Serum Osteoprotegerin Levels in Healthy Controls and
Cancer Patients
Allan Lipton,
1Suhail M. Ali, Kim Leitzel,
Vernon Chinchilli, Lois Witters, Linda Engle,
Donna Holloway, Pirow Bekker, and
Colin R. Dunstan
Division of Hematology/Oncology [A. L., S. M. A., K. L., L. W.] and Department of Health Evaluation Sciences [V. C., L. E.], Milton S. Hershey Medical Center, Hershey, PA 17033, and Development, Amgen, Inc., Thousand Oaks, California 91320 [D. H., P. B., C. R. D.]
ABSTRACT
Purpose: Osteoprotegerin (OPG) is a novel secreted member of the tumor necrosis factor receptor superfamily. In vitro, OPG blocks osteoclastogenesis in a dose-dependent manner. Serum OPG levels were assayed in cancer patients and healthy control subjects using an ELISA.
Results: OPG levels in healthy controls were signifi-cantly higher in sera (0.17 ng/ml) than in plasma (0.14 ng/ml). OPG levels did not differ by age in either control group. Serum was available from patients with solid tumors (n ⴝ 145), hematological malignancies (n ⴝ 111), benign hematological disorders (n ⴝ 35), and rheumatologic dis-eases (nⴝ60). When adjusted for age and sex, there was no significant OPG elevation in the sera of patients with solid tumors compared with controls (0.2 versus 0.18 ng/ml). When analyzed by site of primary malignancy within the solid tumor patient group, serum OPG elevations were ob-served only in patients with colorectal cancer (0.29 ng/ml; P<0.0001) and pancreatic cancer (0.35 ng/ml; P<0.0001). When analyzed by site of metastasis within the solid tumor patient group, significant elevations in serum OPG were observed only in patients with liver metastases (0.29 ng/ml) and soft tissue metastases (0.21 ng/ml) but not in patients with bone or lung metastases. Within the hematological malignancy group, serum levels of OPG were significantly lower in patients with multiple myeloma (0.12 ng/ml) but were elevated in patients with Hodgkin’s disease (0.29 ng/ ml) and Non-Hodgkin’s Lymphoma (0.24 ng/ml; Pⴝ0.048). Conclusions: Although some patients with malignancy have significant elevations of circulating OPG, these
concen-trations do not approach the level that would be expected to suppress osteoclast function.
INTRODUCTION
Bone remodeling is a normal process that involves the synthesis of bone matrix by osteoblasts and the resorption of bone by osteoclasts. Bone synthesis and resorption are highly coordinated (coupled) and are regulated by osteotrophic and calciotropic hormones. Increased bone resorption and turnover mediated by activated osteoclasts occur in various disorders of bone, such as osteoporosis and osteolytic bone metastasis.
Recently, two extracellular regulators of osteoclast differ-entiation and activation have been identified: (a) OPG2
(1); and (b) OPGL (2). OPGL is a TNF-related cytokine that stimulates osteoclast differentiation from hematopoietic precursor cells and activates mature osteoclasts in vitro and in vivo. Mice lacking OPGL lack osteoclasts and have defects in bone remodeling processes that lead to severe osteopetrosis (3).
OPG was identified independently by two groups and subsequently confirmed by other studies as a novel member of the TNFR superfamily (2, 4). OPG is a secreted protein that binds to and neutralizes OPGL bioactivity. Transgenic mice that overexpress OPG have defects in osteoclastogenesis similar to OPGL mice. In contrast, mice lacking OPG develop severe, early onset osteoporosis (5).
Compared with that of OPGL, steady-state levels of OPG mRNA are higher and have a wider tissue distribution, which is not restricted to bone or immune tissues. High levels of OPG mRNA have been detected in lung, heart, kidney, liver, stomach, intestine, skin, brain, spinal cord, thyroid gland, and bone (1, 6, 8, 9). OPG mRNA levels have also been detected in a variety of osteoblastic lineage cells, including marrow stromal (6, 8, 10), osteoblastic (11–13), and osteosarcoma cell lines (8 –11). In addition, high OPG mRNA levels have also been detected in endothelial cells, aortic smooth muscle cells, fibroblastic cells, ovarian (CAOV-3) and breast cancer cell lines (MCF7), and monocytic dendritic and B lymphocytic cell lines (6 –9).
OPG is synthesized as a propeptide (401 amino acids for the human, mouse, and rat forms), of which the signal peptide (21 amino acids) is cleaved, thus generating the mature peptide (380 amino acids; Refs. 1, 6, and 8). In contrast to all other TNFR superfamily members, OPG lacks transmembrane and cytoplasmic domains and is secreted as a soluble protein (1, 6, 8, 9).
The purpose of this study was to determine the levels of circulating OPG in the serum of healthy controls and patients with a variety of hematological malignancies and solid tumors. Received 10/23/01; revised 2/25/02; accepted 3/22/02.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
1To whom requests for reprints should be addressed, at Milton S. Hershey Medical Center, Department of Medicine, Division of Hema-tology/Oncology HO46, 500 University Drive, P. O. Box 850, Hershey, PA 17033. Phone: (717) 531-8677; Fax: (717) 531-5076; E-mail: alipton@psu.edu.
2The abbreviations used are: OPG, osteoprotegerin; OPGL, osteopro-tegerin ligand; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor.
MATERIALS AND METHODS
Subjects. Serum (n⫽112) and matched plasma (n⫽61) were available from healthy volunteers who did not take drugs known to affect bone and calcium metabolism. Serum was available from patients with solid tumors (n ⫽145), hemato-logical malignancies (n⫽111), benign hematological disorders (n⫽35), and rheumatologic diseases (n⫽60). All sera were obtained either at the time of diagnosis or when there was progressive disease despite treatment. All specimens were ob-tained between 1990 and 1996 and were stored frozen at⫺80°C until thawed for this study.
ELISA For OPG. OPG was measured by a sandwich ELISA at Amgen, Inc. All assays were performed on coded samples without knowledge of the clinical status of patients by the person performing the assay. A mouse monoclonal antibody was used as a capture antibody, and a rabbit polyclonal antibody against human OPG was used for detection. Both antibodies were raised against intact mature recombinant human OPG. The limit of detection of OPG was 0.05 ng/ml, and the interassay variability was 15%.
Statistical Analysis. Data were analyzed using ANOVA and regression analysis to compare between groups. A level of P⬍ 0.05 was considered statistically significant. Calculations were done using PROC GLM of SAS, and all analysis used the natural log of serum OPG or the natural log of plasma OPG as the response. Because of the exploratory nature of the analysis, no adjustments were made for multiple comparisons. Paired analysis for serum and plasma OPG was done using the Wilcoxon signed rank test. Gender and age were included in all models analyzing patients. Menopausal status was defined as premenopausal ⬍52 years old and postmenopausal ⬎53 years old.
RESULTS
OPG Levels in Healthy Controls. Serum was available from 112 healthy volunteers and matched plasma samples drawn at the same time from 61 of these individuals. These were volunteers who had a mean age of 40⫾13.6 years and did not have any serious diseases and were not on drugs known to affect bone and calcium metabolism (Table 1). The OPG levels in sera were significantly higher than the levels in plasma (0.17 versus 0.14 ng/ml; P⬍0.0001; Fig. 1). Because only serum samples were available from patients with various oncological and rheu-matologic disorders, all additional analyses were performed on sera.
For the 112 healthy individuals (72 female and 40 male),
there was no difference in serum OPG level according to age (P⫽0.48; Fig. 2). Serum levels of OPG were higher in females than in males (0.18 versus 0.15 ng/ml; P ⫽ 0.016; Fig. 3). However, there was no difference observed between serum levels of OPG in premenopausal (n ⫽ 89) versus postmeno-pausal healthy women (n⫽20; P⫽0.96; Fig. 4).
Table 1 Demographic data on patients in this studya
Age (years) Female (n) Male (n) Controls 40⫾13.6 72 40 Solid tumors 59⫾12.4 94 50 Hematologic malignancy 55⫾15.7 51 60 Rheumatologic disorders 56⫾13 42 18 Benign hematology 62⫾12.4 16 19
aWe studied OPG levels in sera collected from patients and
con-trols at Hershey Medical Center between 1990 and 1996.
Fig. 1 Serum versus plasma OPG levels in healthy controls.
Fig. 2 Serum OPG level versus age in healthy controls.
Serum OPG Levels in Patients with Solid Tumors. Sera were available from 145 patients with a variety of solid tumors. Their mean age was 59⫾12.4 years. When adjusted for age and gender, there was no significant difference in OPG levels between patients with solid tumors and healthy volunteers (0.2 versus 0.18 ng/ml; P⬎0.05). When looked at by site of primary malignancy, elevations were observed only in patients with colorectal cancer (0.29 ng/ml; P⬍0.0001) and pancreatic cancer (0.35 ng/ml; P ⬍ 0.0001) but were lower in sarcoma patients (0.12 ng/ml; P⫽0.04; Fig. 5). When site of metastasis was analyzed (each site in different model), significant eleva-tions in serum OPG levels were observed in patients with liver metastases (0.29 ng/ml; P⬍0.0001) and soft tissue metastasis (0.21; P⫽0.013) but not in patients with bone metastases (0.19 ng/ml; P⫽0.11) or lung metastasis (Fig. 6).
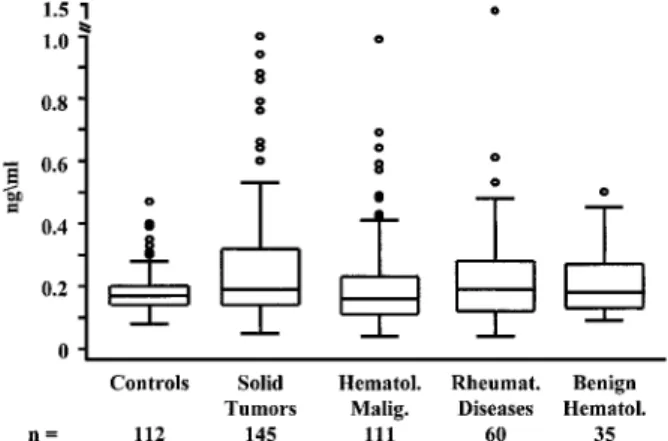
Serum OPG Levels in Patients with Hematological Dis-orders. Serum was available from 111 patients with hemato-logical malignancies and from 35 patients benign hematohemato-logical disorders. Their mean ages were 55⫾15.7 and 62⫾12.4 years, respectively. Serum OPG levels did not significantly differ between patients with malignant and benign hematological dis-orders and the healthy controls (Fig. 7). Within the
hematolog-ical malignancy group, serum levels of OPG were significantly lower in patients with multiple myeloma (n⫽34; 0.12 ng/ml; P ⫽ 0.0005) but were elevated in patients with Hodgkin’s disease (n⫽9; 0.29 ng/ml; P⫽0.0003) and Non-Hodgkin’s Lymphoma (0.24 ng/ml; P⫽0.048; Figs. 8 and 9). There was no difference in serum OPG levels between controls and pa-tients with rheumatologic disorders.
DISCUSSION
OPG is a member of the TNFR family of proteins and plays an important role in the negative regulation of osteoclastic bone resorption. Several members of the TNFR family of proteins have been detected in the serum. A naturally occurring soluble form of FAS, another member of the TNFR family, has been detected in the serum of patients with systemic lupus erythema-tosus, suggesting that the soluble FAS protein functions as an antagonist for the membrane-bound FAS ligand (14). In addition, a soluble form of TNFR generated by limited pro-teolysis inhibits the biological activity of both TNF-␣ and TNF-(15–17).
OPG is synthesized as a propeptide, of which the signal peptide is cleaved, thus generating the mature peptide (380 amino acids). OPG mRNA can be detected in many different Fig. 4 Serum OPG levels in premenopausal versus postmenopausal
healthy controls.
Fig. 5 Serum OPG levels in solid tumor patients according to site of primary malignancy.
Fig. 6 Serum OPG levels by site of metastases.
Fig. 7 Serum OPG levels in healthy controls, solid tumor patients, malignant and benign hematological patients, and rheumatic disease patients.
tissues, including lung, heart, kidney, liver, stomach, intestine, skin, brain, spinal cord, thyroid gland, and bone. In contrast to all of the other TNFR superfamily members, OPG lacks trans-membrane and cytoplasmic domains and is secreted as a soluble protein. OPG can exist in either monomeric or dimeric forms. It has been recently demonstrated that OPG can be detected in the serum, and OPG circulates mainly as a monomer (18).
We have used an ELISA assay that detects both the ho-modimeric and monomeric forms of OPG. The ELISA uses a mouse monoclonal antibody as the capture antibody and a rabbit polyclonal antibody for detection. Both antibodies were raised against intact mature recombinant human OPG. The limit of detection of OPG was 0.05 ng/ml.
We found that OPG levels in healthy controls were signif-icantly higher in sera (0.17 ng/ml) than in plasma (0.14 ng/ml). In our study, OPG values did not differ significantly by age in either serum or plasma. Serum OPG levels were higher in females than in males, but the levels in a few postmenopausal women (n⫽20) were not significantly different from premeno-pausal healthy females. Yano et al. also used an ELISA assay to measure OPG in the serum. These investigators found that there was an age-dependent increase in serum OPG in both healthy men and women. Serum OPG levels in their study ranged between 1 and 4 ng/ml. These authors noted that the increase in serum OPG concentrations appeared to accelerate after 50 – 60 years in both men and women. One possible difference in results is that the mean age of our healthy controls was 40⫾13.6 years, whereas the healthy controls in the paper by Yano et al. were 67.1⫾8.6 years. Thus, we had much fewer patients in the 60⫹ years age range. Because of younger age in the control group, we assessed the effect of age on serum OPG levels in all subjects in this study. Older patients had higher serum OPG levels than younger patients (P⬍ 0.0001), when patients and controls were analyzed together. This supports the effect of age on OPG levels, and we have included age in all our statistical models.
In our series, we found no difference in serum OPG levels between healthy controls and patients with solid tumors. Within the solid tumor patient group, there was a trend for higher levels of OPG in patients with metastatic disease compared with localized malignancy (P⫽ 0.07). Significant elevations in
se-rum OPG levels were observed only in patients with liver and soft tissue metastases but not in patients with bone or lung metastases with each site in a different model. At present, we have no explanation for the effect of liver metastases on serum OPG levels except possibly liver metastases alter the liver clearance of OPG, resulting in higher serum levels.
Within the hematological malignancy group, serum levels of OPG were significantly lower in patients with multiple my-eloma than healthy controls but were elevated in patients with Hodgkin’s disease. However, overall in both the patients with solid tumors and hematological disorders, it was quite uncom-mon for a patient to have a serum OPG level⬎1 ng/ml. Using a spleen/bone marrow stromal cell coculture system, Simonet et al. (1) have shown that the human recombinant OPG dimer inhibited osteoclast differentiation by 50% at a concentration of 1 ng/ml and completely suppressed osteoclast differentiation at concentrations of 10 –100 ng/ml. By using a monomeric and dimeric OPG preparation derived from conditioned medium of fetal pulmonary fibroblasts, Tsuda et al. (7) have reported that osteoclast differentiation was inhibited at concentrations from 1 to 40 ng/ml with a half-maximal effect at 4 – 6 ng/ml. Thus, it would appear that although some patients with malignancy have significant elevations of circulating OPG, these levels are not enough to uniformly suppress osteoclast formation. Indeed, in multiple myeloma serum, OPG levels are decreased compared with healthy controls. Thus, it is unlikely that endogenous OPG is systemically active in any of these disease conditions. Re-combinant human OPG deserves testing as a therapeutic agent in patients with osteolytic and perhaps also osteoblastic bone metastases. A possible goal is to achieve serum OPG levels of 10 –15 ng/ml that are required in transgenic mice to have anti-resorptive activity (19).
ACKNOWLEDGMENTS
We thank Eileen Kenney for her excellent help in the preparation of this manuscript.
REFERENCES
1. Simonet, W. S., Lacey, D. L., Dunstan, C. R., Kelley, M., Chang, M. S., Luthy, R., Nguyen, H. Q., Wooden, S., Bennett, L., and Boone,
Fig. 9 Serum OPG levels in patients with multiple myeloma. Fig. 8 Serum OPG levels in patients with hematological malignancies.
T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell, 89: 309 –319, 1997.
2. Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., Elliott, R., Colombero, A., Elliot, G., Scully, S., et al. Osteoprotgerin ligand is a cytokine that regulates osteoclast differenti-ation and activdifferenti-ation. Cell, 93: 165–176, 1998.
3. Kong, Y. Y, Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Cappar-elli, C., Morony, S., Olivero-dos-Santos, A. J., Van, G., Itie, A. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte develop-ment, and lymph-node organogenesis. Nature (London), 397: 315–323, 1998.
4. Yasuda H., Shima, N., Nakagawa N., Yamaguchi, K., Kinosaki, M., Mochizuki, S-I. Tomoyasu, A., Yano, K., Goto, M., Murakami, A., Tsuda, E., Morinaga, T., Higashio, K., Udagawa, N., Takahashi, N., and Suda, T. Osteoclast differentiation factor is a ligand for osteoprotegerin/ osteoclastogenesis-inhibitory factor and is identical to TRANCE/ RANKL. Proc. Natl. Acad. Sci., 95: 3597–3602, 1998.
5. Bucay, N., Sarosi, I., Dunstan, C. R., Morony, S., Tarpley, J., Capparelli, C., Scully, S., Tan, H. L., Xu, W., Lacey, D. L., Boyle, W. J., and Simonet, W. S. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev., 12: 1260 –1268, 1998.
6. Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S-I. Yano, K., Fujise, N., Sato, Y., Goto, M., Yamaguchi, K., Kuriyama, M., Kanno, T., Murakami, A., Tsuda, E., Morinaga, T., and Higashio, K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology, 39: 1329 –1337, 1998.
7. Tsuda, E., Goto, M., Mochizuki, S-I. Yano, K., Kobayashi, F., Morinaga, T., and Higashio, K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem. Biosphys. Res. Commun., 234: 137–142, 1997.
8. Tan, K. B., Harrop, J., Reddy, M., Young, P., Terrett, J., Emery, J., Moore, G., and Truneh, A. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene, 204: 35– 46, 1997.
9. Kwon, B. S., Wang, S., Udagawa, N., Haridas, V., Lee, Z. H., Kim, K. K., Oh, K-O., Greene, J., Li, Y., Su, J., Gentz, R., Aggarwai, B. B., and Ni, J. TR1, a new member of the tumor necrosis factor receptor family, induces fibroblast proliferation and inhibits osteoclastogenesis and bone resorption. FASEB J., 12: 845– 854, 1998.
10. Vidal, N. O. A., Brandstrom, H., Jonsson, K. B., and Ohlsson, C. Osteoprotegerin mRNA is expressed in primary human osteoblast-like
cells: down-regulation by glucocorticoids. J. Endocrinol., 159: 191–195, 1998.
11. Hofbauer, L. C., Lacey, D. L., Dunstan, C. R., Spelsberg, T. C., Riggs, B. L., and Khosla, S. Interleukin-1and tumor necrosis factor-␣, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone, 25: 255–259, 1999.
12. Hofbauer, L. C., Dunstan, C. R., Spelsberg, T. C., Riggs, B. L., and Khosla, S. Osteoprotogerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun., 250: 776 –781, 1998. 13. Hofbauer, L. C., Khosla, S., Dunstan, C. R., Lacey, D. L., Spels-berg, T. C., and Riggs, B. L. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. En-docrinology, 140: 4367– 4370, 1999.
14. Cascino, I., Fiucci, G., Papoff, G., and Ruberti, G. Three functional soluble forms of the human apoptosis-induced Fas molecule are pro-duced by alternative splicing. J. Immunol., 154: 2706 –2713, 1995. 15. Nophar, Y., Kemper, O., Brakebusch, C., Engelmann, H., Zwang, R., Aderka, D., Holtmann, H., and Wallch, D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J., 9: 3269 –3278, 1990.
16. Ashkenazi, A., Mardtars, S. A., Capon, D. J., Chamow, S. M., Figari, I. S., Pennica, D., Goeddel, D. V., Paladino, M. A., and Smith, D. H. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc. Natl. Acad. Sci. USA, 88: 10535– 10539, 1991.
17. Peppel, K., Crawford, D., and Beutler, B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antago-nist of TNF activity. J. Exp. Med., 174: 1483–1489, 1991.
18. Yano, K., Tsuda, E., Washida, N., Kobayashi, F., Goto, M., Harada, A., Ikeda, K., Higashio, K., and Yamada, Y. Immunological character-ization of circulatory osteoprotegerin/osteoclastogenics inhibitory fac-tor: increased serum concentration in postmenopausal women with osteoporosis. J. Bone Miner. Res., 14: 518 –527, 1999.
19. Min, H., Morony, S., Sarosi, I., Dunstan, C. R., Capparelli, C., Scully, S., Kaufman, S., Kostenuik, P., Lacey, D., Boyle, W., and Simonet, S. Osteoprotegerin reverses osteoporosis by inhibiting en-dosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med., 192: 463– 474, 2000.