0099-2240/81/121103-08$02.00/0
Cellulose
Fermentation by
aRumen
Anaerobic
Fungus
in
Both the Absence and the
Presence
of Rumen Methanogens
THOMAS BAUCHOP'AND DOUGLAS 0. MOUNTFORT2t*
Applied Biochemistry Division,Department of Scientific and IndustrialResearch, PalmerstonNorth,'and
Cawthron
Institute,
Nelson,2
NewZealandReceived6May 1981/Accepted4August1981
The fermentation of cellulose by an ovine rumen anaerobic fungus in the
absence andpresence ofrumen methanogens is described. In the monoculture,
moles of productasapercentageof the moles of hexose fermentedwere:acetate, 72.7; carbon dioxide,37.6;formate,83.1;ethanol,37.4;lactate,67.0;andhydrogen, 35.3.Inthe coculture,acetatewasthe major product(134.7%), and carbon dioxide
increased (88.7%). Lactate and ethanol production decreased to 2.9 and 19%,
respectively, little formatewasdetected (1%),and hydrogen didnotaccumulate.
Substantial amounts ofmethanewereproducedinthe coculture (58.7%).Studies
with
[2-14C]acetate
indicated that acetatewas nota precursorof methane. Thedemonstration ofcellulose fermentationbyafungusextends therangeof known
rumen organisms capable of participating in cellulose digestion and provides further supportforarole of anaerobic fungi inrumenfiber digestion. The effect
of themethanogensonthepatternoffermentation is interpretedas ashift in flow
of electrons away from electron sink products to methane via hydrogen. The
study providesa new example of intermicrobialhydrogen transfer and thefirst
demonstration ofhydrogenformationbyafungus.
Cellulose
digestion
is a common propertyof manyfungi, but untilrecently
virtually all
fungi werefound to require oxygen for growth. As aresult
arole
forfungi
inrumenfermentation hadnotbeen considered
previously. However,
obli-gately anaerobic fungi
wererecently
discoveredin the rumen
(18),
and thefinding
thatlarge
populations
were present attached to fibrousplant fragments (3) led
to thesuggestion
that thereshould be re-examination of
accepted
con-cepts of fiber
digestion.
Thesubsequent
dem-onstrationof
cellulosedigestion by
rumenan-aerobic
fungi (4,
5,19)
wasadditional
evidence forarole infiberdigestion. However,
cellulosefermentation
hasnotbeendescribed and forms partof the presentinvestigation.
Ourdiscovery
thatrumen
methanogens
formedastablecocul-ture with the rumen
fungus
led totheinvesti-gation
of theireffects
on the fermentation ofcellulose.
Recent studies
involving
intermicrobialH2
transferto
methanogens (8,
16)
have indicated that incomplex
ecosystems,suchasthe rumen, microbial interactions may haveprofound
ef-fects on thepattern
of fermentationproducts.
Coculture of
H2-utilizing
methanogens
withru-men
carbohydrate-fermenting
bacteriapro-tPresent address:DepartmentofDairy Science, Univer-sity ofIllinois, Urbana,IL 61801.
duced a shift in the flow of electrons generated in
glycolysis,
awayfrom the formation ofelec-tron sink
products (lactate
and succinate) to methane via hydrogen. In each case this was accompanied byanincrease in acetate.The pat-ternof fermentation products
inthe cocultures
could not account for the
regeneration
of oxi-dizedpyridine
nucleotiderequired
inglycolysis.
It has beenassumed,
therefore,
that thecarbo-hydrate fermenters
wereable
toproduce H2
directly from reduced pyridine nucleotide. How-ever,
calculations
have shown that the reaction reduced nicotinamide adenine dinucleotide(NADH)
+H+-- oxidized nicotinamide adeninedinucleotide
(NAD+)
+ H2 isthermodynami-cally
unfavorable atpartial
pressures ofH2
above
10'
atm (0.1kPa) (26). Thusonly
under conditions of lowpartial
pressures of H2 main-tained by the presence ofmethanogens
could theproduction
ofH2
occurfrom reducedpyri-dine nucleotide. The coculture studies
(8, 16)
provided useful insight into the intermicrobial H2 transfer mechanisms that couldoccurinthe
rumenwhere the
steady-state
hydrogen
concen-tration is low
(12),
and theresultswereconsist-entwith
Hungate's hypothesis (11)
thatmeth-anogenesis
mayprovide
an alternative electron sinktotheproduction
of reducedproducts
from pyruvate.1103
on January 29, 2021 by guest
http://aem.asm.org/
BAUCHOP MOUNTFORT
The present results show that therumen
an-aerobic fungi can be grouped physiologically with the fewspecies of bacteria capableof
par-ticipating in the primary fermentation ofplant fiber in therumen.Cocultureof thefungus with methanogens resultedin an enhancedcellulose fermentationrateandan elevated acetate
pro-duction, an indication of the importance of in-termicrobial H2 transfer in the rumen
fermen-tation.
MATERIALS AND METHODS
Isolationprocedures.Sheeprumencontents were
strainedthroughcheesecloth, anda5% inoculumwas
transferred into7ml of standard medium(3)
contain-ing an antibiotic mixture(benzylpenicillin, 103 IU/ml;
streptomycin sulfate, 102 IU/ml). The culture was
incubated at 38°C, and growth of the fungus was
monitored by direct examination with a dissecting
microscope(Olympus). After three subculturesasmall
inoculum fromanactively growingfungal culture was
transferred to anaerobic broth (standard medium
without agar).Asingle thalluswasthen removedfrom
the culturewith a1-ml syringe witha 1.5-in (ca. 3.8
cm) 22-gauge needle and washed by transferring it
fourtimes through successive tubesto remove
con-taminating zoospores. The thallus was then
trans-ferredtostandard mediumandincubated.Microscopic
examination of theresultant culture indicatedasingle
fungus, based onthemorphology of the thallus and
zoospores.However, further examination by light and
ultraviolet epifluorescence microscopy revealed that
thefunguswasin coculture withmethanogenic
bac-teria. The coculturewasmaintained and kept to
in-oculateexperimental media.Pureculture of the
fun-gus, free from methanogens, was obtained by the
treatmentof thecoculture withchloramphenicol (30
,ug/ml) andsubsequent passage through three
subcul-tures.Thefungus was depositedinthe culture
collec-tion at the Department of Scientific and Industrial
Research, Palmerston North, New Zealand and
des-ignatedasstrainPN1.Taxonomy of the organism has
yettobecompleted.
Culture conditions.Culturesweregrown
anaero-bicallyat 38°C in tubes ofmediumprepared bythe
method ofHungate (13). The gas phase was 70% N2
and30%CO2.
Salt solutions (11) wereused in medium
prepara-tion. Solution A contained (wt/vol): KH2PO4, 0.3%;
NaCl,0.6%; (NH4)2SO4, 0.3%; CaCl, 0.03%;andMgSO4,
0.03%.SolutionB contained0.3%(wt/vol)K2HPO4. Themediumused was modified from Bauchop (3)
and hadthefollowingcomposition:solution A, 165 ml;
solutionB,165ml;cell-freeovinerumen fluid, 170ml;
distilled water, 500 ml; NaHCO3, 5 g; yeast extract
(BBLMicrobiology Systems,Cockeysville,Md.), 1g;
peptone(DifcoLaboratories,Detroit,Mich.), 1g;
cys-teine-HCl,0.2g;Na2S *9H2O,0.1g;and resazurin, 0.001
g.Medium (12 ml) wasdispensed anaerobicallyinto
anaerobic culture tubes(140 by 16mm;A.H.Thomas)
eachcontaining100mgofWhatmanno.1filterpaper
strips (about 15 by2 mm). Tubes were sealed with
butylrubberstoppers,and themediumwassterilized
byautoclaving. Sodium sulfidewasautoclaved
sepa-rately and added to culture media when cooled to
room temperature. The final pH of the culture
me-diumwas6.9.
Culturetechniques. The techniques described by
Hungate (13) were adapted for the maintenance and
subculturing of the fungus alone and
fungus-plus-methanogen cultures.Experimental mediawere
inoc-ulatedby transferring 1/20 the volume ofa6-day-old
cultureoffungus aloneorfungus plus methanogens.
For the former inoculation, this was equivalent to
approximately2.5 x 103colony-forming units (CFU)
offungus per ml ofinoculating culture, and for the
latter, itwasequivalentto 3 x103 CFU of thefungus
and1.4 x107CFU ofmethanogens per ml of inoculum.
Colony-forming units of thefungus inmono- or
cocul-ture weredetermined after4days of incubationinroll
tubes inoculated with 5 x 10-2 to 5 x 10-4 ml of
culture. Colony-forming units of the methanogens
were determined after 21 days of incubation in roll
tubes inoculated with 5 x 10-5 to 5 x 10-7 ml of
coculture. Glucose (0.1%; wt/vol) was used as the
growth substrate for the fungus and H2-CO2 (80:20
[vol/vol]inthe gasphase) for themethanogensinthe
roll tubes.
For studiesonthepurity of thecultures,theywere
roll-tubed in media described above, but with 0.2%
glucosereplacing celluloseasthesubstrate, orin AC
medium. The agarconcentrationwas2%(wt/vol).
Quantitative analysis of substrate and
prod-ucts in culture media. Cellulosewasdetermined by
the methodof Updegraff(21). Whatmanno. 1 filter
paper contained 99.7% cellulose. Soluble
carbohy-drates weredeterminedbyamodification of the
an-throne method (1), in whichglucosewasusedasthe
standard.
Forthe determination ofmethane, hydrogen, and
CO2 (headspace), 1-ml gas samples were removed from
culturetubes and analyzedon aFisher-Hamilton gas
partitioner equipped withathermalconductivity
de-tector.For methane andCO2 analyses, gaswas
frac-tionated at 25°C on dual columns; column 1 was
packed with2-ethylhexylsebacateonColumnpak, and
column 2waspacked with molecular sieve 13x.
He-lium was the carrier gas. For hydrogen analysis, gas
was fractionated on column 2, and argon was the
carrier gas.Gaseswerequantified by comparison with
thepeakheights of known standards.
Total CO2 in culture tubes wasdetermined
gravi-metrically asBaCO3. Culture medium inthe sealed
culture tubes wasacidified topH2byinjection of 0.4
ml of50% (vol/vol) H2SO4 to ensure that all of the
CO2wasliberated from the medium. The stopper was
piercedwith twosyringe needles (19 gauge), and CO2
was flushedfromthe culture tubes byslowly bubbling
nitrogen through the medium for 1 h; CO2 in the
effluent gaswastrappedin 3 MNaOH(10ml). Alkali
was then quantitatively removed from the trap and
madeupto 20ml withC02-freewater. Trapped CO2
wasprecipitatedasBaCO3 by the addition of 1 ml of
12% (wt/vol) BaCl2. The precipitate wascollectedby
centrifugation at 5,000 xg for 10min at 0 to 2°C,
suspended in 10 ml ofC02-free ethanol-H20 (50:50
[vol/vol]),andcentrifugedas before.TheBaCO3pellet
on January 29, 2021 by guest
http://aem.asm.org/
was suspended in 2 ml ofethanol-H20 mixture and
collected on tared glass fiber filter disks (Millipore
Corp., Bedford, Mass.). The disks plus BaCO3 were
dried toconstant weight in a desiccator at 60°C. In
culture tubes where gas samples were removed for
analysis by gas chromatography, CO2 determined in
these samples was added to the CO2 determined as
BaCO3 to give totalCO2.
Formeasurement of volatile fatty acids and ethanol,
cells were removed by centrifugation at 5,000 x g for
20 min at 0 to20C. A 2-ml portion of thesupernatant
was acidified with 0.1 ml of 6 NHCI, and the volatile
fatty acids (except formic acid) and ethanol were
de-terminedby gas chromatography. A glass column (2
mby4mm)packed with 3% (wt) Carbowax20M-0.5%
(wt)H3PO4on60/80 meshCarbopak B (Supelco, Inc.,
Bellefonte, Pa.) wasused in aTracor 560 gas
chro-matographequipped with a flame ionization detector.
The column temperature was 160°C, and nitrogen was
the carrier gas.Fatty acids and ethanol were identified
andquantitated by comparison of the retention times
andpeak height with those of known standards.
For-mate was determined as described previously (10).
Lactatewasconvertedtomethyl-lactate and
deter-mined bygas-liquid chromatography. A 2-ml sample
ofculture mediumsupernatantorlactic acidstandard,
1mlof 0.2%(wt/vol) malonic acid as internal standard,
1mlof 50%(vol/vol) sulfuric acid, and2ml ofdistilled
water were added to the chamber ofa liquid-liquid
extraction apparatus.Carboxylic acidswereextracted
with diethylether for6h, and the extract was then
reducedto avolume of-1mlby rotaryevaporation.
Anhydrous sodium sulfatewasadded(approximately
20mg), andmethylationwasachievedbythe addition
of diazomethane in ether. The methyl esters were
separatedat 1200Con aglass column (2mby4mm)
packed with 10%diethylene glycol succinate on
100-to 120-meshGasChromQ, connectedto aflame
ioni-zation detector.Nitrogenwasthe carrier gas.
Methyl-lactate insampleswasdetermined from the ratios of
the peak heights of dimethylmalonate standards to
those ofmethyl-lactate samplesand standards.
Radioactive incubations with
[2-'4C]acetate
andanalyses.To determineacetate as aprecursorof
methane during cellulose fermentation by the
cocul-tures, 2,ICi of
[2-14C]acetate
(56 mCi/mmol) wasaddedtoculture tubes atzeroincubation time.
Fer-mentation wasterminated at variousstages of
incu-bationbetween0and 168 hby theinjection of1mlof
3NNaOH into culturemedia,and theCO2inthe gas
phasewasabsorbed.Aftercompleteabsorption ofC02,
asascertainedby gaschromatographicprocedures, the
resulting vacuum in the tubes was relieved by the
injection of nitrogen with a glass piston syringe to
allow quantitative gas sampling. Tubes were
main-tained at2°C,and the total volume ofgaswas
deter-mined by recording the excess gas forced into the
syringe. For analysis of radioactive methane, 1-ml
volumesof gas were injectedinto scintillation vials
sealedwithbutyl septum stoppers and countedin 20
ml oftoluene-based scintillantasdescribedbyZehnder
etal.(27).To ensurethatamaximal amountof
meth-anewasdissolved,vials wereagitated vigorously.
As-say ofthegasatmosphereabove thescintillationfluid
revealed that 80 ± 3% of the methane added was
absorbed and therefore counted. Corrections were
madefor methane not absorbed and for quenching.
Counting efficiencywas 86±2%.
Foranalysis of radioactive acetate, media were
cen-trifuged at 5,000 x g for 15 min at 0 to 2°C. The
proceduresfor the isolation ofradioactiveacetatefrom
thesupernatant and the determination of radioactivity
were based onpreviously described methods (17).
Quantitativemeasurementsof methane and acetate
forspecific radioactivity determinations were asinthe
preceding section.
Microscopy. Examination of the monoculture of
the fungus and coculture with methanogens was by
light microscopy and scanning electron microscopy.
Details ofsample preparation for scanning electron
microscopy were as previously described (3). Because
of their characteristic fluorescence at 420 nm, the
methanogenswere alsoexamined byultraviolet,
epi-fluorescence microscopy.
Chemicals. All chemicalswereobtained from
com-mercial sources andwereof reagentgrade. The
radi-oisotope,
[2-14C]acetate
(specific activity, 56 mCi/mmol), wasobtained from the RadiochemicalCenter,
Amersham,England.
RESULTS
Description of anaerobic fungus.
The an-aerobicfungus strain
PN1 appearsclosely
re-lated
totherumen phycomycete Neocallimastix
frontalis
(18). Thephylogenetic
position
of thesefungi
remains to bedetermined, although
the modeofzoosporogenesis
from the monocentric, rhizoid-bearingthallus
iscomparable
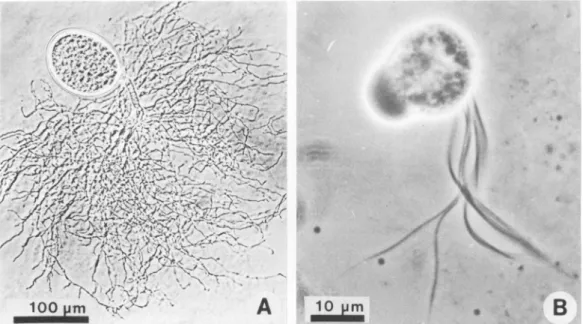
withthat of manychytrids. Figure
1A shows athallus
of thefungus consisting
of asporangium
with ahighly
branched rhizoid. The sizeof the
spo-rangiavaried depending
on the stageof
devel-opment
of the
thalli,
but inglucose medium (3)
it was up to 200 ,um in
length
and 140 t,m in width. Withcellulose
assubstrate,
sporangia
up to180t,m
long
wereobtained.Figure
1B shows asingle
zoospore. The zoospores were oval to beanshaped
(length,
14 to 18,im;
width,
12 to14,um)
and contained between9and12flagella.
The body of the zoospore in
Fig.
1B isslightly
out
of
focus,
which makes it appearlarger.
In oldercultures
zoosporescharacteristically
roundoff.
Colonies in roll tubes inoculated with
actively
fermenting
fungal
cultureprobably
developed
from zoospores, since these were numerous in
the culture fluid
(>103
per ml of6-day-old
cul-ture), whereas freesporangia
(detached
from solidcellulose substrate)
were eitherabsent,
orin low
numbers,
andcellulose
was notusually
transferred in the inoculum.
Stability
offungus-methanogen
cocul-ture. The
fungus-methanogen
cocultureiso-lated was stable and was maintained in
liquid
42,
on January 29, 2021 by guest
http://aem.asm.org/
mX
',-A
p
___
B
FIG. 1. (A) Light micrograph of rumen anaerobic fungus. Thallusshowing
sporangium
and highlybranched rhizoid. (B)Multiflagellatedzoosporeoftherumenanaerobicfungus.
medium
containing cellulose
forover 1yearby routine transfer every6days.
During this time therewas noloss ofcellulolytic
ormethanogenic
activity.
Methanogenic population
inthe
cocul-ture. Colonies inroll tubes with
H2-CO2
(80:20
in the gas
phase),
inoculated with 5 x10-5
to 5x
i0'
mlof stablefungus-methanogen coculture
(6
days
old)
wereof
onetypeandbecame visible
after1week of
incubation.
Surface colonies
weretranslucent, circular
withentire
margins,
off-whitetoyellow in
color,
and reachedadiameter
of
approximately
2 mmafter21days
of incuba-tion. Deepcolonies
werelenticular
anddidnotincrease in size
beyond
1mmindiameter.
Whencolonies
wererepeatedly picked
andexamined
by ultraviolet
epifluorescence microscopy,
the bacteria exhibited characteristicblue-green
flu-orescence of
methanogens
at420nm.The cells
weregram
positive,
nonmotile oval rodsorcocci,usually
0.7yminwidth,
withsomecellsreaching1 ,um, and 0.8 to 1.6
,um
inlength,
occurringpredominantly in pairs but occasionally in chains. These bacteriacan
clearly
beidentified in thescanning
electronmicrograph
of the co-culture (Fig. 2). Isolated colonies grewwell
on H2-CO2 (80:20 in the gas phase), slowly on for-mate, butnotonacetate,although acetate stim-ulatedgrowth
onH2-CO2.
Thecolony-type,
mor-phology,
and substrates utilized indicated that the methanogens were most likely Methano-brevibacterruminantium.
Also present in the mixed culture but not shown in Fig. 2 were largeFIG. 2. Scanning electron micrographof coculture
of anaerobicfunguswithrumenmethanogens
show-ingmethanogens andfungal hyphaegrownonpaper.
cocci (about 1.5
Am
insize),
occurringsingly,
in pairs, and inclusters,
andexhibiting
blue-green fluorescence at 420 nm. We wereunsuccessful inisolating
these organisms, but their morpholog-ical features andfluorescence
at 420 nm sug-gested they weremethanogens
resembling Methanococcus. Direct counts of these orga-nisms byepifluorescence microscopy
indicated thatthey
accountedfor<1% of the totalmeth-anogenic population.
The organism resembling M. ruminantium designated as Methanobrevi-bactersp. strainRAl,
accountedfor >99% of theon January 29, 2021 by guest
http://aem.asm.org/
H2 FUNCTION IN FUNGUS-METHANOGEN INTERACTION 1107
total
methanogens inthe
coculture,
consistentwith the
presenceof only
onecolony
type intheroll
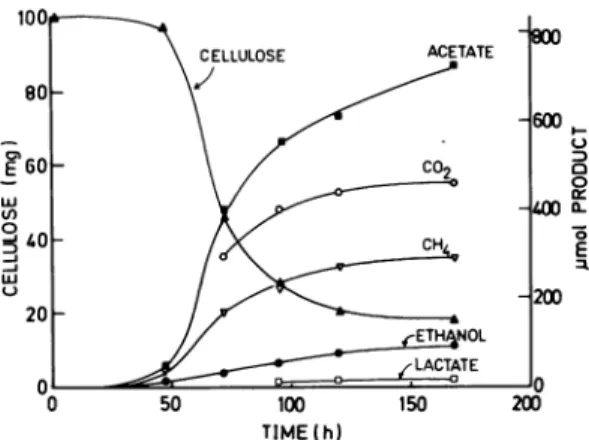
tubes.Fermentation of cellulose. The fermenta-tion ofcellulose by the rumen anaerobic fungus alone
resulted
in theformation of six products, acetate,lactate, formate,
ethanol, C02, and H2 (Fig. 3). The lag time for lactate production was longer than for the other products.Fermentation terminated about 300 h after inoculation. In the coculture, the major products were acetate, car-bon dioxide, and methane (Fig. 4). Ethanol and lactate production decreased. Hydrogen was not detected duringfermentation (detection limit, 5 x10-4
atm), and formate occurred in only trace amounts. The coculture fermentation termi-natedafter
about 200 hof incubation. For both the mono- andcocultures, the pHof
the medium decreased from 6.9 at thebeginning
of incuba-tion to 6.0 atthe end of fermentation. The rate and extentof cellulose degradation
weregreater in thecoculture
than in the monoculture(Fig. 3 and 4). In thecoculture, after 100 h, more than 70% of the initial cellulose had beendegraded,
and in the monoculture, less than 10% had been
degraded.
At theendof
fermentation, 82%
of the initialcellulose
hadbeendegraded
inthe cocul-ture, and 53% had beendegraded
inthemono-culture.
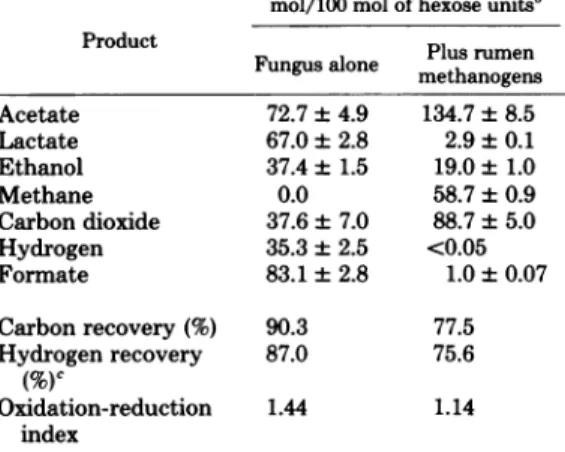
Table
1 comparesthe fermentationproducts
of cellulose
in the mono-and cocultures.
Inthe
O 100 200 300
TIME(h)
FIG. 3. Fermentationofcellulosebyrumen
anaer-obic fungus. Cellulose is expressed as total milli-grams, andproducts areexpressed astotal
micro-moles in thegrowth medium (12 ml). Media were
inoculated with 0.6 mlof fungalculture (2.5 x 103
CFUper ml). Values for acetate, formate, ethanol,
and lactate werecorrectedforamounts transferred
in the inoculum, andwith theexception ofethanol and lactate, the amountspresent in uninoculated media.
TIME (h)
FIG. 4. Fermentation of cellulose by cocultureof
anaerobicfungus withrumenmethanogens. Cellulose
is expressed as totalmilligrams, andproducts are
expressedastotal micromoles in thegrowth medium
(12ml).Mediawereinoculatedwith 0.6 mlof
fungus-methanogencoculture (3x103CFUoffungusperml;
1.4 x 107 CFU of methanogensper ml). Valuesfor
acetatewerecorrectedfor theamountspresent in the
inoculumand inuninoculatedmedia.
coculture, carbon dioxide and acetate increased
by
51 and 62mol/100
ofhexose,
respectively. Lactate and ethanol decreased by 64 and 18.4 mol/100 mol of hexose, respectively. The de-creaseinhydrogen and formate in the coculture reflected their utilization in methane production. To determine whether acetate, the only otherlikely
methaneprecursor, contributed to meth-ane in the coculture, fermentation was carried out in the presence of[2-14C]acetate.
Data onspecific radioactivity
ratios(Table
2) indicated that acetate contributed less than 1% of the methane formed duringcellulose
fermentation and was therefore not a significant precursor. Thus theamountofH2required
for the forma-tion of methane(58.7
mol ofCH4/100
mol ofhexose),
including
H2produced
via formate cleavage, would be 235mol
(4H2+CO2
-- CH4+
2H20).
Other
possible products
ofcellulose
fermen-tationby
thefungus,
propionate, butyrate, or succinatewere notdetected(<0.1
mol/100
mol of hexosefermented),
and there was noaccu-mulation
ofsoluble carbohydrates
inthemono-or
cocultures
during
the fermentations.Recombined culture
experiments.
Iso-lated colonies ofMethanobrevibacter
sp. strainRA1,
were transferred to culture tubes inocu-lated with thefungus alone. Aftercompletion
of growth(approximately
170h),
asascertainedby
methane production, the recombined cultures
wereinoculated into fresh media. The inoculum contained
approximately
3 x 103CFU offungus
per ml and 1.2 x
107
CFU ofmethanogen
perVOL. 42,1981
on January 29, 2021 by guest
http://aem.asm.org/
1108
ml. At the completion ofmethanogenesis (168 h), products in the culturemediawereanalyzed, and unutilized cellulose was determined. The formationofproductswassimilar to the stable coculture (Table 1), except that ethanol
de-creased to9.6mol/100 mol of hexose. CO2was not determined. Formate and hydrogen were
absent, and lactate was low (3.5 mol/mol of hexose). Mean values foracetate and methane
were 138.5 and 59 mol/100 mol ofhexose,
re-spectively. At the end offermentation, 87% of
the initialcellulosehadbeendegraded. DISCUSSION
The gut anaerobicfungipossessseveral
prop-erties not found in otherfungithatare
undoubt-edly related to their obligately anaerobic
life-style, perhaps itself the most unusual of their
properties. Much of the interest in thesefungi
TABLE 1. Fermentationof cellulose by anaerobic
fungus in the absence andpresenceofrumen
H2-formate-utilizingmethanogensa
mol/100mol of hexoseunits'
Product Plusrumen
Fungusalone methanogens
Acetate 72.7±4.9 134.7±8.5 Lactate 67.0±2.8 2.9±0.1 Ethanol 37.4 ± 1.5 19.0±1.0 Methane 0.0 58.7±0.9 Carbondioxide 37.6±7.0 88.7±5.0 Hydrogen 35.3±2.5 <0.05 Formate 83.1±2.8 1.0±0.07 Carbonrecovery(%) 90.3 77.5 Hydrogenrecovery 87.0 75.6 (%) Oxidation-reduction 1.44 1.14 index
a Determinedatthecompletionoffermentation,as
ascertained bynofurtherincrease inhydrogen
(mon-oculture) ormethane (coculture). Thepercentagesof
cellulose degradedinthemono-andcoculture were 53
and82%, respectively.
bValuesare meansof duplicatedeterminations, and
where theerror ispresented, +1standarddeviation.
cDetermined according to Barker (2).
sofarhas concerned the extent of their role in
ruminantdigestion.But since thesearetheonly fungiknowntobeobligate anaerobes,the com-plete spectrum of theirproperties is of
consid-erableintrinsic interesttomicrobiologists. Thedegradationofcellulosebythe monocul-ture providesthe firstexampleofa mixed-acid
typeoffermentation infungi,and theproducts
weresimilartothose thatareproduced by
coli-form bacteria during anaerobic growthon
glu-cose.Hydrogenformation has not beenreported previously infungi, andtheunderlying
mecha-nism of hydrogenformationneedstobestudied
in detail. Inaddition, thepathwaysforlactate,
acetate, and ethanol alsorequire studyto
deter-mine whetherornottheyaresimilar to known pathways for the formation of these products.
All of the fungal fermentation products are
known to be formedbyrumenbacteriaalso,and
in therumentheymaybeutilizedbyeither the
hostanimal or further fermented by other
ru-menmicrobes.
The present work confirms reports (4, 5, 19)
of cellulose digestion by the rumen anaerobic
fungiand thus extends the knownrange of
cel-lulolyticmicrobescapableofparticipatinginthis
primary fermentative process central to rumi-nant digestion. Glucose did not accumulate in
the medium during growth on cellulose,which
may indicate that the cellulase is associated
closely with the fungal hyphae and is not
re-leased into the medium. Thisagreeswith results
of microscopic examination of strips of paper undergoing fermentation, which showed that
digestion occurred only in the areas in close proximity to fungal rhizoid (4). Until recently the major cellulolytic microbes found in the
rumen havebeen anaerobic bacteria, and they
havelongbeenaccepted asthe main agents of
cellulosedigestion.The close association offungi
with fibrous plant fragments suggested that
fungihadarole in fiberdigestionintherumen (3). Theextentofthis role required
demonstra-tion of the appropriate enzymatic activities, as
wellasdetermination ofthemassoffungal
veg-etative tissues within the tissues of digested
plant fragments.The firstof theserequirements,
TABLE 2. Comparison of specificradioactivities ofmethane with [2-
4C]acetate
during the fermentation of cellulosebycocultureof anaerobic fungus with rumen H2-formate-utilizingmethanogensaIncubation Acetate CH4
14CH4
(dpm)
[2-'4C]acetate Sp act of CH4 Sp act of acetate Sp act ratio time(h) (pmol) (smol() (dpm) (dpm/,umol)(dpm//imol)
CH4/acetate0 10.8 -b 4.30 x 10 - 3.9 x 105
-72 395 178 1.37 x 104 3.72 x106 76.9 9.1 x103 8.4 x 10-3
168 537 335 1.83x 104 3.37 x 106 54.6 6.3 x 103 8.7 x10-3
aTubes contained12mlofmedium with 100 mg of Whatman no. 1filter paper strips as substrate (cellulose).
A
2-,Ci
amountof[2-'4C]acetate(56mCi/mmol) was added at zero time. Results are mean values of duplicatetubes.
b-,Not done.
on January 29, 2021 by guest
http://aem.asm.org/
H2 FUNCTION FUNGUS-METHANOGEN INTERACTION the ability to ferment cellulose, has now been
demonstrated. It may be important also that
fungi,
by nature of their mode of growth involv-inghyphal extension, possess the ability to pen-etratedeeply into tissuesnormally inaccessible to bacteria, and this suggests a special role for anaerobicfungi in rumen fiber digestion.The
coculture
consisting of an anaerobic fun-gusand
methanogenic bacteria presumably re-sulted from the insensitivity of the methanogensto the antibiotics used in the isolation of the fungus. However, in the rumen it seems likely that the methanogens would be metabolically associated with the anaerobic fungi as well as other H2-producing organisms. The coculture interaction provides the first example of H2
transfer
between a fungus and a bacterium. Pre-viousstudies on H2 transfer have involvedinter-bacterial
systems (7, 8, 15, 16, 20, 23), although the association of methanogenic bacteria with rumen ciliates suggests the possibility of H2transfer
between protozoa and bacteria (22). Production of hydrogen is the key to theco-metabolism
of the fungus with methanogens, resulting inadecrease inelectron sink products,lactate
and ethanol. To explain this decrease, it is necessary topostulate
the presence of a hy-drogenase whichcatalyzes the production ofhy-drogen
from reduced pyridine nucleotide at lowpartial
pressureof
the gas (26). Thus the meth-anogens,by
removing H2 and maintaining lowpartial
pressures,would facilitate the production of H2 from reducedpyridine nucleotide by thefungus. Reducing
equivalents
whichwould oth-erwise beused
in the formationof lactate
andethanol would
bediverted
to methane via hy-drogen in the coculture. The proportion ofmeth-ane
expected
from theelectron shift via reduced pyridine nucleotide may be calculated by assum-ingthat theonly
other source of hydrogen was from reactionsleading
to the formation ofethanol
andacetateand that thiswasequivalent
tothesumof these
products
(153.7mol/100
molof hexose).
Theassumption
wasbasedonmon-oculture data
(Table
1), from which thesumof formate andhydrogen
wasalmost
equal
tothesumofacetateand
ethanol), suggesting
acom-mon
intermediate
for the formation of the2-carbon
products, perhaps
acetyl
coenzyme A. Thus, out of thetotal
hydrogen required
for methaneproduction
(235 mol for 58.7 mol ofCH,),
about 35% would beexpected
tobepro-vided from reduced
pyridine
nucleotide from electron shift.The carbon and
hydrogen
recoveries in the coculture were less than in the monoculture. Onepossible explanation
is that one or moreproducts
from thefungus
were assimilated into cell biomass of themethanogens.
Previousstud-ies have shown that both
CO2
and acetate may contribute tocell
carbonof methanogens (6, 9,24,
25) and that as much as 60% of thecell
carbon in M.
ruminantium
maybederived from acetate (6). Theoxidation-reduction
index for bothculture fermentations was higher than thetheoretical
value of 1.0. The deficiency inre-duced products
mayhavebeen due
tothefor-mation
ofcells
withanoxidation-reduction
statemorereduced than that of cellulose (14). In the
coculture,
cellulose degradation
com-menced
earlier,
the rate wasfaster,
and thequantity digested
was greaterthan in themon-oculture
(82versus53%).
These effectsmight
beexplained
by increasedgrowth resulting
from higher energyyields
via the "acetate"path-way.Inaddition,
coculture
withthemethanogen
resulted in the
removal
ofanumber of thefungal
fermentation products,
some of whichmight
have
been
inhibitory
togrowth
of thefungus.
Mostof the
cellulose degradation
inthecocul-ture
occurred
in theperiod
between 50and
100 h;perhaps application of
this systemtobiocon-version processes,
including cellulase
produc-tion, maywarrantfuture
investigation.
Inthe rumen many aspectsof
cellulose
diges-tion are
still inadequately understood,
and the presentwork nowraisesquestions
onaccepted
concepts. The
demonstration
of cellulosefer-mentation is further support forarole of
anaer-obic
fungi
infiber
digestion
in the rumen and extends the known range ofcellulose-fermenting
microbes there. The
knowledge
that similarfungi
arepresentin theforegut
andhindgut
ina
wide
rangeof
herbivorousanimals
(4) likewise
raises the
question
of the extentof therole
of thesefungi
infiberdegradation.
Thediscovery
of
afungus-methanogen
combination instable
coculture and the resultant
altered fermentation
pattern
obtained
bring additional information
tounderstanding
some of thecomplexities
of therumen
fermentation.ACKNOWLEDGMENTS
WethankRodAsher andRayWillsfortheir expert tech-nical assistance.
LITERATURE CITED
1. Bailey,R.W. 1958. The reaction of pentoses with
an-throne.Biochem.J. 68:669-672.
2. Barker,H. A.1944.On thefermentationofsomedibasic C4acidsbyAerobacteraerogenes.K.Akad. Wet. Am-sterdamProc. 39:674-683.
3. Bauchop,T. 1979. Rumen anaerobicfungiofcattleand sheep.Appl.Environ.Microbiol.38:148-158. 4. Bauchop,T. 1979. The rumenanaerobicfungi:colonizers
ofplantfiber. Ann.Rech.Vet.10:246-248.
5. Bauchop,T. 1980.Scanningelectronmicroscopyin the studyof themicrobialdigestionofplant fragmentsin the gut, p.305-32'5.In D. C.Ellwood,J. N.Hedger,M. J. Latham, J. M.Lynch,and J. H. Slater(ed.), Contem-porarymicrobialecology.AcademicPress,London.
42,1981
on January 29, 2021 by guest
http://aem.asm.org/
6. Bryant, M. P.,S. F. Tzeng,I.M.Robinson,and A. E. Joyner. 1971.Nutrient requirementsof methanogenic bacteria,p. 23-40. In F. G. Pohland (ed.), Anaerobic biologicaltreatmentprocesses.AdvancesinChemistry series165. American ChemistrySociety, Washington, D.C.
7. Bryant, M. P.,E. A. Wolin, M.J.Wolin, and R. S. Wolfe.1967.Methanobacillus omelianskii,a symbiotic association oftwospecies of bacteria.Arch. Microbiol. 59:20-31.
8. Chen,M., and M. J.Wolin.1977. Influence of methane production by Methanobacteriumruminantium on the fermentation ofglucose and lactate by Selenomonas ruminantium. Appl. Environ. Microbiol.34:756-759. 9. Fuchs, G., E. Stupperich, andR. K.Thauer. 1978.
Acetateassimilation and synthesis of alanine,aspartate andglutamatein Methanobacterium thermoautotroph-icum. Arch.Microbiol.117:61-66.
10.Howlett,M.R., D.0. Mountfort,K. W. Turner, and A. M. Roberton.1976. Metabolism and growth yields inBacteroides ruminicola strain B,4. Appl. Environ. Microbiol.32:274-283.
11. Hungate, R. E. 1966. The rumen and its microbes. Aca-demicPress, Inc., New York.
12.Hungate, R. E. 1967. Hydrogenas an intermediate in rumenfermentation.Arch. Mikrobiol. 59:158-164. 13. Hungate, R. E. 1969. A roll-tube method for the
cultiva-tion ofstrictanaerobes,p. 117-132. In J. R. Norris and D. W. Ribbons (ed.), Methods in microbiology. Aca-demicPress, Inc., London.
14. Hungate, R. E. 1975. The rumen microbial ecosystem. Annu.Rev.Ecol.Syst.6:39-66.
15. lannotti, E. L., D. Kafkewitz,M. J. Wolin, and M. P. Bryant. 1973. Glucosefermentationproducts of Rum-inicoccusalbusgrown in continuous culture with Vibrio succinogenes:changes caused by interspecies transfer ofH2. J. Bacteriol.114:1231-1240.
16. Latham,M. J., and M. J. Wolin. 1977. Fermentation of celluloseby Ruminicoccus flavefaciensin the presence
and absence ofMethanobacterium ruminantium. Appl. Environ. Microbiol. 34:297-301.
17. Mountfort,D.O.,and R. A. Asher. 1978.Changes in proportionsof acetateandcarbondioxide usedas meth-aneprecursorsduring the anaerobicdigestion ofbovine waste.Appl.Environ. Microbiol. 35:648-654. 18. Orpin,C. G. 1975. Studies on the rumenflagellate
Neo-callimastixfrontalis.J.Gen.Microbiol. 91:249-262. 19. Orpin, C. G., and A. J. Letcher. 1979. Utilization of
cellulose, starch, xylan, and other hemicelluloses for growth by the rumen phycomycete, Neocallimastix frontalis.Curr. Microbiol. 3:121-124.
20. Scheifinger,C.C.,B.Linehan,and M. J. Wolin. 1975. H2 production by Selenomonas ruminantiumin the presenceofmethanogenic bacteria. Appl.Microbiol. 29: 480-483.
21. Updegraff,D. M. 1969.Semimicro determination of cel-lulose in biological materials. Anal. Biochem. 32:420-424.
22.Vogels, G.D.,W.F.Hoppe,andC.K. Stumm. 1980. Association ofmethanogenicbacteria with rumen cil-iates. Appl.Environ.Microbiol.40:608-612.
23.Weimer,P.J., and J.G. Zeikus.1977.Fermentation of cellulose andcellobioseby Clostridium thermocellum in the absence and presence of Methanobacterium ther-moautotrophicum. Appl. Environ. Microbiol. 33:289-297.
24. Weimer, P. J., and J. G. Zeikus. 1978. One carbon metabolisminmethanogenicbacteria.Cellular charac-terization and growth of Methanosarcina barkeri. Arch.Microbiol.119:49-51.
25. Weimer,P.J., and J.G. Zeikus.1978.Acetate metab-olism in Methanosarcina barkeri. Arch. Microbiol. 119: 175-182.
26. Wolin,M. J. 1974.Metabolicinteractions among intes-tinalmicroorganisms.Am.J.Clin.Nutr. 27:1320-1328. 27. Zehnder, A. J. B.,B.Huser, and T. D. Brock.1979. Measuring radioactivemethane with theliquid scintil-lationcounter.Appl.Environ.Microbiol.37:897-899.