Patient Preference and Adherence
Dove
press
O r i g i n A l r e s e A r c h
open access to scientific and medical research
Open Access Full Text Article
Patient experience with intravenous biologic
therapies for ankylosing spondylitis, crohn’s
disease, psoriatic arthritis, psoriasis, rheumatoid
arthritis, and ulcerative colitis
susan c Bolge1
helen M eldridge2
Jennifer h lofland3
caitlin ravin3
Philip J hart4
Michael P ingham1
1health economics and Outcomes
research, Janssen scientific Affairs, llc, raritan, 2Payer
Provider insights & Analytics, Janssen services, llc, Titusville, nJ,
3health economics and Outcomes
research, Janssen scientific Affairs, llc, horsham, PA, 4Value
communications, Medaxial group, new York, nY, UsA
Objective: The objective of this study was to describe patient experience with intravenous (IV) biologics for ankylosing spondylitis, Crohn’s disease, psoriatic arthritis, psoriasis, rheumatoid arthritis, or ulcerative colitis.
Methods: Semi-structured telephone interviews were conducted in 405 patients with these autoimmune diseases who were receiving an IV biologic to treat their disease.
Results: On a 7-point scale (1= not at all satisfied; 7= very satisfied), mean satisfaction with IV medication was rated 6.1; 77% of patients rated satisfaction as 6 or 7. The most frequently perceived benefits of IV therapy were related to supervision provided by health care
profes-sionals. Most patients (82%, n=332) preferred their IV medication to subcutaneous injection.
The three most common reasons for preferring IV were not wanting to self-inject (43%), less frequent dosing (34%), and preference for administration by a health care professional (24%). African–American/black patients had a stronger preference for IV administration than Caucasian/
white patients (97% vs 80%, P,0.05) and a greater dislike of needles/self-injection (71% vs
40%, P,0.05). Hospital outpatient departments were not rated as well as physician in-office
infusion. Only half (49%) of the patients reported that both they and their physician equally influenced the choice to switch from subcutaneous to IV therapy, and only 30% were given a choice of infusion center.
Conclusion: Users of IV biologics are highly satisfied with their medications and perceive the opportunity for health care provider interaction at their infusion facilities as an advantage of their regimen. These findings support continued need for IV therapeutic options and shared decision-making between patients and physicians while selecting biologic treatments.
Keywords: patient experience, biologic, intravenous, subcutaneous, anti-TNF, preference
Introduction
Ankylosing spondylitis (AS), Crohn’s disease (CD), psoriatic arthritis (PsA), psoriasis (PsO), rheumatoid arthritis (RA), and ulcerative colitis (UC) involve an immune response that is inappropriate or excessive.1,2 These autoimmune diseases can be
caused, signified, or accompanied by systemic disruption that may result in acute or chronic inflammatory injury, sometimes severe, in any organ system.1 They share
common inflammatory pathways; patients with one condition have a greater risk of having another of these conditions relative to the rest of the population.2 Individually,
these autoimmune diseases are rare; however, their combined prevalence in the United States is 5%–8%2,3 and has been increasing.3
correspondence: susan c Bolge health economics and Outcomes Research, Janssen Scientific Affairs, llc, 1000 route 202 – room 3348, raritan, nJ 08869, UsA
Tel +1 908 927 7426 Fax +1 908 927 3166 email sbolge@its.jnj.com
Journal name: Patient Preference and Adherence Article Designation: Original Research Year: 2017
Volume: 11
Running head verso: Bolge et al
Running head recto: Patient experience with intravenous biologics DOI: http://dx.doi.org/10.2147/PPA.S121032
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
For personal use only.
Number of times this article has been viewed
This article was published in the following Dove Press journal: Patient Preference and Adherence
Dovepress
Bolge et al
Treatment guidelines differ between these diseases. Current therapies for inflammatory bowel disease (IBD), which includes CD and UC, include aminosalicylates, cor-ticosteroids, antibiotics, immunomodulators, and biologics.4
For PsO, there are topical emollients and systemic therapies including fumaric acid esters, methotrexate, or biologics.5 In
both IBD4 and PsO,5,6 biologics are generally reserved for
those with moderate-to-severe disease.
Guidelines for moderate-to-severe RA call for initial treatment with disease-modifying anti-rheumatic drugs, most commonly methotrexate.7 If a patient does not respond
adequately to a nonbiologic agent after 3 months of treat-ment, then it is recommended that they switch to a biologic, the largest class of which is the tumor necrosis factor-alpha (TNF-α) inhibitors.7 TNF-α inhibitors (anti-TNFs) are also
recommended for use in newly diagnosed RA patients with high disease activity and poor prognostic indicators.7
The availability of biologic agents has represented a significant advance in the clinical management of certain moderate-to-severe autoimmune diseases.3 Currently, two
modes of administration are available for the biologic therapies that are used to treat these diseases: intravenous (IV) infusion and subcutaneous (SC) injection. In general, only health care professionals administer IV infusions, whereas SC injections can be either self-administered or professionally administered. Current IV biologics include abatacept for the treatment of RA; golimumab for the treatment of RA; infliximab for the treatment of AS, CD, PsA, PsO, RA, and UC; rituximab for the treatment of RA; tocilizumab for the treatment of RA; and vedolizumab for the treatment of CD and UC. SC biologics include abatacept for the treatment of RA; adalimumab for the treatment of AS, CD, PsA, PsO, RA, and UC; certolizumab for the treatment of AS, CD, PsA, and RA; etanercept for the treatment of AS, PsA, PsO, and RA; golimumab for the treat-ment of AS, PsA, RA, and UC; tocilizumab for the treattreat-ment of RA; and ustekinumab for the treatment of PsA and PsO.
The mode of administration may influence a patient’s preference for that treatment. However, there is limited information on patient experience with respect to this attri-bute of therapy. A few studies have examined treatment expectations among patients with RA8 and IBD.9,10 However,
none of these studies captured information from a sample of patients with AS, IBD, PsA, PsO, and RA having actual experience with biologic therapy. Additionally, none of these studies provided detailed information on IV therapy in particular. This leaves a gap in the literature with respect to data that come from patients with firsthand experience of IV biologic therapy, as well as in autoimmune conditions
other than RA and IBD; such data may inform the usage of IV therapy in clinical practice.
To address these gaps, a cross-sectional study was con-ducted in order to analyze the experience of patients with AS, CD, PsA, PsO, RA, or UC who were being treated with IV biologics at the time of study. This research sought to explore the total patient experience with IV therapy. Specific objectives included evaluation of perceived advantages and disadvantages of IV biologic therapy; description of patient experiences at the site of care (SOC); and examination of the decision-making process that led to the selection of IV therapy over other modes of administration.
Methods
study design
This was a cross-sectional study conducted through semi-structured telephone interviews performed from August 4 to September 29, 2010. The sample comprised patients with certain autoimmune diseases who were currently being treated with IV biologic therapy. Patients were asked to describe the advantages and disadvantages associated with their IV infusion experience. This study was approved by the Essex Institutional Review Board, Inc. Informed consent was obtained verbally for telephone interviews or online for web-based screening.
study sample
Study participants were recruited from Internet panels, infusion centers, and social media websites. Inclusion criteria required all patients to self-report: being $21 years of age; having a diagnosis for which IV biologic therapy was indicated (ie, AS, CD, PsA, PsO, RA, or UC); current treatment with an IV bio-logic; and having received at least three infusions of the pre-scribed IV biologic. Demographic quotas were set to ensure a sufficient sample size for statistical analyses across age, gender, household annual income, education level, employment status, current residence, and insurance coverage subcategories.
study measures
To structure the telephone interview, a topic guide was developed and mailed to participants. This topic guide com-prised 45 questions that focused on specific issues surrounding the IV therapy experience. Listed issues included reasons why patients might receive their biologic treatment as an infusion rather than as an injection; what patients might like/ dislike about receiving their medication as an infusion; what incentives there may be for patients to switch from an IV to an SC biologic; why patients might switch from an SC to an
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress Patient experience with intravenous biologics
IV biologic, if applicable; different attributes of their infusion center; and how the patient’s infusion center was selected.
Interviews captured a large quantity of qualitative data about patient experiences with their current IV therapy at the time of the survey. Topics covered in interviews included demographics, health characteristics, the perceived advan-tages and disadvanadvan-tages of IV biologic therapy, reasons for preferring IV biologics, perceptions with respect to the SOC at which patients received infusions, and the decision-making process that led to the selection of IV therapy.
Patients were classed as employed if they self-reported a full-time or part-time job. Individuals were categorized as not employed if they reported being temporarily unemployed, retired, out of work because of disability, a homemaker, or a student. Patients’ education levels were recorded either as college graduates, those with college degrees and post-graduate qualifications, or not college graduates (patients who attended high school or had some college education). SOC was categorized as rheumatology in-office infusion, gastroenterology in-in-office infusion, hospital outpatient department (HOPD), and infusion therapy provider (eg, community infusion centers not owned by hospitals).
During interviews, patients used 7-point Likert scales to rate satisfaction with various aspects of therapy, ease of use, and convenience, with anchors of 1 (extremely dissatisfied, extremely difficult, or extremely inconvenient, respectively) and 7 (extremely satisfied, extremely easy, or extremely convenient, respectively). Patients were also asked about their level of agreement with particular statements about their therapy (1= strongly disagree; 2= disagree; 3= neither agree nor disagree; 4= agree; and 5= strongly agree). Likert scale scores were summarized by counting the number of patients who reported the top two categories (ie, $6 of 7 or $4 of 5).
Descriptive and summary statistics were generated for all data. Statistical analyses were conducted using Student’s t-tests to compare means and independent z-tests to compare percentages.
Results
sample characteristics
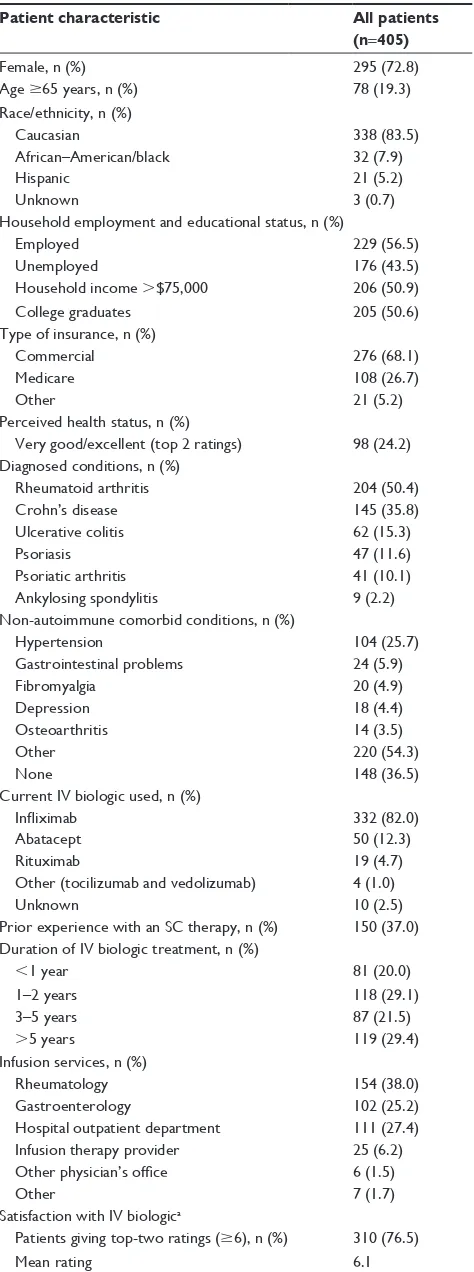
Most of the 405 patients were female (73%) and Caucasian/ white (84%) (Table 1). African–American/black patients accounted for 8% and Hispanic patients 5%, while 1% did not report their race/ethnicity. The mean age of the sample was 50 years, with 19% aged $65 years. A total of 57% of patients were employed, 51% had an annual household income of .$75,000, and 51% were college graduates.
Table 1 Patient characteristics
Patient characteristic All patients (n=405)
Female, n (%) 295 (72.8)
Age $65 years, n (%) 78 (19.3)
race/ethnicity, n (%)
caucasian 338 (83.5)
African–American/black 32 (7.9)
hispanic 21 (5.2)
Unknown 3 (0.7)
household employment and educational status, n (%)
employed 229 (56.5)
Unemployed 176 (43.5)
household income .$75,000 206 (50.9)
college graduates 205 (50.6)
Type of insurance, n (%)
commercial 276 (68.1)
Medicare 108 (26.7)
Other 21 (5.2)
Perceived health status, n (%)
Very good/excellent (top 2 ratings) 98 (24.2) Diagnosed conditions, n (%)
rheumatoid arthritis 204 (50.4)
crohn’s disease 145 (35.8)
Ulcerative colitis 62 (15.3)
Psoriasis 47 (11.6)
Psoriatic arthritis 41 (10.1)
Ankylosing spondylitis 9 (2.2)
non-autoimmune comorbid conditions, n (%)
hypertension 104 (25.7)
gastrointestinal problems 24 (5.9)
Fibromyalgia 20 (4.9)
Depression 18 (4.4)
Osteoarthritis 14 (3.5)
Other 220 (54.3)
none 148 (36.5)
current iV biologic used, n (%)
Infliximab 332 (82.0)
Abatacept 50 (12.3)
rituximab 19 (4.7)
Other (tocilizumab and vedolizumab) 4 (1.0)
Unknown 10 (2.5)
Prior experience with an sc therapy, n (%) 150 (37.0) Duration of iV biologic treatment, n (%)
,1 year 81 (20.0)
1–2 years 118 (29.1)
3–5 years 87 (21.5)
.5 years 119 (29.4)
infusion services, n (%)
rheumatology 154 (38.0)
gastroenterology 102 (25.2)
hospital outpatient department 111 (27.4)
infusion therapy provider 25 (6.2)
Other physician’s office 6 (1.5)
Other 7 (1.7)
satisfaction with iV biologica
Patients giving top-two ratings ($6), n (%) 310 (76.5)
Mean rating 6.1
Note:a1= not at all satisfied and 7= extremely satisfied.
Abbreviations: iV, intravenous; sc, subcutaneous.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress
Bolge et al
In addition, most patients (68%) had commercial health insurance; 27% were covered by Medicare while 5% were covered by other insurance providers. A total of 24% of patients perceived their health status to be “very good” or “excellent.” Over a third (37%) reported no comorbid condi-tions for which they were receiving treatment (discounting comorbid autoimmune diseases). The most common comorbidity for which patients were receiving treatment was hypertension (26%). Self-reported autoimmune diseases of interest in the sample included RA (50%), CD (36%), UC (15%), PsO (12%), PsA (10%), and AS (2%). These were treated with infliximab (82%), abatacept (12%), rituximab (5%), and other biologics (tocilizumab and vedolizumab: 1%). Patients specified that they had been treated for periods of ,1 year (20%); 1–2 years (29%); 3–5 years (22%); and .5 years (29%). Patients reported receiving care at the following sites: rheumatology offices (38%); HOPD (27%); gastroenterology offices (25%); infusion therapy providers (6%); other physician offices (2%); or other centers (2%). Approximately 37% of patients reported having prior experience with an SC therapy.
Perceived benefits and disadvantages
of iV therapy
A total of 310 patients (77%) were very satisfied with their current IV medication (rating their satisfaction as $6 out of 7; mean score: 6.1). The most frequently cited advantage of
infusion therapy, mentioned by 98% of the sample, was that it was administered by a professional, and the staff on site could monitor the patient for side effects (Table 2). Another com-monly perceived advantage was that infusion center staff could medically assess the patients during their treatment; 91% of patients regarded the visit as a valuable consultation in addition to their regular doctor visit; 89% of patients men-tioned staff keeping track of the patient’s dosing schedule as an advantage; 81% of patients considered the emotional sup-port received from the nurses and supsup-port staff at the infusion center as an advantage; and 78% of patients mentioned that the supervising staff could be consulted on medical issues unrelated to the condition for which they were receiving their IV biologic. Over half of the sample (56%) saw the oppor-tunity to learn from other patients at their infusion center as valuable; 55% of patients reported socialization with other patients at the infusion center as an advantage of IV therapy. A total of 55% of patients also stated that administration at an infusion center was convenient as they were able to tie in other activities (eg, shopping and dining out) with their visit. Finally, 22% of patients reported that traveling to and from the infusion center provided the opportunity to spend quality time with family or friends. Trends were similar among patients within each disease state.
The most commonly reported disadvantages of IV infu-sion (Table 2) were related to inconvenience. A total of 41%, 23%, and 19% of patients reported that duration of
Table 2 Perceived advantages and disadvantages of intravenous therapy
All patients (n=405)
Perceived advantage, n (%)
staff at infusion center can monitor for side effects 398 (98.3)
The medication is administered by a professional 397 (98.0)
infusion center visits act as an additional assessment to a regular doctor visit 369 (91.1)
infusion center staff keep track of the patient’s dosing regimen 362 (89.4)
emotional support is provided by infusion center staff 327 (80.7)
center staff can check medical issues beyond the autoimmune disease for which the biologic is being received 314 (77.5)
learning from the experiences of other patients attending the infusion center 228 (56.3)
social interaction with other patients at the infusion center 224 (55.3)
infusion center visits can complement activities such as shopping or dining out 221 (54.6)
Travel time to the infusion center can be spent with family/friends 87 (21.5)
Perceived disadvantage, n (%)
The infusion takes too long 165 (40.7)
scheduling appointments is inconvenient 92 (22.7)
Travel to the infusion center is inconvenient 78 (19.3)
infusion side effects/reactions 52 (12.8)
Multiple attempts may be required to start infusion; veins may be difficult to find 51 (12.6)
cost of infusion (including co-insurance or co-payment costs) 34 (8.4)
infusion is painful 30 (7.4)
sight of needles during infusion 28 (6.9)
no disadvantage 65 (16.0)
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress Patient experience with intravenous biologics
infusion, appointment scheduling, and travel to their SOC, respectively, could be problematic; 13% mentioned the fear of side effects or infusion reaction; a similar percentage cited the difficulty of finding veins, which meant that multiple attempts were sometimes required to start their infusion; 8% of patients reported the cost of infusion (including co-insurance or co-payment costs) as a drawback; 7% of patients saw IV administration as painful; and a similar per-centage of patients were fearful of the needles involved. In total, 16% of patients could think of no disadvantage with IV therapy. Again, trends were similar among patients within each disease state.
Overall, 82% of the patients in the sample preferred IV to SC therapy. The most common reason for preferring IV therapy to SC was a dislike of self-injection (mentioned by 43%; Table 3). In addition, 34% of patients preferred the less frequent dosing regimen associated with IV infusion. Many patients (24%) were concerned that they could not self-inject safely and preferred a health care professional to administer their medication. Some patients (16%) pre-ferred IV infusion because of the opportunity to interact with staff at the infusion center. Patients also perceived the arrangement of having appointments set by SOC staff as beneficial for remembering their doses (14%), while 11% regarded IV infusions as more effective than SC injections. Indeed, 9% of patients described a preference for IV medi-cation because they perceived it to be effective from direct experience and had no experience with SC administration. A small fraction of patients (3%) preferred IV as it was perceived to be logistically easier. Logistical issues for SC injections that were mentioned during interviews included the requirement to carry around equipment/medication as well as the need to dispose of needles properly. Similarly, 3% reported that they preferred IV infusion as it was less costly for them than SC alternatives, and 3% indicated a
preference for IV as they regarded SC injection as either uncomfortable or painful.
African–American/black patients displayed a sig-nificantly stronger preference for IV medication than did Caucasian/white patients (97% vs 80%, respectively; P,0.05) (Table 4). Additionally, more African–American/ black patients reported not wanting to self-inject or not liking needles as a reason for preferring IV medication (71% vs 40% of Caucasian/white vs 39% of Hispanic patients; P,0.05 for comparisons of African–American patients to both Caucasian/white and Hispanic patients). The number of patients reporting other reasons for prefer-ring IV therapy also varied according to ethnicity. African– American/black patients were significantly more likely than Caucasian/white patients (all comparisons P,0.05) to prefer IV medication due to the ease of remembering doses with a scheduled appointment (38% vs 17%); concerns about the safety of self-injection (41% vs 16%); dislike of needles (47% vs 24%); pain of self-injecting (38% vs 23%); and perceptions about the efficacy of IV therapy (50% vs 32%).
Perceptions regarding site of care
The attributes rated highest ($6 of 7 on a subjective scale of excellence) for all SOCs were related to the expertise of (92%), and interaction with (90%), SOC staff. Patients who received care in either rheumatology or gastroenterology offices were more likely to assign higher ratings for the level of staff interaction (94% or 93%, respectively) compared with patients who were infused at HOPDs (82%) (Table 5). Patients who received care in gastroenterology offices were more likely to report favorable ratings for waiting times (89%) compared with patients who received care in rheu-matology offices or HOPDs (78% or 54%, respectively; P,0.05). Patients were most likely to give the highest ratings
Table 3 reasons for the preference of iV therapy to alternative modes of administration
Reasons for preferring an IV biologic, n (%) Patients preferring IV therapy (n=332)
Dislike of self-injection/needles; lack of comfort with self-injection 144 (43.4)
less frequent dosing 114 (34.3)
Administered by a professional; self-injection may not be carried out safely 80 (24.1)
staff interaction at infusion center 54 (16.3)
easier to remember doses when an appointment is scheduled 45 (13.6)
iV infusion is perceived to be more effective than sc injection 38 (11.4)
iV has always been effective/no experience with sc administration 31 (9.3)
infusion is easier/everything is taken care of for you 11 (3.3)
less costly 10 (3.0)
iV infusion is less painful 10 (3.0)
Abbreviations: iV, intravenous; sc, subcutaneous.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress
Bolge et al
for the convenience of scheduling infusions for infusion therapy providers (96%). HOPDs received the lowest ratings of all SOCs for factors such as ease of parking, free parking, waiting time, staff interaction, and staff expertise.
Decision process for the selection
of iV therapy
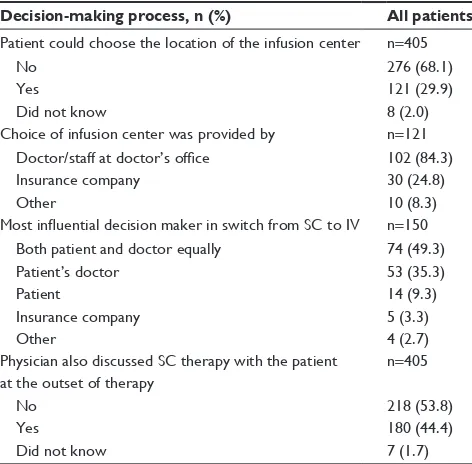
Of the 405 patients in the overall sample, 68% reported that they did not have the opportunity to choose their SOC (Table 6). Of the 121 patients who were given a choice about their SOC, 84% reported that the available options were provided to them either by their doctor or the staff at their doctor’s office. Of the 150 patients who had switched from SC to IV therapy, 49% stated that physicians and patients were equally decisive in the selection of therapy. When asked
whether doctors discussed SC options with them at therapy initiation, over half (54%) of patients reported that their doctor had not done so.
Discussion
The results of this study indicated that patients with AS, CD, PsA, PsO, RA, or UC who were treated with IV biologics were very satisfied with their medication and overall treatment experience. The most prominent perceived advantages of IV infusion were related to patient interactions with the staff at infusion centers. This was perceived to be an advantage as the trained staff could monitor the patient for any side effects during the infusion, could be consulted on other medical issues, and actually administered the medication. The most frequently cited reason for preferring IV infusion Table 4 racial disparities in the preference for iV medication
Characteristic, n (%) A: Caucasian/white (n=338)
B: African–American/black (n=32)
C: Hispanic (n=21)
Prefer iV to sc 270 (79.9)b 31 (96.9)a 18 (85.7)
reasons for the preference of iV n=270 n=31 n=18
Dislike self-injection/needles 108 (40.0)b 22 (71.0)a,c 7 (38.9)b
less frequent dosing 92 (34.1) 8 (25.8) 9 (50.0)
Administered by a professional; self-injection may not be carried out appropriately
60 (22.2) 10 (32.3) 5 (27.8)
staff interaction at infusion site 47 (17.4)b,c 2 (6.5)a 1 (5.6)a
easier to remember doses with an appointment 35 (13.0) 4 (12.9) 5 (27.8)
less costly 10 (3.7) 0 (0.0) 0 (0.0)
Patient interaction at infusion site 8 (3.0) 0 (0.0) 0 (0.0)
Patient perceptions (rating $4 of 5) n=338 n=32 n=21
iV infusion is more effective 107 (31.7)b 16 (50.0)a 11 (52.4)
Unease with self-injection due to dislike of needles 81 (24.0)b 15 (46.9)a,c 3 (14.3)b
iV infusion is less painful 79 (23.4) 12 (37.5)c 3 (14.3)b
easier to remember doses with an appointment 58 (17.2)b 12 (37.5)a 7 (33.3)
Unease about self-injection due to safety concerns 55 (16.3)b 13 (40.6)a 6 (28.6)
Note:a–cP,0.05 when compared to the group with the listed column letter.
Abbreviations: iV, intravenous; sc, subcutaneous.
Table 5 Patients with satisfied or very satisfied perceptions of their infusion, by site of care
Characteristic, n (%) A: Rheumatology office (n=154)
B: Gastroenterology office (n=102)
C: Hospital outpatient department (n=111)
D: Infusion therapy provider (n=25)
staff expertise 144 (93.5)c 96 (94.1)c 95 (85.6)a,b,d 24 (96.0)c
staff interaction 144 (93.5)c 95 (93.1)c 91 (82.0)a,b 22 (88.0)
handling of insurance coverage and paperwork
140 (90.9)c 90 (88.2) 93 (83.8)a 21 (84.0)
convenience of scheduling 124 (80.5)d 84 (82.4)d 97 (87.4) 24 (96.0)a,b
Presence of free parkinge 132 (85.7)b,c 78 (76.5)a 73 (65.8)a 21 (84.0)
Accessibility of parkinge 126 (81.8)c 77 (75.5) 72 (64.9)a,d 22 (88.0)c
Waiting time 120 (77.9)b,c 91 (89.2)a,c 60 (54.1)a,b,d 19 (76.0)c
easy to reach 99 (64.3) 68 (66.7) 77 (69.4) 16 (64.0)
convenience of location 95 (61.7) 62 (60.8) 66 (59.5) 15 (60.0)
Notes:a–dP,0.05 compared with the group with the listed column letter. eThe number of valid respondents for this characteristic was 151 for rheumatology office and 109 for hospital outpatient department.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress Patient experience with intravenous biologics
to SC injection was a dislike of self-injection (cited by 43% of patients). Hence, the availability of medications with dif-ferent routes of administration may be important to provide patients with more options if they are uncomfortable with a particular route. The less frequent dosing required for the administration of IV biologics was also perceived as a rea-son to prefer IV therapy. Therefore, it appears that there are patient subgroups that place particular value on the medical supervision available during infusion, while others see the lack of self-injection and relatively infrequent dosing as more convenient. African–American/black patients preferred IV infusion to SC injection more strongly than Caucasian/white patients. Therefore, it seems that African–American/black patients may be one subgroup for whom access to IV therapy is especially important.
The main results of this study corroborate earlier findings regarding patient preferences for and perceived advantages of IV biologic therapy.8–10 In an Italian study conducted by
Scarpato et al (2010), 802 patients were surveyed to determine their preferred route of administration for biologic medica-tion in RA.8 Similar to the findings of the current study, the
presence of medical professionals was the most frequently cited reason for preferring IV therapy. In their study, Scarpato et al found that of 403 patients sampled who preferred an IV biologic, the perceived safety of hospital administration (77%) and the reassuring effect of the doctor’s presence (66%) were the most common reasons for their preference.8
Also complementing the findings of the present study,
Scarpato et al found that of 399 patients who preferred SC injection, the most common reason was the inconvenience of IV infusion (55%),8 which matches the most frequently
perceived disadvantage of IV therapy in the present study. Notably, Scarpato et al evaluated only RA patients.8 By
contrast, the present study examined patients with various autoimmune diseases. In addition, the patients sampled by Scarpato et al had never received an anti-TNF biologic and could only describe their expectations.8 In the present study,
patients were included only if they were currently receiving an IV biologic and could therefore describe their experience. Indeed, most patients (80%) in the present study had received their IV biologic for .1 year.
Vavricka et al examined the treatment preferences of patients with CD by fielding a survey to patients who were naïve to anti-TNF therapy.9 Patients were asked to choose
from treatment profiles (adalimumab, certolizumab pegol, and infliximab) and describe the influential factors in their decision. Ease of use was cited by 69%, time required by 34%, interval between doses by 31%, efficacy by 19%, and fear of needles by 10%.9 A total of 13% reported that the
route of administration was the most important factor in selecting their drug.9 This complements the findings of the
current study, which highlights route of administration as an important component in patient preference.
In another study, Allen et al surveyed IBD patients to compare their preference for one of two anti-TNF agents.10
Among the 78 individuals surveyed, 33 (42%) preferred the IV infusion and the most commonly reported reasons were the dislike of self-injection (66%) and relatively infrequent dosing frequency (42%).10 Nineteen (24%) preferred SC
injection and the most commonly reported reasons were the convenience of dosing at home (79%) and less frequent visits to the hospital (63%).10
In addition to comparing patient preferences for IV vs SC therapy, the present study provides novel information with respect to patient perception of the SOC for IV infusions. Patients who were infused at HOPDs were less satisfied with many aspects of their experience (particularly in terms of parking, wait time, and staff interaction) compared with patients attending more specialized centers (rheumatology offices, gastroenterology offices, and infusion therapy providers) for their infusions.
Patient satisfaction has been correlated to treatment out-comes previously in the context of RA.11 Although a causal
relationship has not been established, it seems reasonable that there may be a link between satisfaction and outcomes such as adherence to the therapy for autoimmune disease. Table 6 The decision-making process for selecting iV therapy
Decision-making process, n (%) All patients
Patient could choose the location of the infusion center n=405
no 276 (68.1)
Yes 121 (29.9)
Did not know 8 (2.0)
choice of infusion center was provided by n=121
Doctor/staff at doctor’s office 102 (84.3)
insurance company 30 (24.8)
Other 10 (8.3)
Most influential decision maker in switch from SC to IV n=150
Both patient and doctor equally 74 (49.3)
Patient’s doctor 53 (35.3)
Patient 14 (9.3)
insurance company 5 (3.3)
Other 4 (2.7)
Physician also discussed sc therapy with the patient at the outset of therapy
n=405
no 218 (53.8)
Yes 180 (44.4)
Did not know 7 (1.7)
Abbreviations: iV, intravenous; sc, subcutaneous.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Dovepress
Bolge et al
As such, attributes of the patient experience that are perceived as advantages/disadvantages may be useful quality indicators for therapy. Where aspects of this experience are found to be lacking, it is possible that they may be improved to the benefit of therapeutic outcomes.
The current study is limited by its sample size of 405 patients, which means that some subgroups were too small for reliable statistical comparisons. The composition of the sample may also be limited by the recruitment methods used (ie, Internet panels, infusion centers, and social media). By recruiting through infusion centers, which include those in gastroenterology offices, there is an over-representation of patients with CD and UC compared to patients with other conditions. Recruitment through Internet panels and social media may cause an over-representation of patients who are younger or of a higher socioeconomic status. The question-naire has not been rigorously validated. Moreover, the quality of the data presented is reliant upon the accuracy of patients’ anonymous self-reporting of their condition. Nevertheless, it is important to stress that the self-reported nature of this study is also a strength with respect to the evaluation of patient perception.
The current study only included patients currently using IV biologics. Although some patients had previous experience with SC biologics, most did not. Preferences may differ among patients based on actual experience. Those currently using IV biologics may simply be satisfied with their current experience. Further comprehensive research is required to understand patient experience with IV biolog-ics. Complementary studies examining the experience of patients receiving SC therapy would serve as a useful com-parison to the present findings. It would also be interesting to evaluate the experiences of patients who had previously discontinued an IV/SC medication to determine whether their preferences or perceptions of the disadvantages/ advantages of therapy differ. Although 40% of the current sample perceived length of infusion as a disadvantage of IV versus SC therapy, these patients remained on IV therapy at the time of the survey. Understanding why patients persist on IV therapy despite perceived disadvantages is worth exploring further.
Conclusion
The present study adds valuable information in the context of the existing literature, focusing on actual patient experi-ence after receiving IV biologic therapy rather than expec-tations. This study has shown that most patients receiving IV biologics are satisfied with their medication and that these
preferences may be rooted in the presence of medical staff at their infusion center, the perceived injection experience, and the convenience of having their therapy managed for them. This study also revealed that only half of the patients sampled deemed their selection of biologic therapy to have been a shared decision between them and their physician. Therefore, stakeholders should consider IV biologics as an important option for the treatment of patients with certain moderate-to-severe autoimmune diseases and encourage patients to participate in the choice of therapy to optimize their treatment experience.
Key points for decision makers
• Patient preference for route of biologic administration should be considered in treatment decisions.
• Patients with autoimmune diseases view access to health professionals as an advantage of IV biologics.
• Black patients are more likely to prefer IV biologics and dislike self-injection.
Acknowledgments
Jonathan Latham of PharmaScribe, LLC provided assistance with editing and submitting the manuscript. The authors thank Julie Vanderpoel, PharmD, MPA and Samir Mody, PharmD, MBA for their contributions to the study and their critical review of the manuscript. In addition to the authors, Susan Wyant, PharmD (President), and Barbara Roland, MBA (Senior Vice President) of The Dominion Group made substantial contributions to the study. The Dominion Group was responsible for recruitment, data collection, and analysis of the survey data. Janssen Scientific Affairs, LLC provided financial support for this study.
Author contributions
MP Ingham, H Eldridge, SC Bolge, and JH Lofland were involved in the design of the study and the analysis and interpretation of the data. All the authors were involved in drafting, critical revision, and review of the manuscript and approved the final article.
Disclosure
At the time of the research SC Bolge, JH Lofland, C Ravin, and MP Ingham were employees of Janssen Scientific Affairs, LLC and H Eldridge was an employee of Janssen Services, LLC. SC Bolge, JH Lofland, MP Ingham, and H Eldridge are stockholders of parent company Johnson & Johnson. PJ Hart was an employee of the Medaxial Group. The authors report no other conflicts of interest in this work.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020
Patient Preference and Adherence
Publish your work in this journal
Submit your manuscript here: http://www.dovepress.com/patient-preference-and-adherence-journal Patient Preference and Adherence is an international, peer-reviewed, open access journal that focuses on the growing importance of patient preference and adherence throughout the therapeutic continuum. Patient satisfaction, acceptability, quality of life, compliance, persistence and their role in developing new therapeutic modalities and compounds to optimize
clinical outcomes for existing disease states are major areas of interest for the journal. This journal has been accepted for indexing on PubMed Central. The manuscript management system is completely online and includes a very quick and fair peer-review system, which is all easy to use. Visit http://www. dovepress.com/testimonials.php to read real quotes from published authors.
Dovepress
Dove
press
Patient experience with intravenous biologics
References
1. Williams JP, Meyers JA. Immune-mediated inflammatory disorders (I.M.I.D.s): the economic and clinical costs. Am J Manag Care. 2002;8: S664–S681.
2. Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad
Med J. 2007;83:251–260.
3. Carter PH, Zhao Q. Clinically validated approaches to the treatment of autoimmune diseases. Expert Opin Inv Drugs. 2010;19:195–213. 4. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters
Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. 5. Murphy G, Reich K. In touch with psoriasis: topical treatments and
cur-rent guidelines. J Eur Acad Dermatol Venereol. 2011;25(Suppl 4):3–8. 6. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the
management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850.
7. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease- modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625–639. 8. Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the
choice of anti-TNF therapies in rheumatoid arthritis. Results from a ques-tionnaire survey (RIVIERA study). Rheumatology (Oxford). 2010;49: 289–294.
9. Vavricka SR, Bentele N, Scharl M, et al. Systematic assessment of factors influencing preferences of Crohn’s disease patients in selecting an anti-tumor necrosis factor agent (CHOOSE TNF TRIAL). Inflamm
Bowel Dis. 2012;18:1523–1530.
10. Allen PB, Lindsay H, Tham TC. How do patients with inflamma-tory bowel disease want their biological therapy administered? BMC
Gastroenterol. 2010;10:1.
11. Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum. 2007;56:2135–2142.
Patient Preference and Adherence downloaded from https://www.dovepress.com/ by 118.70.13.36 on 26-Aug-2020