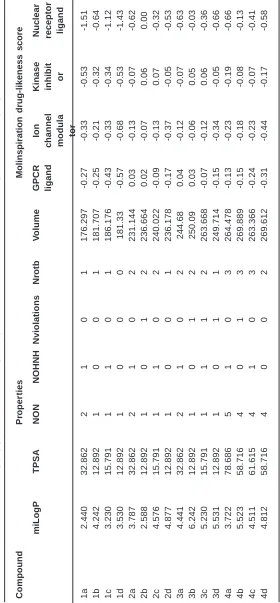
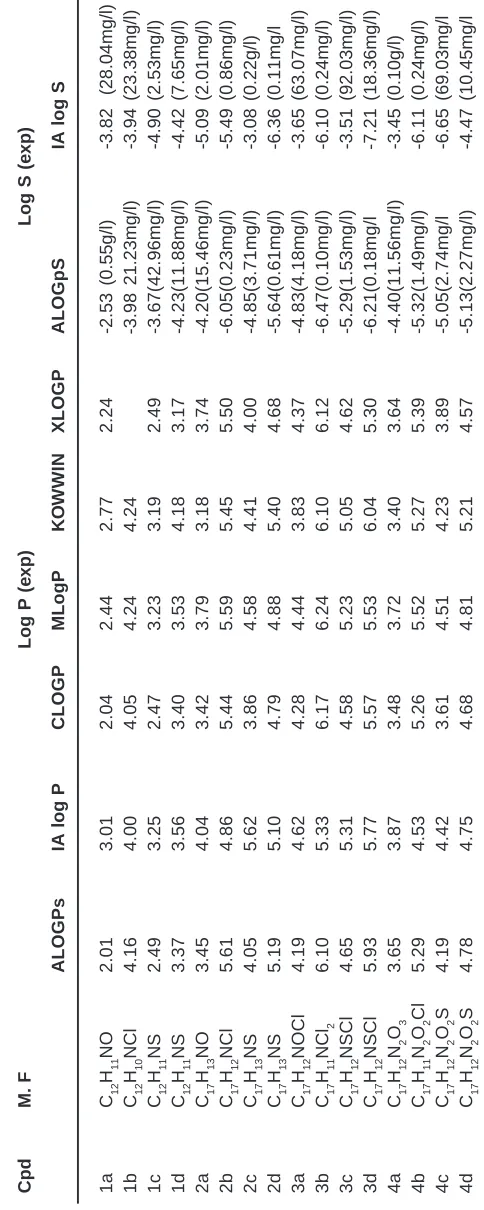
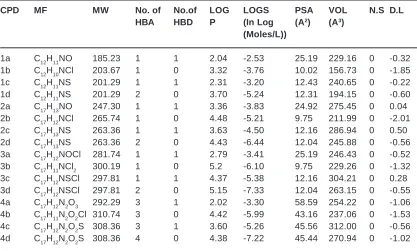
Calculation of Molecular Lipophilicity and Drug Likeness for Few Heterocycles
Full text
Figure
Related documents
signifikant reduziert gegenüber den anderen Patienten. iii) Diese signifikante Reduktion (A-12h) fand sich für STAT1 auch in Granulozyten. (vgl. Abb.22, p69), während STAT3 in
Remote sensing data provides several essential informa- tion about land use/land cover and the topographic fac- tors; therefore, integrating these data with soil characteristics
In this study an attempt has been made to purify and partially characterize the active metabolites of the metabolic extract of the marine sponges, Sigmadocia
The antifungal activity of biosurfactant was examined against different strains of Candida 100.. albicans (7 isolates),
inward hooked apically; Middle and hind tibiae normally widened toward apex and with strong transverse carinae on outer face, apically fimbriate with spinules unequal and
Eighteen cases of meningococcal disease were reported in NSW in September and October 2010 (14 cases were reported in the same period in 2009).. The ages of the affected people
Culex mosquitoes sampled from different habitats with different nutrient concentra- tions (influenced by larval control treatments) also did not reveal significant differences in
The influence of CrVI, 4-OHE2 and 16-OHE1 on thiobarbituric acid reactive substances (TBARS), the hydroxyl radical (•OH), superoxide dismutase (SOD), glutathione peroxidase (GPx)