ORIGINAL ARTICLE
De novo
cancer avoidance after renal
transplantation: A case
e
control study on
low-dose sirolimus combined with a
calcineurin inhibitor
*
Kuo-Hsin Chen
a,b, Chih-Yuan Lee
a, Fe-Lin Lin Wu
c,d,
Ching-Yao Yang
a, Chi-Chuan Yeh
a, Rey-Heng Hu
a,
Meng-Kun Tsai
a,*
a
Department of Surgery, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
bDepartment of Surgery, Far Eastern Memorial Hospital, New Taipei City, Taiwan c
School of Pharmacy, National Taiwan University College of Medicine, Taipei, Taiwan
dDepartment of Pharmacy, National Taiwan University Hospital, Taipei, Taiwan
Received 3 December 2014; received in revised form 6 February 2015; accepted 26 February 2015
KEYWORDS
calcineurin inhibitor; malignancy;
renal transplantation; sirolimus
Background/purpose:Full-dose sirolimus (SRL) therapy without a calcineurin inhibitor (CNI) re-duces the incidence of malignancy after renal transplantation, but with significant side ef-fects. We hypothesized thatde novotherapy with low-dose SRL combined with a CNI could still prevent cancer in renal transplant recipients.
Methods:A retrospective caseecontrol study was performed to assess the cancer incidence among renal transplant patients who had undergone surgery in our transplant centers between January 2000 and June 2012. Patients who received low-dose SRL and a CNI (SRL group, nZ189) were compared with patients receiving conventional CNI-based therapy in the same hospitals (Conventional group,nZ271).
Results:The 5-year graft and patient survival rates were comparable between the two groups. Seven patients in the SRL group and 24 patients in the Conventional group developed malig-nancies during mean follow-up periods of 68.237.5 months and 81.751.4 months, respec-tively. The cancer incidence at 5 years was significantly lower in the SRL group (1.9%), than that in the Conventional group (6.7%;pZ0.04). By multivariate analyses, SRL therapy
*An abstract containing preliminary data of this article was accepted and presented at the World Transplant Congress, San Francisco, 2014.
Conflicts of interest: The authors have no conflicts of interest relevant to this article.
* Corresponding author. Department of Surgery, National Taiwan University Hospital, Number 7, Chung-Shan South Road, Taipei 100, Taiwan.
E-mail address:mengkuntsai@ntu.edu.tw(M.-K. Tsai). http://dx.doi.org/10.1016/j.jfma.2015.02.008
0929-6646/Copyrightª2015, Elsevier Taiwan LLC & Formosan Medical Association. All rights reserved. Available online atwww.sciencedirect.com
ScienceDirect
(pZ0.04), male sex (pZ0.04), and younger age (pZ0.01) were significantly associated with a lower risk of malignancy after kidney transplantation.
Conclusion: De novotherapy with low-dose SRL combined with a CNI was associated with reduced risk of post-transplant cancer in renal transplant recipients.De novocancer preven-tion using a low-dose proliferapreven-tion signal inhibitor such as SRL could be effective for renal transplant recipients.
Copyrightª2015, Elsevier Taiwan LLC & Formosan Medical Association. All rights reserved.
Introduction
Renal transplantation is associated with an increased risk of cancer, which is probably caused by prolonged immuno-suppression due to the use of calcineurin inhibitors (CNIs).1,2Research and clinical studies have reported that sirolimus (SRL), a macrocyclic lactone inhibitor of the mammalian target of rapamycin (mTOR) signaling pathway, exerts both anticancer and immunosuppressive effects.3e6
SRL therapy, after early CNI withdrawal, reduced the inci-dence of both skin and nonskin malignancies in renal transplant patients after 5 years.7,8Furthermore, SRL pre-vented secondary skin cancer in high-risk renal transplant recipients.9,10 However, SRL-related adverse events occurred frequently, sometimes resulting in the discontin-uation of SRL treatment. For example, Campbell et al11 reported that 46.2% of patients taking full-dose SRL (trough levels > 8 ng/mL) without a CNI discontinued treatment during their follow-up. We reported recently that low-dose SRL (targeting trough levels of 4e8 ng/mL) combined with either tacrolimus (TAC) or cyclosporine (CsA) CNIs might have a reduced SRL discontinuation rate (15%).12
Although low-dose SRL and CNI combinations reduce the recurrence of hepatocellular carcinoma after liver trans-plantation, the efficacy of this drug combination has not yet been assessed thoroughly in renal transplant pa-tients.13,14 Previously, Kreis et al15 demonstrated that, compared with placebo, the combination of SRL and CsA resulted in a significantly lower 2-year incidence of skin cancer. In addition, a low incidence of malignancy (mostly skin tumors) was reported in SRL/CsA-treated renal trans-plant recipients when analyzed using the Surveillance, Epidemiology, and End Results (SEER) database of the general United States population.16 In a large, long-term study performed by Wimmer et al,17 mTOR inhibitor-based regimens did not significantly reduce the risk ofde novomalignancies after renal transplantation. By contrast, Kauffman et al5 used the Organ Procurement and Trans-plantation Network/United Network for Organ Sharing (OPTN/UNOS) database to document the association be-tween mTOR inhibitors and reduced post-transplant ma-lignancies, but did not report the timing and doses of SRL used.
A primary dose of 2 mg/d of SRL is commonly used in combination with a CNI.18,19 We previously performed several prospective trials to assess the efficacy ofde novo SRL therapy (2 mg/d, trough levels of 4e8 ng/mL) against acute rejection.12,20e22We hypothesized that low-dose SRL
and a CNI might help prevent cancer. Therefore, we
performed a caseecontrol study to assess the cancer inci-dence in long-term renal transplant patients treated with or withoutde novoSRL.
Patients and methods
Study group
A caseecontrol study was performed to assess the benefi-cial effect of low-dose SRL and a CNI on cancer prevention after renal transplantation. Data from renal transplant patients who met the criterion of our previous studies for SRL de novo therapy were collected and reviewed. We excluded: patients who had received antibody induction therapy or multiple solid organ transplants; patients who had tested positive for hepatitis B virus surface antigen or anti-hepatitis C virus antibodies; and patients with ABO incompatibility or positive lymphocytotoxicity.12,20e22
Pa-tients with<6 months of follow-up, secondary transplants, or pretransplant cancers were also excluded. The outcomes of patients who underwent renal transplantation with the combination therapy of low-dose SRL and a CNI between January 2000 and June 2012 (SRL group) were compared with outcomes of those who received conventional CNI-based regimens in the same transplant centers (Conven-tional group). The clinical and research activities reported comply with the ethical standards laid down in the Decla-ration of Helsinki and its later amendments.
Immunosuppressive regimens
The immunosuppressive regimens in the SRL group included SRL, corticosteroids, and a CNI (either CsA or TAC). SRL was administered at a loading dose of 6 mg within 48 hours after graft reperfusion, followed by a maintenance dose of 2 mg/d. The initial target trough levels of the CNIs were 100e200 ng/ mL for CsA and 4e8 ng/mL for TAC.
22
The trough levels of SRL were measured regularly and adjusted if side effects occurred. The target blood levels at 12 months were 50150 ng/mL for CsA, 36 ng/mL for TAC, and 48 ng/mL for SRL. In the Conventional group, the CNI doses were initially adjusted to the target trough levels of 200400 ng/ mL for CsA and 816 ng/mL for TAC. The target blood levels at 12 months were 100200 ng/mL for CsA and 58 ng/mL for TAC. In addition, mycophenolate mofetil or mycophenolate sodium was prescribed at an initial dose of 12 g/d or 7201440 mg/d, respectively. White blood cell counts were maintained between 4109/L and 6109/L unless intoler-ance developed or the maximum dose was reached.
Corticosteroids, including intravenous methylpredniso-lone and oral prednisomethylpredniso-lone, were administered, consistent with standard practices. The dose of prednisolone was reduced to 2.55 mg/d at 12 months and could then be discontinued if significant side effects were reported. To prevent cytomegalovirus disease, valganciclovir (450 mg/d) was administered to patients with a functioning graft for 3 months, unless the patient had leukopenia.
Statistical analyses
Intent-to-treat analyses were performed using NCSS 2008 for Windows software (Kaysville, UT, USA). Graft and pa-tient survival, and cancer incidence were estimated using the KaplaneMeier method. For patients with multiple cancers, only the first cancer was recorded for statistical analyses. All values were expressed as the meanstandard deviation. Unpaired two-tailed t tests and Fisher’s exact tests were used for normally distributed continuous vari-ables and categorical varivari-ables, respectively. Univariate analysis using the log-rank test was used to determine the statistical significance of the effects of SRLde novo ther-apy, recipient sex, CNI (CsA or TAC) therther-apy, donor type, and acute rejection on cancer incidence. Cox regression analysis was used to assess the statistical significance of recipient age at transplantation and HLA mismatches on cancer occurrence. Finally, multivariate Cox’s regression analysis was employed to examine the independent effect of each factor that exhibited statistical significance in univariate analyses.
Results
Patient demographics
Patient demographics and outcomes of the SRL and Con-ventional groups are summarized inTable 1. The mean ages of patients in the SRL (42.513.0 years) and Conventional groups (40.5 13.5 years) at transplantation were com-parable (pZ0.10). There were more female patients in the Conventional group (53.9%, 146/271) than in the SRL group (49.7%, 94/189), but the difference was statistically insig-nificant (pZ 0.39). The number of deceased-donor trans-plants (73.5%, 139/189) included in the SRL group was higher than that included in the Conventional group (62.0%, 168/271;pZ0.01). The mean number of HLA mismatches between donors and recipients was also higher in the SRL group (3.21.5 vs. 2.61.4,p<0.001). In addition, the number of patients in the SRL group who received TAC (82.0%, 155/189) was higher than the number of patients in the Conventional group who received TAC (56.1%, 152/271; p<0.001).
Transplant outcomes
The 5-year graft and patient survival rates of the two groups were comparable, although there was a lower inci-dence of acute rejection at 1 year in the SRL group than that in the Conventional group (15.3% vs. 19.2%;pZ0.32). Seven patients in the SRL group and 24 patients in the
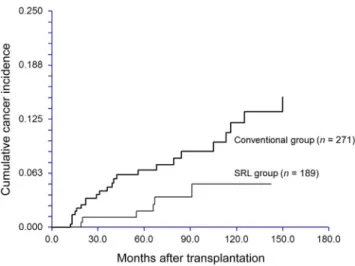
Conventional group developed malignancies during mean follow-up periods of 68.2 37.5 months and 81.7 51.4 months, respectively. During the follow-up period, 22 SRL group patients stopped taking SRL at 2 years (11.6%, 22/ 189).12The incidences ofde novocancers at 5 years post-transplantation in the SRL and Conventional groups were 1.9% and 6.7%, respectively (Fig. 1). The difference in post-transplant cancer incidence between the two groups was statistically significant (pZ0.04).
Urothelial carcinoma was the most common post-transplant malignancy, accounting for 57.1% (4/7) and 54.2% (13/24) of the pathological diagnoses in the SRL and Conventional groups, respectively. Skin cancer was rela-tively rare in our study population: only one and two cases
Table 1 Patient demographics and outcomes. Characteristics SRL group (nZ189) Conventional group (nZ271) pa Age at transplantation (y) 42.513.0 40.513.5 0.10 Sex (M:F) 95:94 125:146 0.39 Donor type (D:L) 139:50 168:103 0.01 HLA mismatches 3.21.5 2.61.4 <0.001 Initial CNI (TAC:CsA) 155:34 152:119 <0.001 1-y acute rejection 29/189
(15.3%)
52/271 (19.2%)
0.32 5-y graft survival 86.5% 84.7% 0.25 5-y patient survival 97.0% 97.5% 0.43 5-y cancer incidence 1.9% 6.7% 0.04 Follow-up (mo) 68.237.5 81.751.4 0.002 CNI Z calcineurin inhibitor; CsA Z cyclosporine; D Z deceased; F Z female; L Z living; M Z male; SRLZsirolimus; TACZtacrolimus.
aTwo-tailed Fisher’s exact test was used for categorical var-iables; two-tailed unpairedt-test was used for continuous var-iables; log-rank test was used for survival analysis.
Figure 1 KaplaneMeier estimates of the cumulative cancer incidence (intent-to-treat analysis). The sirolimus (SRL) group exhibited a significantly lower risk of malignancy than the Conventional group (pZ0.04).
of nonmelanoma skin cancer were reported in the SRL and Conventional groups, respectively. There were two cases of post-transplant lymphoproliferative disease in the Con-ventional group but none in the SRL group. Even though patients with hepatitis B and C viral infections were excluded, there was one case of hepatocellular carcinoma in the Conventional group. The pathological diagnoses of post-transplant malignancy are summarized by immuno-suppressive regimens inTable 2.
Univariate and multivariate analyses
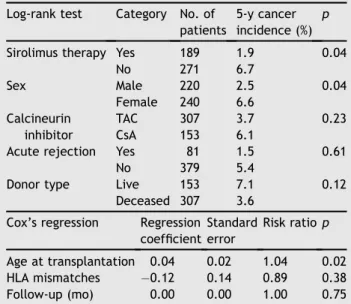
Univariate analyses were performed to identify risk factors for post-transplant de novo malignancies. Data revealed that SRLde novotherapy (pZ0.04), sex (pZ0.04), and age at transplantation (p Z 0.02) were significant prog-nostic factors. Patients in the SRL group had a significantly lower risk of post-transplant cancer than those in the Conventional group. Interestingly, male patients were found to have lower incidence of cancer after renal trans-plantation than female patients. Besides, the risk ratio of post-transplant cancer was found to be 1.038 per year of increased age in the univariate analysis. Donor type, CNI therapy, acute rejection, and follow-up duration were not significantly associated with the development of post-transplant cancer. Results of the univariate analyses are shown in Table 3. Cox’s multivariate regression analysis revealed that de novo SRL therapy (p Z 0.04), sex (p Z 0.04), and age at transplantation (p Z 0.01) were significant factors for post-transplant malignancies. De novo SRL therapy (risk ratio Z 0.38) and male sex (risk ratio Z 0.43) were independently associated with decreased risk of cancer development, whereas older age at transplantation increased the risk of malignant tumors by a ratio of 1.04 per year (Table 4).
Risk factors for urothelial carcinoma
Since urothelial carcinoma accounted for most cancer pa-thology, survival analyses were further conducted to reveal prognostic factors for post-transplant urothelial carcinoma.
By log-rank tests and Cox’s regression, female sex was identified as the only risk factor (p Z 0.03) for post-transplant urothelial carcinoma. Age at post-transplantation and SRL therapy were not significant factors for urothelial carcinoma, possibly because of inadequate patient and cancer numbers.
Discussion
Determining the minimal dosage of SRL that could effec-tively prevent post-transplant cancers is worthwhile. Pre-vious studies reported that high-dose SRL (trough levels> 8 ng/mL) without CNI was associated with an increased incidence of adverse events, which could lead to the discontinuation of SRL treatment. Nevertheless, the effects of SRL on cancer prevention were evident in patients who received SRL-based therapy initiated early post-trans-plantation.7,11This mTOR inhibitor could be regarded as an agent forde novocancer prevention, akin to ganciclovir for the prevention of cytomegalovirus, if the minimal effective dose of mTOR inhibitors could be identified. There might be an inverse relationship between the dose of mTOR inhibitor and the incidence of cancer after transplantation. Howev-er, the efficacy of SRL was difficult to assess in retrospec-tive studies that included long-term follow-up of patients who were converted to variable doses of SRL at different time points post-transplantation.23 In addition, SRL was only transiently effective, or even ineffective, in treating renal transplant recipients with severe Kaposi’s sarcoma or urothelial carcinoma.24,25De novoSRL therapy at 2 mg/d, which targeted a trough level of 4e8 ng/mL, could reduce the incidence of post-transplant malignancies in the cur-rent study, even in combination with a CNI.
The reduced cancer incidence in the current study might result from the antiproliferative effect of SRL, as well as the reduced doses of CNIs. A previous study reported that patients receiving CNI-based therapies had a significantly
Table 2 Cancer pathology grouped by immunosuppressive regimens.
Cancer pathologya Sirolimus group nZ7 Conventional group nZ24 Urothelial carcinoma 4/7 (57.1) 13/24 (54.2) Skin cancer (nonmelanotic) 1/7 (14.3) 2/24 (8.3)
PTLD 0/7 (0.0) 2/24 (8.3) Gastrointestinal carcinoma 1/7 (14.3) 1/24 (4.2) Breast cancer 1/7 (14.3) 1/24 (4.2) Hepatocellular carcinoma 0/7 (0.0) 1/24 (4.2) Gynecological cancer 0/7 (0.0) 1/24 (4.2) Others 0/7 (0.0) 3/24 (12.5)
Data are presented asn/N(%).
PTLDZpost-transplant lymphoproliferative disease.
aIn case of multiple cancers, only the first cancer was counted.
Table 3 Univariate analyses of prognostic factors for cancer incidence.
Log-rank test Category No. of patients
5-y cancer incidence (%)
p Sirolimus therapy Yes 189 1.9 0.04
No 271 6.7 Sex Male 220 2.5 0.04 Female 240 6.6 Calcineurin inhibitor TAC 307 3.7 0.23 CsA 153 6.1
Acute rejection Yes 81 1.5 0.61
No 379 5.4
Donor type Live 153 7.1 0.12
Deceased 307 3.6 Cox’s regression Regression
coefficient Standard error Risk ratiop Age at transplantation 0.04 0.02 1.04 0.02 HLA mismatches 0.12 0.14 0.89 0.38 Follow-up (mo) 0.00 0.00 1.00 0.75 CsAZcyclosporine; TACZtacrolimus.
higher risk of post-transplant cancer than those treated with azathioprine and steroids (ST).2 Campistol et al7 re-ported that immunosuppressive regimens converted from combined SRL (troughs of 515 ng/mL) with CsA and ST to high-dose SRL (troughs > 15 ng/mL) with ST reduced the rates of nonskin cancer from 9.6% to 4.0% at 5 years. However, the overall 5-year cancer incidence (1.9%) observed in our SRL group, which also included patients receiving the SRLþCsAþST regimen, was much lower than the risk of nonskin cancer in patients treated with SRLþST (4.0%) in the study by Campistol et al.7This suggests that a SRL þ CsAþ ST regimen could still be effective for pre-venting nonskin cancer.
The pathological diagnoses of post-transplant malig-nancies vary among different countries. Nonmelanoma skin cancer and lymphoproliferative disorders are preva-lent in Western, but not Asian, countries.16,26By contrast, urothelial carcinoma is relatively common among Asian renal transplant patients, especially when those with viral hepatitis are excluded.27 The underlying mechanism for this effect was suggested to be oncogenic viral infections with concurrent suppressed immune surveillance of tumor antigens.28 In addition, several nonviral risk factors, including age, sex, race, and duration of dialysis have been reported.29Therefore, we chose to exclude patients with viral hepatitis from our study population to minimize bias toward cancer occurrence, although the true inci-dence of post-transplant cancer could be underestimated. Based on the substantial evidence suggesting that SRL has antitumor properties, a primary strategy to reduce cancer-related complications after renal transplantation might bede novotherapy or early conversion to SRL (or another mTOR inhibitor), although conversion to SRL has not been proved effective in preventing recurrence of malignant cancers except for those derived from the skin.9,11
Female recipients of renal transplantation, in this study, had a higher risk of post-transplant cancer, especially urothelial carcinoma. Actually, in the general population, the incidence of bladder cancer was similar in both sexes.30 Female sex was reported to be associated with higher grade and stage of urothelial carcinoma, although the transplant outcome of female patients (as we reported recently) seemed better than that of our male patients.31e33 The
survival of female patients was lower after radical cys-tectomy for bladder urothelial carcinoma.34 While the contributing factors to worse prognosis of urothelial carci-noma in females remain to be identified, immunosuppres-sive therapy could possibly enhance the underlying oncogenic processes in female patients and uncover the sex differences in cancer biology. Future multicenter studies would determine if the prognosis of female renal transplant
recipients with urothelial carcinoma is worse than that of male patients.
The early use of SRL, which can improve renal function and graft survival, for treating renal transplant recipients is controversial.35 There was a latent predisposition to use SRL in our study patients receiving transplants from deceased donors, although donor type was not identified as a significant factor for cancer occurrence in the univariate analysis. The possibility of wound complications, delayed graft function, and interstitial pneumonitis has concerned both patients and transplant surgeons.36 In addition, SRL exerts a paradoxical stimulatory effect on innate immunity; thus, a CNI might be indispensable for suppressing acute allograft rejection.37,38Therefore, a regimen including low-dose SRL and a CNI, proposed by us and Campistol et al,39 might be an appropriate early immunosuppressive therapy for renal transplant recipients. However, it will be chal-lenging to perform long-term randomized controlled trials, even in large-volume transplant centers, to address the role of immunosuppressive agents in post-transplant malig-nancies.16As such, retrospective caseecontrol cohorts are the method of choice.
In conclusion, compared with conventional CNI-based therapy, low-dose SRL combined with a CNI was associated with reduced risk of post-transplant cancer in renal trans-plant recipients. Accordingly, we propose a concept ofde novo cancer prevention using a low-dose proliferation signal inhibitor, such as SRL, for renal transplant recipients.
References
1. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation.JAMA2006;296:2823e31.
2. Berardinelli L, Messa PG, Pozzoli E, Beretta C, Montagnino G. Malignancies in 2753 kidney recipients transplanted during a 39-year experience.Transplant Proc2009;41:1231e2. 3. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S,
Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor.Nat Med2002;8:128e35.
4. Koehl GE, Andrassy H, Guba M, Richter S, Kroemer A, Scherer MN, et al. Rapamycin protects allografts from rejec-tion while simultaneously attacking tumors in immunosup-pressed mice.Transplantation2004;77:1319e26.
5. Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin in-hibitors is associated with a reduced incidence of de novo malignancies.Transplantation2005;80:883e9.
6. Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma.Transplantation2010;89:227e31.
Table 4 Multivariate Cox’s regression analysis of the factors with statistical significance in the univariate analysis. Cox’s regression Regression coefficient Standard error Risk ratio Lower 95% CL Upper 95% CL p
Sirolimus therapy 0.96 0.46 0.38 0.16 0.95 0.04
Male sex 0.85 0.41 0.43 0.19 0.96 0.04
Age at transplantation 0.04 0.02 1.04 1.01 1.08 0.01
7.Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal trans-plantation.J Am Soc Nephrol2006;17:581e9.
8.Alberu´ J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Sirolimus CONVERT Trial Study Group: Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin-inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011;92:303e10.
9.Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer preven-tion in kidney transplantapreven-tion.N Engl J Med2012;367:329e39. 10. Salgo R, Gossmann J, Scho¨fer H, Kachel HG, Kuck J, Geiger H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients and nonmelanoma skin can-cer in a prospective randomized assessor-blinded, controlled clinical trial.Am J Transplant2010;10:1385e93.
11. Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer.Am J Transplant2012; 12:1146e56.
12. Tsai MK, Wu FL, Lai IR, Lee CY, Hu RH, Lee PH. Decreased acute rejection and improved renal allograft survival using sirolimus and low-dose calcineurin inhibitors without induction therapy. Int J Artif Organs2009;32:371e80.
13. Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl2009;15:1834e42.
14. Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl2008;14:633e8.
15. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies.Clin Transplant2004;18:446e9. 16. Kahan BD, Yakupoglu YK, Schoenberg L, Knight RJ, Katz SM,
Lai D, et al. Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation2005;80:749e58.
17. Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppressiondde novo malignancy after renal transplantation; the experience of the Transplantation Center Munich.Kidney Int2006;71:1271e8. 18. Kahan BD. Efficacy of sirolimus compared with azathioprine for
reduction of acute renal allograft rejection: a randomized multicenter study.Lancet2000;356:194e202.
19. MacDonald AS, RAPAMUNE Global Study Group. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation2001;71:271e80.
20. Tsai MK, Chueh SC, Hu RH, Lee PH. Effect of sirolimus in combination with low-dose cyclosporine and steroids on acute renal allograft rejection.J Formos Med Assoc2003;102:91e6. 21. Wu FL, Tsai MK, Chen RR, Sun SW, Huang JD, Hu RH, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy2005;25:646e53.
22.Chen KH, Tsai MK, Lai IR, Wu Lin FL, Hu RH, Lee PH. Favorable results of concomitant tacrolimus and sirolimus therapy in Taiwanese renal transplant recipients at 12 months.J Formos Med Assoc2008;107:533e9.
23.Naik MG, Heller KM, Arns W, Budde K, Diekmann F, Eitner F, et al. Proteinuria and sirolimus after renal transplantation: a retrospective analysis from a large German multicenter data-base.Clin Transplant2014;28:67e79.
24.Lebbe´ C, Euvrard S, Barrou B, Pouteil-Noble C, Garnier JL, Glotz D, et al. Sirolimus conversion for patients with post-transplant Kaposi’s sarcoma.Am J Transplant2006;6:2164e8. 25.Chung SD, Lai MK, Wang SM, Liao CH, Yu HJ, Chueh SC. Uro-thelial carcinoma in kidney transplant recipients conversion from calcineurin inhibitor to proliferation signal inhibitor?Am J Kidney Dis2008;52:630.
26.Imao T, Ichimaru N, Takahara S, Kokado Y, Okumi M, Imamura R, et al. Risk factors for malignancy in Japanese renal transplant recipients.Cancer2007;109:2109e15.
27.Wu MJ, Lian JD, Yang CR, Cheng CH, Chen CH, Lee WC, et al. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan.Am J Kidney Dis2004;43:1091e7.
28.Agraharkar ML, Cinclair RD, Kuo YF, Daller JA, Shahinian VB. Risk of malignancy with long-term immunosuppression in renal transplant recipients.Kidney Int2004;66:383e9.
29.Kauffmann HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novomalignancies in renal transplant re-cipients: the past and present.Transplant Int2006;19:607e20. 30.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality.Urol Oncol2004;22: 86e92.
31.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differ-ences in bladder cancer presentation and mortality in the US. Cancer2009;115:68e74.
32.Mungan NA, Kiemeney LA, van Dijck JAAM, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer.Urology2000;55:368e71.
33.Chen PD, Tsai MK, Lee CY, Yang CY, Hu RH, Lee PH, et al. Gender differences in renal transplant graft survival.J Formos Med Assoc2013;112:783e8.
34.Messer JC, Shariat SF, Dinne CP, Novara G, Fradet Y, Kassouf W, et al. Female gender is associated with a worse survival after radical cystectomy.Urology2014;83:863e7.
35.Flechner SM. Reviewing the evidence for de novo immuno-suppression with sirolimus.Transplant Proc2008;40:S25e8. 36.Mehrabi A, Fonouni H, Wente M, Sadeghi M, Eisenbach C,
Encke J, et al. Wound complications following kidney and liver transplantation.Clin Transplant2006;20:97e110.
37.Cravedi P, Ruggenenti P, Remuzzi G. Sirolimus for calcineurin inhibitors in organ transplantation: contra.Kidney Int2010;78: 1068e74.
38.Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open-label study of siroli-mus versus cyclosporine in primary de novo renal allograft recipients.Transplantation2013;95:1233e41.
39.Campistol JM, Cockwell P, Diekmann F, Donati D, Guirado L, Herlenius G, et al. Practical recommendations for the early use of m-TOR inhibitors (sirolimus) in renal transplantation. Transplant Int2009;22:681e7.