with intrauterine hypoxia), often accompanied by thrombocytopenia, not obviously improved by rG-CSF or IVIG, and associated with an increased risk for NEC.
WHAT’S KNOWN ON THIS SUBJECT:Small for
gestational age neonates (weight ,10th percentile) are at risk for neutropenia during the first days after birth. However, the duration, responsible mechanism, and outcomes of this variety of neonatal neutropenia are not precisely known.
WHAT THIS STUDY ADDS:Six percent of small for
gestational age neonates had neutrophils ,1000/mL, with an average neutropenia duration of 7 days. Neutropenia was more closely linked with small for gestational age status than maternal hypertension. This neutropenia is associated with elevated nucleated red blood cell count and increased odds of necrotizing enterocolitis.
aWomen and Newborn’s Clinical Program, Intermountain Healthcare,bDivision of Hematology/Oncology, and cDivision of Neonatology, Department of Pediatrics, University of Utah School of Medicine and Primary Children’s
Hospital, Salt Lake City, Utah; anddStatistical Data Center, LDS Hospital, Salt Lake City, Utah
Drs Christensen and Yoder and Ms Baer conceptualized and designed the study; Drs Christensen and Yoder drafted the initial manuscript; Ms Baer carried out the initial data assembly analyses and display; Dr Snow and Ms Butler carried out the statistical analysis; Ms Baer, Dr Snow, and Ms Butler reviewed and revised the manuscript; and all authors approved thefinal manuscript as submitted. www.pediatrics.org/cgi/doi/10.1542/peds.2015-1638
DOI:10.1542/peds.2015-1638 Accepted for publication Aug 5, 2015
Address correspondence to Robert D. Christensen, MD, University of Utah Department of Pediatrics, 295 Chipeta Way, Salt Lake City, UT 84108. E-mail: robert.christensen@hsc.utah.edu
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:No external funding.
Neonates who are born small for gestational age (SGA) (,10th percentile birth weight for a reference population) are at risk for having neutropenia in thefirst days after birth.1–6Various definitions have
been used for neonatal neutropenia, leading to diversity in the reported incidence of neutropenia among SGA neonates. Definitions have included an absolute neutrophil count,5th percentile reference range6or a count
of,1500/mL or,1000/mL.7
Surely some cases of neutropenia among SGA infants, regardless of the definition used for neutropenia, are the result of bacterial sepsis8,9or
neonatal alloimmune neutropenia,10
but most neutropenic SGA neonates have a different variety of
neutropenia that is sometimes termed“neutropenia of SGA”or neutropenia of infants of mothers with hypertensive disorders.1–6,11–13
Many aspects of that variety of neonatal neutropenia remain unclear.
As a step toward enhancing the knowledge base of neutropenia of SGA, we conducted a retrospective analysis of neonates hospitalized in our health care system during the past 10 years. This period was selected to coincide with improvements in our electronic data system dating to 2003. Our study aims were (1) to identify all SGA neonates who had a blood neutrophil concentration,1000/mL during thefirst week after birth (a stringent definition for neutropenia) and, within that group, to identify and exclude from further analysis those with other explanations for neutropenia such as early-onset sepsis, alloimmune neutropenia, or any other recognized form of neutropenia, defining the residual group as having the exclusionary diagnosis of the neutropenia of SGA; (2) to determine the duration of this variety of neutropenia; (3) to assess the mechanism causing this variety of neutropenia; and (4) to evaluate the outcomes of neonates with this condition.
METHODS
Patient Information
Data were collected retrospectively as deidentified limited data sets from archived Intermountain Healthcare records. Intermountain Healthcare is a not-for-profit healthcare system that owns and operates 19 hospitals with labor and delivery units in Utah and Idaho. The information collected was limited to the information in this report. Patient records were accessed if the neonate had a date of birth from January 1, 2004, through December 31, 2013. The Intermountain Healthcare Institutional Review Board approved this deidentified data–only study as not requiring the consent of individual subjects.
Blood Cell Counts
Leukocyte counts, neutrophil counts, and ratios of immature to total neutrophils (I/T ratios) were determined in all hospitals with the Beckman Coulter LH750 Hematology Analyzer (Fullerton, CA) from 2004 through mid-2012. After mid-2012, blood cell counts were determined using Sysmex counters (Sysmex America, Lincolnshire, IL). It is standard operating procedure in all Intermountain Healthcare Clinical Laboratories to include a manual differential cell count on all complete blood counts (CBCs) of infants in the first 90 days after birth. All blood tests were performed in accordance with Intermountain Healthcare Laboratory Services standard operating procedures. Leukocyte differential counts were performed by enumeration by certified medical technologists on Wright-stained blood smears, counting a minimum of 100 nucleated cells per test. The reference intervals for CBC
parameters are those we published from Intermountain Healthcare databases.14
Gestational age was determined by obstetrical assignment unless this
was changed by the neonatologist on the basis of gestational age
assessment (physical examination and neurologic-neurodevelopmental findings). Neonates were classified as SGA if their weight at birth was ,10th percentile for gestational age, using normative values from our Intermountain Healthcare
population.15Severity of SGA was
classified according to 3 categories: severe, less thanfirst percentile; moderate,first tofifth percentile; and mild, sixth to 10th percentile. Cases of maternal hypertension were
identified from case-mix records usingInternational Classification of
Diseases, Revision 9definitions 6425 (severe preeclampsia), 6426 (eclampsia), or 6427 (preeclampsia or eclampsia superimposed on existing hypertension). We did not include women with theInternational
Classification of Diseases, Revision 9
code 6424 (mild or nonspecific), because in our previous studies we found that this definition includes transient, resolved, or mild hypertension and that eliminating this code reduces heterogeneity.16No
specific guidelines for administering intravenous immunoglobulin (IVIG) and recombinant granulocyte colony-stimulating factor (rG-CSF) were sanctioned by the Intermountain Healthcare NICUs during this period, but general guidelines were
available.7Early thrombocytopenia
was defined as$2 platelet counts ,150 000/mL during thefirst week after birth.16
neutropenia, such as early-onset sepsis or immune-mediated neutropenia.
A diagnosis of necrotizing
enterocolitis (NEC) was recorded if it qualified as Bell stage$2.17
Spontaneous intestinal perforation was not included if this entity was recognized as the cause of the bowel dysfunction.18Late-onset
bacterial sepsis (LOS) was recorded if, after day of life 3,$1 blood cultures were positive in concert with clinical signs interpreted by the attending clinician as clinical sepsis and entered into the clinical record as a case of late-onset culture-positive sepsis.19
Data Collection and Statistical Analysis
The program used for data collection was a modified subsystem of Clinical Workstation (3M, Minneapolis, MN); 3M approved the structure and definitions of all data points for use within the program. Data were managed and accessed by authorized data analysts. Means and SDs were used to express values in groups that were normally distributed, and medians and interquartile ranges to express values in groups that were not. Differences in categorical variables were assessed by using the Fisher exact test orx2test for normally distributed data and the Tukey biweight estimator for groups that were not. Statistical analysis used Statit (Midas, Tucson, AZ) or the R Foundation package (Statistical Computing, Vienna, Austria). The mixed-effects model used NIME, version 3.1-105, also from the R package. Statistical significance was set asP,.05.
RESULTS
Severity and Duration of Neutropenia During the 10-year period studied, 24 036 neonates were admitted to an Intermountain Healthcare NICU, 3964 of whom were SGA (Fig 1). Of these,
3650 had $1 neutrophil counts measured in thefirst week, and 207 (6%) of those had$1 counts,1000/mL, thereby qualifying for the
definition of “early” (first-week) neutropenia. Neutropenia was more common among SGA neonates than gestational age–matched control neonates who were not SGA (P, .001) (Fig 1). The 3650 non-SGA controls were well matched with the 3650 SGA neonates on the basis of gestational age (Table 1), but the non-SGA control group had a higher birth weight (as expected) as well as lower rates of Cesarean delivery, eclampsia or preeclampsia, and death in the NICU compared with the SGA neonates.
Four of the 207 neutropenic SGA neonates had early-onset culture-positive bacterial sepsis. In addition, 1 had alloimmune neutropenia, and 1 had Barth syndrome. These 6 neonates, who had alternative explanations for their neutropenia, were excluded from further analyses, leaving 201 with a condition we termed neutropenia of SGA.
Neutrophil counts of the 201 neonates are shown in Fig 2. These 201 had a gestational age of 3064 weeks and a birth weight of 10046578 g. The reference intervals for blood neutrophils during theirfirst 9 days (5th to 95th percentile limits) for neonates of 28 to 36 weeks’gestational age range, which we published previously,14are shown for
comparison in the shaded area. The median value of all neutrophil counts in this group, during the first 4 days, was 1400/mL (first quartile 700/mL, third quartile 2500/mL). The lowest neutrophil counts (median nadir of 570/mL, first quartile 400/mL, third quartile 800/mL) was also during thefirst 4 days. By day of life 7, the neutrophil counts of those
with the neutropenia of SGA (95% confidence interval [CI] 1800 to 10 100/mL) were approximately within the reference interval of the gestational age–matched controls (CI 2000 to 12 000/mL14). Neonates
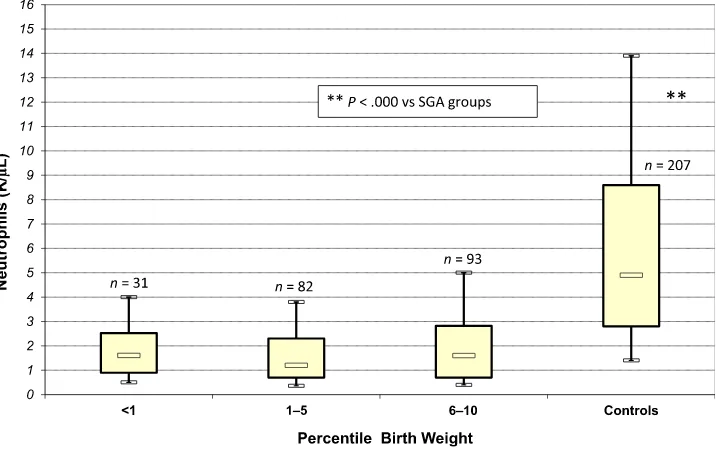
with severe SGA (weight less than first percentile) had neutrophil counts in the same range as those
FIGURE 1
with moderate (first tofifth percentile) or mild (sixth to 10th percentile) SGA (Fig 3).
SGA Versus Maternal Hypertension Associations of neutropenia with SGA status versus maternal hypertensive disorders were sought by comparing the lowest neutrophil counts during thefirst 4 days of life among 4 groups of NICU patients matched for gestational age and month of birth (Fig 4). Group 1 consisted of neonates who were SGA but had no record of a maternal hypertensive disorder. These neonates had lower neutrophil counts than did those of Group 2, which consisted of neonates who were not SGA but were born to women with hypertensive disorders during pregnancy (P,.005). Among SGA neonates, the presence or absence of hypertension during pregnancy made no difference in the neutrophil count. Thus maternal hypertension may not be associated with a risk of neonatal neutropenia over and above the risk associated with being SGA. Of 201 infants with neutropenia of SGA, 129 (64%) also had early thrombocytopenia, defined as$2 platelet counts,150 000/mL during thefirst week.16
Nucleated Red Blood Cells, I/T Ratios, IVIG, and rG-CSF
Among the 3650 SGA neonates, 2981 had a nucleated red blood cell count (NRBC/mL) recorded on the day of birth. The NRBC count inversely correlated, somewhat, with the neutrophil count during thefirst days after birth (Fig 5) (r= 0.262;P, .001). The subgroup of the most severely weight-restricted (less than first percentile) SGA neutropenic neonates had a trend toward higher NRBC counts (mean6SEM 22006 420/mL) than did those in thefirst to 10th percentile (17006160/mL,P= .06). The weak to moderate
association between fetal hypoxia and neonatal neutropenia is similar to the relationship we reported between NRBC and platelet counts in thefirst
week, which we speculated was an effect of intrauterine hypoxia on increased red blood cell production with a subsequent reduction in platelet and neutrophil
production.15,20
The 201 neonates with neutropenia of SGA had I/T ratios, as a measure of the leukocyte“left shift,”within the normal reference range of 0.00 to 0.25. Moreover, the I/T ratios of these 201 were not different from those of the group of 3443 SGA neonates who did not have neutropenia.
Thirty-four of the 201 neutropenic neonates were treated, during their first days of life, with either IVIG or rG-CSF. These treatments were not by protocol, but rather at the discretion of the various attending
neonatologists. Eighteen neonates were treated with IVIG alone (500 to 1000 mg/kg), 11 were treated with rG-CSF alone (1 to 3 doses of 10mg/kg), and 5 were treated with both medications. Neither IVIG nor rG-CSF treatment appeared to improve outcomes (see Supplemental Table 3). In fact, the 167 neutropenic neonates who received IVIG or rG-CSF were more likely to subsequently develop LOS or NEC (P= .014). Treatment of this neutropenia using IVIG or rG-CSF became progressively less common over the 10-year period studied: of the 34 treated with these
medications, 11 were treated in 2004 and 2005, 10 were treated in 2006 and 2007, 7 in 2008 and 2009, 5 in 2010 and 2011, and only 1 in 2012 and 2013.
Outcomes
NEC and LOS were diagnosed more commonly among the 201 neonates with neutropenia of SGA than among the 3443 SGA neonates who did not have neutropenia (Table 2). Because of the well-described associations between gestational age and NEC and between gestational age and LOS, we performed
regression analysis focusing on the independent contribution of
neutropenia to these outcomes. As expected, among SGA neonates, higher gestational age correlated with lower odds of acquiring NEC
(estimate20.15, OR 0.86,P,.001) and LOS (estimate20.16, OR 0.87,
P,.001). Neutropenia was
independently associated with higher odds of acquiring NEC (odds ratio [OR] 4.01, 95% CI 2.08–7.35,P, .001). Thrombocytopenia alone (without neutropenia) was not
associated with higher odds of acquiring NEC (OR 1.16, 95% CI 0.72–1.79,P= .60). Neutropenia was not independently associated with higher odds of acquiring LOS (OR 1.44, 95% CI 0.73–2.61,P= .342). FIGURE 2
Blood neutrophil concentrations of 201 neonates with neutropenia of SGA during thefirst 9 days after birth (mean neutrophil count and SD). The shaded area shows the normal reference interval based on.100 000 neutrophil counts of neonates during thefirst 9 days after birth (modified from Henry and Christensen14).
FIGURE 3
DISCUSSION
Neutropenia is classically defined as a blood neutrophil concentration,2 SDs of the appropriate reference-population mean.21,22However,
problems arise in applying this definition to newborn infants. First, the normal rise in neutrophil counts during thefirst day after birth would indicate that neonates who are 6 to 12 hours old are neutropenic with counts as high as 5000/mL, and
between the second and fourth days after birth, counts ,3000/mL would be termed neutropenic.22,23It seems
very unlikely to us that neutrophil counts as high as 3000 to 5000/mL would constitute a host defense deficiency, even if those counts are statistically abnormally low. Perhaps a more clinically meaningful
definition of neonatal neutropenia would be a neutrophil count sufficiently low to cause an
antibacterial host-defense deficiency, but that condition is difficult to recognize precisely. In fact, the risk of infection imparted by neutropenia is a complex issue, being influenced not only by the neutrophil count, but also by the duration of the low count,21
the size of the marrow neutrophil storage pool,24neutrophil function,21
and the underlying cause of the neutropenia.21
Because of these complexities, rather arbitrary definitions for neonatal neutropenia have been used. One such definition is a count,1500/mL, but it is not clear that counts in the 1000 to 1500/mL range impart any significant host defense
deficiency.25,26 We desired, in the
present analysis, to select
a biologically meaningful definition of neutropenia, and chose the definition of a count,1000/mL typically used in older children and adults.7,21We
realized before beginning our study that by selecting this definition, our proportion of neonates with neutropenia would be much lower than in all previous reports that defined neutropenia using higher cutoff values. Using this definition, we FIGURE 4
Blood neutrophil concentrations (mean and SD) during thefirst 4 days after birth among 4 groups of NICU admissions: (1) SGA but no eclampsia or preeclampsia (PE); (2) PE but not SGA; (3) both SGA and PE; and 4) neither SGA nor PE.n= 100 in each group.
FIGURE 5
found neutropenia in 6% of SGA neonates versus 1% of matched non-SGA controls. Six of the 207
neutropenic SGA neonates had other conditions that likely could have generated early neutropenia. Excluding these 6 neonates from further analysis, we labeled 201 as having the neutropenia of SGA. We found that their low neutrophil counts remained rather unchanged during thefirst 4 days after birth and then increased into the normal reference range by day 7 to 9. We recognize a potential problem in applying our neutrophil counts to other published values. Our hospitals are at altitudes ranging from 2661 to 4649 feet (881 to 1417 m) above sea level. We previously reported that our reference intervals have a lower border (5th percentile) similar to counts reported at sea level,23but
ours have a much higher upper border (95th percentile).22The
reasons for this difference, and the effect on applicability to other data, remain unknown.
In a previous study, we confirmed that thrombocytopenia is more common in SGA neonates than in matched non-SGA controls.16We
found that the thrombocytopenia of SGA was more severe in neonates with the most severe growth
restriction (birth weight less than first percentile) than in those with moderate or mild growth restriction (first to 10th percentile). However, we did not see that same pattern in neutrophil counts in the current study.
We speculate that the neutropenia of SGA is due to reduced neutrophil production, not accelerated
neutrophil utilization or destruction. This speculation is based on the normal I/T ratios. The neutropenia resulting from accelerated neutrophil usage or destruction (eg, sepsis, alloimmune) is typically accompanied by a left shift.8,9,23,27–29
Several previous studies reported that neutropenia associated with either maternal hypertension or SGA resulted in an elevated risk for subsequently developing
LOS.1,28,30–33However, other studies
did notfind that association.25,26
Using a regression analysis focused on those with a neutrophil count ,1000/mL, our data suggest that this variety of early neutropenia is associated with a 4-fold increased risk for the development of NEC, and this was the case for the subgroups weighing$1500 g and,1500 g at birth. However, although univariate analysis also suggested a higher risk
of LOS, this association was explained by other variables, particularly gestational age. In concordance with ourfindings, Ree et al reported that SGA neonates were.2-fold more likely to develop NEC than appropriate for gestational age–matched controls.34Luig et al
also found a relationship between SGA and a risk of NEC, but reported that maternal hypertensive disorders of pregnancy were associated with a reduced risk for NEC.35Our
findings suggest that the subset of SGA infants with neutropenia are at greater risk for NEC than the entire group of SGA neonates. The mechanism by which the NEC risk occurs is not known. It might not be due to the early neutropenia per se, but could result from other
immunologic or morphologic abnormalities that accompany the neutropenia of SGA.36,37For instance,
perhaps the intestine is more severely injured among the subset of SGA neonates who develop neutropenia than among the SGA neonates who maintain normal neutrophil counts. Additional work is needed to clarify this issue.
Our studies.20 years ago suggested to us that an inhibitor of neutrophil production was present in the placentas of women with
preeclampsia, and the resolution of neonatal neutropenia was the result of removing that source of inhibitor at birth.30This issue remains
unresolved, but the fairly consistent resolution of neutropenia in 6 to 7 days seems consistent with the elimination of an inhibitor at birth. Tsao et al posed the alternative hypothesis that neutropenia of SGA could be the result of inadequate fetal G-CSF production.38This is a rational
alternative explanation, but it seems to us that if G-CSF production were defective in utero and then
reestablished after birth, it might take much longer to generate neutrophils than would the immediate release of an inhibitor from an otherwise ready-to-go system.
TABLE 2 Associations Between Early Neutropenia (,1000/mL) and Subsequent Development of Either NEC or LOS in 3644 SGA Neonates
Factor n NECa LOSb
SGA and neutropenia 201 19 (9.5) 56 (27.9)
SGA and neutropenia,1500 g 170 15 (8.8) 53 (49.5)
SGA and no neutropenia 3443 69 (2.0) 161 (4.7)
SGA and no neutropenia,1500 g 501 23 (4.6) 43 (8.5)
Univariate analysis, SGA and neutropenia vs SGA and no neutropenia,P
,.0001 ,.001
Univariate analysis
SGA,1500 g and neutropenia vs SGA,1500 g and no neutropenia,P
.039 ,.001
Regression analysis, SGA neutropenia vs SGA no neutropenia
OR (95% CI) 4.01 (2.08–7.35) 1.44 (0.73–2.61)
P ,.001 .342
Data are expressed asn(%) unless noted otherwise.
aBell stage$2.
Our previous study on
thrombocytopenia in SGA neonates16
and the present work suggest to us that the neutropenia and
thrombocytopenia associated with SGA are more directly linked with fetal growth restriction than with maternal hypertension. This
conclusion is also the one reached by Wirbelauer et al, namely that it is SGA status, not high maternal blood pressure, that is associated with neonatal neutropenia.11In fact, we
suggest that what has been termed neutropenia of pregnancy-induced hypertension and thrombocytopenia of pregnancy-induced hypertension might more properly be called neutropenia of SGA and thrombocytopenia of SGA.
The value of administering IVIG and/ or rG-CSF to neonates with
neutropenia of SGA remains unproven, but from our present analysis, a substantial benefit is unlikely. Although IVIG was given to 9% of our neonates, rG-CSF was given to 5%, and both were given to 2%, we were unable to identify a benefit. A review by Ohlsson and Lacy
concluded that administering IVIG to low birth weight neonates reduces their odds of developing LOS, but only by about 3%.39LaGamma and
colleagues32,40 and Ahmad et al41
convincingly demonstrated that administering rG-CSF to neutropenic neonates increases their circulating neutrophil counts, but Carr et al found no evidence that prophylactic administration of rG-CSF or rGM-CSF to very low birth weight neonates reduces their odds of subsequently developing a late-onset infection.42
Whether neonates with neutropenia of SGA would derive any protection from NEC from treatment with IVIG or rG-CSF is unknown and cannot be concluded from these studies, because the numbers treated with IVIG or rG-CSF were so small. However, even if no medications can currently be identified as preventing NEC in these patients, it seems conceivable to us that just the
awareness that these neonates are at increased risk could be of some value in their daily care and expectant management.
ACKNOWLEDGMENTS
The authors thank Joyce M. Koenig, MD, Professor of Pediatrics, Molecular Microbiology, and Immunology, Department of Pediatrics, Saint Louis University School of Medicine, Saint Louis, MO, and Roger G. Faix, MD, Professor of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT, for helpful discussions and manuscript editing. We also thank Erick Henry, MPH, for assistance with data analysis and display.
ABBREVIATIONS
CBC: complete blood count CI: confidence interval I/T ratio: immature to total
neutrophil ratio
IVIG: intravenous immunoglobulin LOS: late-onset sepsis
NEC: necrotizing enterocolitis NRBC: nucleated red blood cell
count OR: odds ratio
rG-CSF: recombinant granulocyte colony-stimulating factor SGA: small for gestational age
REFERENCES
1. Koenig JM, Christensen RD. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension.N Engl J Med. 1989;321(9):557–562
2. Engle WD, Rosenfeld CR. Neutropenia in high-risk neonates.J Pediatr. 1984; 105(6):982–986
3. Davies N, Snijders R, Nicolaides KH. Intra-uterine starvation and fetal leucocyte count.Fetal Diagn Ther. 1991;6(3-4): 107–112
4. Juul SE, Haynes JW, McPherson RJ. Evaluation of neutropenia and neutrophilia in hospitalized preterm infants.J Perinatol. 2004;24(3):150–157
5. Ozyürek E, Cetintas¸ S, Ceylan T, et al. Complete blood count parameters for healthy, small-for-gestational-age, full-term newborns.Clin Lab Haematol. 2006; 28(2):97–104
6. Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Lambert DK. Low blood neutrophil concentrations among extremely low birth weight neonates: data from a multihospital health-care system.J Perinatol. 2006;26(11):682–687
7. Calhoun DA, Christensen RD, Edstrom CS, et al. Consistent approaches to procedures and practices in neonatal hematology.Clin Perinatol. 2000;27(3): 733–753
8. Greenberg DN, Yoder BA. Changes in the differential white blood cell count in screening for group B streptococcal sepsis.Pediatr Infect Dis J. 1990;9(12): 886–889
9. Christensen RD, Shigeoka AO, Hill HR, Rothstein G. Circulating and storage neutrophil changes in experimental type II group B streptococcal sepsis.Pediatr Res. 1980;14(6):806–808
10. Lewin S, Bussel JB. Review of fetal and neonatal immune cytopenias.Clin Adv Hematol Oncol. 2015;13(1):35–43
11. Wirbelauer J, Thomas W, Rieger L, Speer CP. Intrauterine growth retardation in preterm infants#32 weeks of gestation is associated with low white blood cell counts.Am J Perinatol. 2010;27(10): 819–824
12. Sharma G, Nesin M, Feuerstein M, Bussel JB. Maternal and neonatal
characteristics associated with neonatal neutropenia in hypertensive
pregnancies.Am J Perinatol. 2009;26(9): 683–689
13. Park YH, Lee GM, Yoon JM, et al. Effect of early postnatal neutropenia in very low birth weight infants born to mothers with pregnancy-induced hypertension.
Korean J Pediatr. 2012;55(12):462–469
14. Henry E, Christensen RD. Reference intervals in neonatal hematology.Clin Perinatol. 2015;42(3):483–497
15. Christensen RD, Baer VL, Henry E, Snow GL, Butler A, Sola-Visner MC.
16. Christensen RD, Henry E, Kiehn TI, Street JL. Pattern of daily weights among low birth weight neonates in the neonatal intensive care unit: data from a multihospital health-care system.J Perinatol. 2006;26(1):37–43
17. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria.Pediatr Clin North Am. 1986;33(1):179–201
18. Gordon PV, Attridge JT. Understanding clinical literature relevant to
spontaneous intestinal perforations.Am J Perinatol. 2009;26(4):309–316
19. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments.Arch Dis Child Fetal Neonatal Ed. 2015;100(3): F257–F263
20. Christensen RD, Baer VL, Yaish HM. Thrombocytopenia in late preterm and term neonates after perinatal asphyxia.
Transfusion. 2015;55(1):187–196
21. Jacobson CA, Berliner N. Neutropenia. In Greer JP, Arber DA, Glader B, List AF, Means Jr, RT, Paraskevas F, Rodgers GM, eds. Wintrobe’s Clinical Hematology, 13th Ed. Philadelphia: Lippincott, Williams & Wilkins, 2014:1279
22. Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited.J Perinatol. 2008;28(4): 275–281
23. Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells.J Pediatr. 1979; 95(1):89–98
24. Erdman SH, Christensen RD, Bradley PP, Rothstein G. Supply and release of storage neutrophils. A developmental study.Biol Neonate. 1982;41(3–4): 132–137
25. Teng R-J, Wu T-J, Garrison RD, Sharma R, Hudak ML. Early neutropenia is not associated with an increased rate of
nosocomial infection in very low-birth-weight infants.J Perinatol. 2009;29(3): 219–224
26. Paul DA, Leef KH, Sciscione A, Tuttle DJ, Stefano JL. Preeclampsia does not increase the risk for culture proven sepsis in very low birth weight infants.
Am J Perinatol. 1999;16(7):365–372
27. Schelonka RL, Yoder BA, desJardins SE, Hall RB, Butler J. Peripheral leukocyte count and leukocyte indexes in healthy newborn term infants.J Pediatr. 1994; 125(4):603–606
28. Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Effect of maternal hypertension on neonatal neutropenia and risk of nosocomial infection.Pediatrics. 1992; 90(3):430–435
29. Doron MW, Makhlouf RA, Katz VL, Lawson EE, Stiles AD. Increased incidence of sepsis at birth in neutropenic infants of mothers with preeclampsia.J Pediatr. 1994;125(3):452–458
30. Koenig JM, Christensen RD. The mechanism responsible for diminished neutrophil production in neonates delivered of women with pregnancy-induced hypertension.Am J Obstet Gynecol. 1991;165(2):467–473
31. Cadnapaphornchai M, Faix RG. Increased nosocomial infection in neutropenic low birth weight (2000 grams or less) infants of hypertensive mothers.J Pediatr. 1992;121(6):956–961
32. La Gamma EF, Alpan O, Kocherlakota P. Effect of granulocyte colony-stimulating factor on preeclampsia-associated neonatal neutropenia.J Pediatr. 1995; 126(3):457–459
33. Kocherlakota P, La Gamma EF.
Preliminary report: rhG-CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia-associated neutropenia.Pediatrics. 1998;102(5): 1107–1111
34. Ree IM, Smits-Wintjens VE, Rijntjes-Jacobs EG, et al. Necrotizing enterocolitis
in small-for-gestational-age neonates: a matched case-control study.
Neonatology. 2014;105(1):74–78
35. Luig M, Lui K; NSW & ACT NICUS Group. Epidemiology of necrotizing enterocolitis, Part II: Risks and susceptibility of premature infants during the surfactant era: a regional study.J Paediatr Child Health. 2005;41(4):174–179
36. Li J, Li H, Mao H, et al. Impaired NK cell antiviral cytokine response against influenza virus in small-for-gestational-age neonates.Cell Mol Immunol. 2013; 10(5):437–443
37. Neta GI, von Ehrenstein OS, Goldman LR, et al. Umbilical cord serum cytokine levels and risks of small-for-gestational-age and preterm birth.Am J Epidemiol. 2010;171(8):859–867
38. Tsao PN, Teng RJ, Tang JR, Yau KI. Granulocyte colony-stimulating factor in the cord blood of premature neonates born to mothers with pregnancy-induced hypertension.J Pediatr. 1999;135(1): 56–59
39. Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants.Cochrane Database Syst Rev. 2013;7:CD000361
40. La Gamma EF, De Castro MH. What is the rationale for the use of granulocyte and granulocyte-macrophage colony-stimulating factors in the neonatal intensive care unit?Acta Paediatr Suppl. 2002;91(438):109–116
41. Ahmad M, Fleit HB, Golightly MG, La Gamma EF. In vivo effect of recombinant human granulocyte colony-stimulating factor on phagocytic function and oxidative burst activity in septic neutropenic neonates.Biol Neonate. 2004;86(1):48–54
42. Carr R, Modi N, Doré C. G-CSF and GM-CSF for treating or preventing neonatal infections.Cochrane Database Syst Rev
DOI: 10.1542/peds.2015-1638 originally published online October 12, 2015;
2015;136;e1259
Pediatrics
Allison Butler
Robert D. Christensen, Bradley A. Yoder, Vickie L. Baer, Gregory L. Snow and
Early-Onset Neutropenia in Small-for-Gestational-Age Infants
Services
Updated Information &
http://pediatrics.aappublications.org/content/136/5/e1259 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/136/5/e1259#BIBL This article cites 40 articles, 4 of which you can access for free at:
Subspecialty Collections
_sub
http://www.aappublications.org/cgi/collection/hematology:oncology
Hematology/Oncology
http://www.aappublications.org/cgi/collection/neonatology_sub
Neonatology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2015-1638 originally published online October 12, 2015;
2015;136;e1259
Pediatrics
Allison Butler
Robert D. Christensen, Bradley A. Yoder, Vickie L. Baer, Gregory L. Snow and
Early-Onset Neutropenia in Small-for-Gestational-Age Infants
http://pediatrics.aappublications.org/content/136/5/e1259
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2015/10/06/peds.2015-1638.DCSupplemental Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.