Emerging therapies for the treatment of relapsed or
refractory multiple myeloma
Meletios A. Dimopoulos1, Jesus F. San-Miguel2, Kenneth C. Anderson3
1Department of Clinical Therapeutics, University of Athens School of Medicine, Athens, Greece;2University Hospital of Salamanca, Salamanca, Spain;3Dana-Farber Cancer Institute, Boston, MA, USA
Multiple myeloma (MM) is the second most common hematologic malignancy and was responsible for an estimated 21 000 deaths in the European Union in 2008 and more than 10 000 deaths in the United States in 2009 (1, 2). Newly diagnosed MM is responsive to treatment with combinations of melphalan, prednisone, dexametha-sone, doxorubicin, immunomodulatory drugs (IMiDs; such as thalidomide and lenalidomide), and proteasome inhibitors (PIs, such as bortezomib) (3) or autologous stem cell transplant following high-dose chemotherapy in appropriate patients (4, 5). However, most patients even-tually relapse or become refractory to treatment, owing in part to the changing biology of the tumor and develop-ment of aggressive, drug-resistant phenotypes within the tumor. Although some agents used as initial therapy (including thalidomide, lenalidomide, and bortezomib) have also shown activity and improved outcomes in patients with relapsed or refractory MM (6–11), these responses are often of limited duration (12–14). Thus, there is an urgent unmet need to develop targeted agents that provide durable disease control and symptomatic relief in patients with MM that has relapsed or is refrac-tory to currently approved agents.
There are three distinct patient populations within the relapsed⁄refractory MM setting: patients who are relapsed but not refractory to treatment, patients with primary refractory disease, and patients who are relapsed and refractory (15). Historically, the definition of relapsed vs. refractory disease was based on sensitivity to the vincristine, doxorubicin, and dexamethasone regimen, but the introduction of bortezomib, thalidomide, and lenalidomide has outdated this distinction. A more relevant definition of relapse is the presence of clinically active disease in patients who have received one or more prior therapies. Similarly, it has been suggested that refractory MM be defined as either progressive disease (PD) or stable disease (SD) while on prior therapy or PD within 3 months of the last dose of prior therapy. Patients with relapsed and refractory disease would be those who had achieved at least a minor response (MR) before disease progression within 60 d of the last treat-ment (15).
This review is focused on the current management of patients with relapsed or refractory MM who have expe-rienced disease progression within 60 d of the most recent treatment, although some of the studies reviewed Abstract
Encouraging progress has been made in the treatment of patients with relapsed⁄refractory multiple mye-loma (MM). The rapidly evolving understanding of key pathways responsible for tumor growth and survival has led to the development of novel agents (including immunomodulatory drugs, proteasome inhibitors, histone deacetylase inhibitors, and other targeted agents) with the potential to provide significant improve-ments in response and survival, and influence treatment guidelines. This review summarizes recent advances in understanding of the biology of relapsed⁄refractory MM and clinical trials with novel targeted agents that are currently under investigation for patients with this disease.
Key wordsmultiple myeloma; immunomodulatory drug; proteasome inhibitor; Akt inhibitor; histone deacetylase inhibitor
CorrespondenceMeletios A. Dimopoulos, MD, Department of Clinical Therapeutics, University of Athens School of Medicine, Alexandra Hospital, 80 Vas. Sofias, Athens 11528, Greece. Tel: +(30210) 3381541; Fax: + (30210) 3381511; e-mail: mdimop@med.uoa.gr
Accepted for publication 10 October 2010 doi:10.1111/j.1600-0609.2010.01542.x R E V I E W A R T I C L E
may have used the older definitions (15). The aims of this review are to discuss the biology of the advanced stages of the disease, examine the current therapeutic options for patients, and review clinical data on currently approved and emerging treatment options for patients who have relapsed or become refractory to treatment.
Biology of relapsed/refractory multiple myeloma
Multiple myeloma is characterized by the accumulation of clonally identical plasma cells in the bone marrow that appear to develop from post-germinal center B cells (16). Current criteria for the diagnosis of MM have been sum-marized elsewhere (17).
The growth, survival, adhesion, migration, and apop-totic resistance of MM cells are mediated by a large number of cytokines and adhesion molecules found in the bone marrow and tumor microenvironment (18). Very late antigen 4 and intercellular adhesion molecule 1 are important for the adhesion of MM cells to extracellu-lar matrix bone marrow stromal cells (BMSCs) (19, 20). Interleukin-6 (IL-6), IL-21, tumor necrosis factor-a
(TNF-a), insulin-like growth factor 1, vascular endothe-lial growth factor (VEGF), and stromal cell-derived fac-tor 1-a have been shown to mediate MM cell survival, growth, and⁄or resistance to apoptosis (18).
In addition to clonal expansion of myeloma cells, the complex interplay of soluble factors in the bone marrow microenvironment and various receptor-mediated signal-ing pathways on BMSCs, osteoblasts⁄osteoclasts, and myeloma cells [e.g., the receptor activator of nuclear fac-tor-kappa B (NF-jB)⁄osteoprotegerin system and NF-jB pathway] mediates the development of destructive bone disease and potentially life-threatening hypercalcemia (21, 22). Surrogate cytokine markers of time-to-event outcomes have been investigated in relapsed⁄refractory MM and may have prognostic potential. For example, low baseline levels of VEGF were shown to be an inde-pendent prognostic factor for reduced response and shorter progression-free survival (PFS) in patients treated with thalidomide (23).
Different somatic genetic abnormalities reflect the complex biology and pathogenesis of MM and have prognostic value in patients with newly diagnosed MM. Chromosomal translocations such as t(4;14) and the 17p13 deletion (del17p13; associated with low expression of TP53 gene) are associated with early relapse in newly diagnosed patients treated with high-dose therapy (24). The presence of t(4;14) and deletion of chromosome 13 (del13) have been associated with a significantly lower likelihood of response (defined as a >90% reduction in M-protein concentration) to up-front thalidomide plus dexamethasone in newly diagnosed MM patients (25).
Similarly, the combination of lenalidomide and dexa-methasone was not able to overcome the adverse prog-nostic effects of del(13) or t(4;14) in patients with relapsed⁄refractory MM (26).
The influence of cytogenetics has also been examined in patients with relapsed⁄refractory MM, with varying results. As an example, bortezomib in combination with doxorubicin and dexamethasone showed comparable activity in relapsed⁄refractory patients with or without del13q (27). In contrast, relapsed⁄refractory MM patients treated with lenalidomide plus dexamethasone exhibited comparable time to progression (TTP) and overall survival (OS) regardless of del13q or t(4;14) sta-tus, whereas patients with del17p13 experienced worse time-to-event outcomes (28). Similarly, the presence of a non-hyperdiploid karyotype, other poor-risk cytogenetic abnormalities [i.e., presence of del13q, del17p, add1q21, t(4;14), or t(14;16)], and thalidomide-refractory disease were associated with reduced responses [less than a par-tial response (PR)] to treatment with lenalidomide and dexamethasone alone or in combination with bortezomib (29). These findings suggest that the presence of high-risk karyotypic abnormalities may define subsets of patients more likely to benefit from targeted therapies. Further-more, new agents that improve PFS or OS in high-risk patients are of particular interest in the treatment of relapsed⁄refractory MM.
Current treatment options for relapsed/ refractory multiple myeloma
Several factors should be considered in the selection of appropriate treatment options for patients with relapsed⁄refractory MM, including response to prior therapies, type of relapse (e.g., aggressive), and individ-ual patient characteristics (e.g., comorbidities, life expec-tancy, and quality of life) (30). Currently, multiple targeted agents, such as IMiDs and bortezomib, are approved for the treatment of relapsed⁄refractory MM.
Immunomodulatory drugs
Before the advent of immunomodulatory drugs, few effective treatment options were available for patients with relapsed⁄refractory MM. As an example, vincristine in combination with doxorubicin and dexamethasone (VAD) was associated with overall response rates (ORR) ranging from 25% to 61% in patients with relapsed⁄refractory MM.(31–34) Although the VAD regi-men was an improveregi-ment over high-dose melphalan (35, 36), the clinical benefits were limited, with response dura-tion and OS £12 months (32–34). Thus, the development of novel targeted agents with tumor-specific mechanisms of action is an important advance in the treatment of
relapsed⁄refractory MM, as it has led to a significant improvement in 5-yr survival (increasing from approxi-mately 29% in 1990–1992 to 35% in 2002–2004, P< 0.001) and 10-yr survival rates (increasing from approximately 11–17% during the same time periods, P< 0.001) (37).
Thalidomide and lenalidomide
Immunomodulatory drugs target similar pathways – including inhibition of cytokine expression (e.g., IL-6, TNF-a) by BMSCs and inhibition of angiogenesis – that contribute to decreased growth of MM cells (38). For example, thalidomide stimulates T lymphocytes with IL-2 and interferon-crelease and subsequent NK cell activa-tion, leading to the destruction of myeloma cells (38).
A review of the literature shows that treatment of relapsed⁄refractory MM with thalidomide (alone or in combination with dexamethasone) is associated with ORR ranging from 25% to 65% (39). When combined with bortezomib, melphalan, and prednisone or dexa-methasone, thalidomide produced ORR ranging from 55% to 67% (40–42). However, peripheral neuropathy occurred in 6–16% of relapsed⁄refractory MM patients who received thalidomide alone or in combination with high-dose chemotherapy and was the primary cause of thalidomide dose reduction (39). Moreover, an increased incidence of thromboembolic events was observed, par-ticularly when thalidomide was given in combination with dexamethasone and doxorubicin (39). Consequently, the thalidomide analog lenalidomide was developed in an effort to reduce the toxicity associated with thalidomide, while maintaining or improving its efficacy.
Studies have shown that combined treatment of relapsed⁄refractory MM with lenalidomide and dexa-methasone was associated with an ORR [complete response (CR)⁄PR] of approximately 60%, significantly improved TTP, and significantly longer OS, regardless of previous exposure to thalidomide (8, 9). Although low rates of grade ‡3 peripheral neuropathy were observed (<2% of patients), venous thromboembolic events were reported in approximately 10–15% of patients receiving lenalidomide plus dexamethasone (8, 9).
More recently, lenalidomide has shown clinical activity in combination with bortezomib and dexamethasone in patients with relapsed⁄refractory MM. In a phase II study in 64 patients (77% had previously received thalid-omide and 55% had received bortezomib therapy), the combination of lenalidomide (15 mg on days 1–14), bort-ezomib (1.0 mg⁄m2, days 1, 4, 8, and 11), and dexameth-asone (40 mg⁄20 mg, cycles 1–4⁄5–8, days of⁄after bortezomib dosing) produced an objective response (21% CR⁄near CR, 68% at least PR, 84% at least MR) in 62 evaluable patients, irrespective of high-risk disease fea-tures and prior therapies (43). The median duration of
response was 24 wk. Adverse events (AEs) were generally manageable (primarily grade 1–2 myelosuppression); two patients developed deep vein thrombosis while receiving aspirin prophylaxis, two patients had grade 3 atrial fibril-lation, and one patient experienced grade 3 peripheral neuropathy.
Other recent clinical studies in patients with relapsed⁄refractory MM suggest that the ORR to lena-lidomide in combination with targeted agents (e.g., bev-acizumab, dacetuzumab, and dasatinib) may vary widely depending on the patient population and molecular drug target involved (44–47).
Proteasome inhibitors
In MM, it is thought that the ubiquitin proteasome system may affect tumor growth and progression via proteolysis of key proteins, including NF-jB signaling pathways; proapoptotic caspases; and various cytokines involved in the regulation of tumor cell growth, apopto-sis reapopto-sistance, and angiogeneapopto-sis (48).
Bortezomib
The reversible PI bortezomib affects expression of a num-ber of proteins involved in cell cycle arrest and apoptosis (e.g., NF-jB, caspase-9) (49). In patients with relapsed⁄ refractory MM, bortezomib alone significantly improved TTP (6.2 vs. 3.5 month; hazard ratio, 0.55; P< 0.001) and the response rate (CR + PR, 38% vs. 18%; P< 0.001) compared with high-dose dexamethasone (50).
Alternating combination regimens have been explored in an effort to improve the response to bortezomib-based salvage therapy. In a study in 20 patients with relapsed⁄refractory MM (30% had previously received bortezomib; 5% had received previous IMiD therapy), 28-d cycles of bortezomib (1.3 mg⁄m2, days 1, 4, 8, and 11) in combination with melphalan (9 mg⁄m2, days 1–4), prednisone (60 mg⁄m2, days 1–4), and doxorubicin (con-ventional, 40 mg⁄m2 on day 1; liposomal, 30 mg⁄m2 on day 1) were alternated with 28-d cycles of thalidomide (200 mg daily, days 1–28) in combination with cyclo-phosphamide (50 mg daily, days 1–28) and dexametha-sone (40 mg daily, days 1–4). This approach resulted in an ORR of 95% (immunofixation-negative CR, 42%; near CR, 16%; PR, 47%) in nine evaluable patients, including CR in three of seven patients (42%) with high-risk cytogenetic abnormalities [e.g., t(4;14) or delRB] (51). The use of alternating combination therapy regi-mens was associated with manageable toxicities, includ-ing grade ‡3 thrombocytopenia (30%), neutropenia (30%), and infection (16%); grade 1–2 peripheral neu-ropathy occurred in three patients (15%) (51).
The synergistic effects of combined treatment with bortezomib and pegylated liposomal doxorubicin (PLD;
a novel formulation of doxorubicin with improved car-diac safety) (52) have been evaluated in patients with relapsed⁄refractory MM. In a phase III study in bortezo-mib-naive patients (none had progressed on anthracy-cline-based therapy), 21-d cycles of bortezomib (1.3 mg⁄m2, days 1, 4, 8, and 11) in combination with PLD (30 mg⁄m2 on day 4) produced a significantly greater quality of response [i.e., CR + very good partial response (VGPR) rate] (27% vs. 19%;P= 0.0157), TTP (9.3 vs. 6.5 month; hazard ratio, 1.82; P= 0.000004), duration of response (10.2 vs. 7.0 month; P= 0.0008), and 15-month survival (76% vs. 65%; P= 0.03) com-pared with bortezomib alone (11).
Several subgroup analyses have also been conducted in this study population to determine whether patient-related factors influence the response to treatment. These analyses have shown that the significantly longer TTP seen with the bortezomib-PLD regimen (relative to bortezomib alone) is consistent, even in relapsed⁄ refrac-tory MM patients with prior IMiD exposure, prior stem cell transplant, and poor prognostic factors (e.g., serum beta-2 microglobulin ‡5.5 mg⁄mL, refractory disease) (53–55). Moreover, the bortezomib-PLD regimen is associated with significant improvements in TTP in both elderly (276 vs. 205 d; hazard ratio, 1.82; P= 0.0056) and younger patients (295 vs. 190 d; hazard ratio, 1.75; P= 0.0008) (56). Importantly, combined treatment with bortezomib and PLD did not increase the inci-dence of grade ‡3 cardiac events, thromboembolic events, or peripheral neuropathy compared with bort-ezomib alone (11, 54). Further investigations should establish the clinical benefits of bortezomib-based com-binations with liposomal doxorubicin in elderly and high-risk MM patients.
Investigational options for relapsed/refractory multiple myeloma
Although currently available agents can provide clinical benefit in relapsed MM, not all patients will respond, and even those who do respond will ultimately relapse or become refractory to salvage therapy. Consequently, sev-eral new agents from a range of therapeutic classes are being examined in the relapsed⁄refractory setting. Spe-cific agents in development for the treatment of bortezo-mib- or lenalidomide-resistant MM include new IMiDs (e.g., pomalidomide), second-generation PIs (e.g., car-filzomib, NPI-0052), the signal transduction modulator perifosine, monoclonal antibody therapy (e.g., elot-uzumab), and histone deacetylase (HDAC) inhibitors (e.g., panobinostat, romidepsin, and vorinostat). Although the goal with all of these newer agents is to improve patient outcomes, the rationale for use in MM varies with the drug class.
Immunomodulatory drugs
Pomalidomide
Pomalidomide, an IMiD derived from thalidomide, has demonstrated greater activity in vitro (e.g., inhibition of osteoclast formation, cell cycle arrest) than thalidomide (57, 58). In a phase I⁄II study, pomalidomide (2–5 mg daily on days 1–21 of each 28-d cycle) demonstrated a 38% ORR and up to 46% SD when administered alone or in combination with dexamethasone in 32 patients with relapsed⁄refractory MM (59). In a phase II study in 60 relapsed MM patients (62% had received prior thalid-omide or lenalidthalid-omide treatment), the combination of pomalidomide (2 mg daily during each 28-d cycle) and dexamethasone (40 mg daily, days 1, 8, 15, and 22) resulted in an ORR of 63% (5% CR, 28% VGPR, and 30% PR), including confirmed responses in 74% of patients classified as high risk (60). The primary toxicity was grade‡3 myelosuppression; grade 3 neuropathy and a thromboembolic event were each reported in a single patient. Taken together, these studies suggest that poma-lidomide may overcome resistance to the IMiDs cur-rently used as initial or second-line therapy, with a lower incidence of neurotoxic and thromboembolic events. Preliminary results from recent clinical studies of pomalidomide are summarized in Table 1.
Proteasome inhibitors
The rationale for developing new PIs is similar to the rationale for developing new IMiDs: potential improve-ments in efficacy and⁄or tolerability and potentially incomplete cross-resistance within the drug class.
Carfilzomib
In patients who have become resistant to bortezomib, the use of a new PI with a different chemical backbone could overcome this resistance. Carfilzomib is a second-generation PI that is structurally similar to epoxomicin. Unlike bortezomib, which has a reversible effect, carfilzo-mib irreversibly targets the same proteasomal subunit (20S chymotrypsin-like b5 subunit) and has shown activ-ity (e.g., caspase activation, inhibition of proliferation) against bortezomib-resistant MM cell lines, as well as cells from MM patients with clinical evidence of bortezo-mib resistance (61).
In a phase I study, 19 patients who had relapsed fol-lowing or became refractory to previous bortezomib and IMiD therapy received carfilzomib 15–27 mg⁄m2on days 1, 2, 8, 9, 15, and 16 of each 28-d cycle. Treatment with carfilzomib resulted in an ORR of approximately 17%, with 33% of patients achieving an MR or better. No treatment-related or newly emergent peripheral neuropa-thy was reported in response to carfilzomib (62). Two
Table 1 Targeted agents in clinical investigation for the treatment of relapsed⁄refractory multiple myeloma Agent Author N⁄n Dosing regimen Confirmed responses, % Time-to-event
outcome Most common toxicities, %
Pomalidomide Lacy (116) 34
28-d cycles
POM 2 mg daily, days 1–28 DEX 40 mg, days 1, 8, 15, 22
ORR, 26 (all PR) NR Grade 3–4
Neutropenia, 21; anemia, 12; thrombocytopenia, 9; fatigue, 9; non-infectious pneumonitis, 3;
hyperglycemia, 3; edema, 3; skin rash, 3; no TEEs observed
Richardson (71) 28-d cycles
POM 2–5 mg, days 1–21 POM 2–5 mg, days 1–21 DEX 40 mg, weekly (after 4 cycles for lack of response or PD)
POM alone: ORR‡MR, 38 POM + DEX: ORR‡MR, 38 DOR, 11 wk TTP, 8.3 wk DOR, 14.2 wk TTP, 20 wk Grade 3–4 Neutropenia, thrombocytopenia Carfilzomib Niesvizky (117) 32⁄20 28-d cycles CFZ 15–27 mg⁄m2, days 1, 2, 8, 9, 15, 16 LEN 10–27 mg, days 1–21 DEX 40 mg, days 1, 8, 15, 22 (monthly after cycle 5)
CR⁄VGPR⁄PR, 55 NR Grade 3–4 Thrombocytopenia 15; anemia 15; neutropenia 8 Siegel (118) 35⁄33 28-d cycles CFZ 20 mg⁄m2, days 1, 2, 8, 9, 15, 16 CR⁄PR, 18 NR Grade 3–4 Anemia, 14; neutropenia, 11; peripheral neuropathy, 3 Wang (119) 57⁄51 28-d cycles CFZ 20 mg⁄m2, days 1, 2, 8, 9, 15, 16 CR⁄VGPR⁄PR, 45 NR Grade 3–4 Thrombocytopenia, 9; fatigue, 9; neutropenia, 7; lymphopenia, 7; anemia, 5; pneumonia, 5; hyperglycemia, 5 NPI-0052 Richardson (66) 27 28-d cycles NPI 0.025–0.7 mg⁄m2, days 1, 8, 15 NR 1 unconfirmed PR (71%flM-protein after 3 cycles) 8 SD NR DLTs observed (grade 3 fatigue, mental status change and loss of balance, 1 patient each at highest dose) Perifosine Richardson (59) 84⁄73 21-d cycles PER 50 mg daily BTZ 1.3 mg⁄m2, days 1, 4, 8, 11 DEX 20 mg, day of and day after BTZ for PD ORR‡PR, 38 CR⁄PR, 20 TTP, 6.4 mo OS, 22.5 mo Grade 3–4 Thrombocytopenia; neutropenia; anemia; hyponatremia; diarrhea (‡5% each) Tanespimycin Richardson (87) 72 21-d cycles TSP 100–340 mg⁄m2, days 1, 4, 8, 11 BTZ 0.7–1.3 mg⁄m2, days 1, 4, 8, 11 ORR‡MR: BTZ-naive (n= 21), 48 BTZ-pretreated (n= 23), 22 BTZ-refractory (n= 23), 13 DOR, 12 mo Grade 3–4 thrombocytopenia, 25; neutropenia, 3 Badros (86) 22 21-d cycles TSP 50–340 mg⁄m2, days 1, 4, 8, 11 BTZ 1.3 mg⁄m2, days 1, 4, 8, 11 VGPR⁄PR⁄MR, 14 NR Grade 3–4 Thrombocytopenia, 27; neutropenia, 18; peripheral neuropathy 5 Panobinostat Berenson (99) 15⁄12 28-d cycles PAN 20 mg, days 1, 3, 5, 8, 10 MLP 0.05 mg⁄kg, days 1, 3, 5 CR⁄PR, 33 NR Grade 3–4 Neutropenia; thrombocytopenia San Miguel (101) 29⁄28 21-d cycles
PAN 10–30 mg, 3 times weekly BTZ 1.3 mg⁄m2, days 1,4, 8, 11
CR⁄PR, 50 NR Grade 3–4
Thrombocytopenia; neutropenia; anemia; pneumonia; fatigue; significant QTcdid not occur
ongoing phase II trials are investigating the efficacy, safety, and tolerability of carfilzomib as monotherapy in patients with relapsed⁄refractory MM and prior treat-ment with bortezomib and thalidomide or lenalidomide (63, 64). Preliminary data from ongoing clinical studies are summarized in Table 1.
NPI-0052
Like carfilzomib, the non-peptide-based inhibitor NPI-0052 also targets all three proteasome units (i.e., the caspase-, chymotrypsin-, and trypsin-like subunits) and irreversibly inhibits the 20S proteasome (65). In a phase I study, NPI-0052 (0.025–0.075 mg⁄m2, days 1, 8, and 15 of a 28-d cycle) exhibited more potent protea-some inhibitory activity than bortezomib, with no reports of peripheral neuropathy or myelosuppression in patients (N= 27) with relapsed⁄refractory MM (Table 1) (66).
Inhibitors of signal transduction and cell adhesion
Although conventional and targeted agents have dramat-ically improved response rates, MM remains incurable. As noted earlier, the interactions of MM cells within the bone marrow microenvironment are complex and depend on a number of cell–ligand and cell–cell interactions that
activate signal transduction processes controlling cell migration, growth, and survival. Signal transduction modulators affect a variety of cellular processes, includ-ing cell growth, differentiation, and death, makinclud-ing them rational targets for new therapies.
Perifosine
Perifosine is thought to target cell membranes and indi-rectly affect the phosphatidylinositol 3-kinase⁄Akt path-way, which is a critical regulator of cell survival and cell growth and may underlie the pathogenesis of resistance to conventional agents (e.g., dexamethasone, doxorubi-cin) in MM (67, 68).
In a phase I dose-escalation study in 32 heavily pre-treated patients (94% received prior dexamethasone, 83% prior thalidomide, and 47% prior bortezomib ther-apy), treatment with perifosine (50 or 100 mg daily dur-ing a 28-d cycle) in combination with lenalidomide (15 or 25 mg, days 1–21) and dexamethasone (20 mg, days 1–4, 9–12, and 17–20 for 4 cycles; days 1–4 thereafter) resulted in a 50% ORR (PR or better) in evaluable patients (n= 30) (69). Patients who achieved a PR or better exhibited a longer median TTP (31 vs. 23 wk in all evaluable patients). The most common grade 3–5 AEs were neutropenia, hypophosphatemia, thrombocytopenia, anemia, and fatigue (69).
Table 1 (Continued) Agent Author N⁄n Dosing regimen Confirmed responses, % Time-to-event
outcome Most common toxicities, %
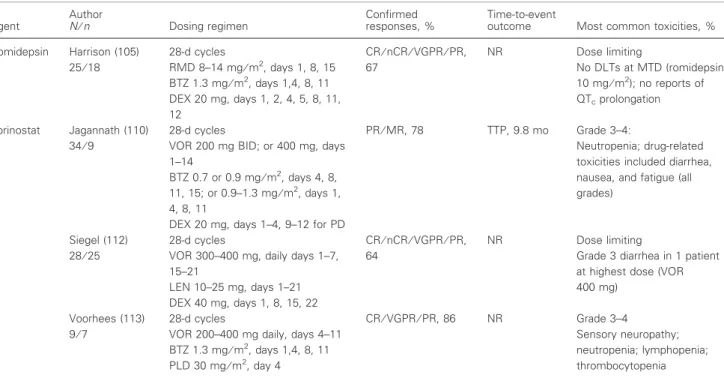
Romidepsin Harrison (105) 25⁄18 28-d cycles RMD 8–14 mg⁄m2, days 1, 8, 15 BTZ 1.3 mg⁄m2, days 1,4, 8, 11 DEX 20 mg, days 1, 2, 4, 5, 8, 11, 12 CR⁄nCR⁄VGPR⁄PR, 67 NR Dose limiting No DLTs at MTD (romidepsin 10 mg⁄m2); no reports of QTcprolongation Vorinostat Jagannath (110) 34⁄9 28-d cycles
VOR 200 mg BID; or 400 mg, days 1–14
BTZ 0.7 or 0.9 mg⁄m2, days 4, 8, 11, 15; or 0.9–1.3 mg⁄m2, days 1, 4, 8, 11
DEX 20 mg, days 1–4, 9–12 for PD
PR⁄MR, 78 TTP, 9.8 mo Grade 3–4:
Neutropenia; drug-related toxicities included diarrhea, nausea, and fatigue (all grades)
Siegel (112) 28⁄25
28-d cycles
VOR 300–400 mg, daily days 1–7, 15–21 LEN 10–25 mg, days 1–21 DEX 40 mg, days 1, 8, 15, 22 CR⁄nCR⁄VGPR⁄PR, 64 NR Dose limiting
Grade 3 diarrhea in 1 patient at highest dose (VOR 400 mg)
Voorhees (113) 9⁄7
28-d cycles
VOR 200–400 mg daily, days 4–11 BTZ 1.3 mg⁄m2, days 1,4, 8, 11 PLD 30 mg⁄m2, day 4 CR⁄VGPR⁄PR, 86 NR Grade 3–4 Sensory neuropathy; neutropenia; lymphopenia; thrombocytopenia
BID, twice daily; BTZ, bortezomib; CFZ, carfilzomib; CR, complete response; DEX, dexamethasone; DLT, dose-limiting toxicity; DOR, duration of response; LEN, lenalidomide; MLP, melphalan; MR, minimal response; MTD, maximum tolerated dose; nCR, near complete response; NPI, NPI-0052; NR, not reported; ORR, overall response rate; OS, overall survival; PAN, panobinostat; PER, perifosine; PD, progressive disease; PLD, pegylated liposomal doxorubicin; POM, pomalidomide; PR, partial response; QTc, corrected QT interval; RMD, romidepsin; SD, stable disease; TEE, thromboembolic events; TSP, tanespimycin; TTP, time to progression; VGPR, very good partial response; VOR, vorinostat.
In a phase II study in 64 patients with relapsed or relapsed⁄refractory MM (95% received prior dexametha-sone, 89% prior thalidomide, 73% prior bortezomib, and 30% prior lenalidomide), perifosine alone (150 mg daily for a 21-d cycle) showed modest clinical activity, producing best responses [according to European Blood and Marrow Transplant (EBMT) criteria] of MR (n= 1) and SD (n= 22).(70) However, when adminis-tered in combination with dexamethasone 20 mg twice weekly to patients with PD, perifosine showed greater clinical activity (38% PR + MR) in 12 of 31 evaluable patients; an additional 15 patients (47%) achieved SD (70). The most common grade 3–5 AEs were nausea, vomiting, fatigue, anemia, increased creatinine, and reversible neutropenia. Peripheral neuropathy and deep vein thrombosis were not reported (70).
Perifosine has also been evaluated in combination with bortezomib and dexamethasone in 84 relapsed⁄refractory MM patients previously treated with bortezomib (71). As shown in Table 1, this regimen was associated with an ORR of 38% (CR⁄PR, 20%) and an OS of 22.5 months (median not yet reached); myelosuppression, hyponatre-mia, and diarrhea were the most common grade 3–5 events.
Elotuzumab
Further improvements in the management of relapsed and refractory MM may be achieved using monoclonal antibody (MAb) therapy. Elotuzumab (HuLuc63) is a humanized MAb that targets CS1, a cell surface glyco-protein involved in cell adhesion that is selectively expressed on MM cells and colocalizes with CD138 in these cells (72, 73).
High rates of tumor cell lysis were observed when CD138+ cells isolated from patients with refractory (and newly diagnosed) MM were treated with elot-uzumab in the presence of autologous peripheral blood mononuclear cells, including natural killer cells (72). Importantly, elotuzumab-induced tumor cell lysis was enhanced in MM cells that had been pretreated with sub-therapeutic doses of diverse types of targeted agents (i.e., bortezomib, lenalidomide, perifosine) (72, 74).
These promising preclinical findings have been vali-dated in early clinical trials in patients with relapsed⁄refractory MM. In a phase I study in 28 patients with relapsed⁄refractory MM (31% had received prior bortezomib), 21-d cycles of elotuzumab (2.5– 20 mg⁄kg, days 1 and 11) in combination with bortezo-mib (1.3 mg⁄m2, days 1, 4, 8, and 11) produced a best response (‡MR) of 60% (40% ‡PR) in 20 evaluable patients who had completed at least 2 treatment cycles (75). Elotuzumab has also been evaluated in combination with lenalidomide in a phase I⁄II study in 29 patients (69% had received prior bortezomib, 59% received
tha-lidomide, and 21% received lenalidomide). Treatment with elotuzumab (5–20 mg⁄kg weekly for the first 2 28-d cycles, then every other week) combined with lenalido-mide (25 mg, days 1–21) produced an ORR of 82% (18% VGPR; 64% PR) in 28 evaluable patients (76). Interestingly, an ORR of 95% (23% VGPR; 73% PR) was seen in the 22 lenalidomide-naive patients enrolled in the study. Further investigation is needed to determine the optimal role of elotuzumab in the treatment of MM.
High-dose chemotherapy and targeted agents
Bendamustine, a bifunctional alkylating agent that cross-links DNA and induces apoptosis and mitotic catastro-phe (77), may be another option for salvage therapy in patients with relapsed⁄refractory MM. This agent has shown some clinical activity as monotherapy (ORR, 36– 55%) (78, 79), prompting the evaluation of combination regimens in the treatment of MM.
In a phase I study in 28 evaluable patients with relapsed⁄refractory MM (14% had received prior bort-ezomib and 7% received thalidomide), 28-d cycles of bendamustine (60 mg⁄m2, days 1, 8, and 15) in combina-tion with prednisolone (100 mg, days 1, 8, 15, and 22) and thalidomide (50, 100, or 200 mg, days 1–28) pro-duced an ORR of 86% (CR + PR), including those patients who had relapsed on prior conventional chemo-therapy or high-dose chemochemo-therapy and autologous stem cell transplant (SCT) (80). Overall, the median duration of response was 11 months, and median OS was 19 months; however, OS was longer in patients who had relapsed on prior chemotherapy (32+ vs. 16 month; P= 0.03) compared with SCT (80). The most common grade‡3 AEs were hematologic in nature, and thrombo-embolic events were not observed (80).
The feasibility of adding bendamustine to bortezomib and dexamethasone therapy has been explored in patients with <MR to 1 cycle of bortezomib plus dexa-methasone. In this study, a total of seven patients with relapsed⁄refractory MM who failed to respond ade-quately to bortezomib plus dexamethasone received 21-d cycles of bortezomib (1.3 mg⁄m2, days 1, 4, 8, and 11) combined with dexamethasone (40 mg, days 1, 4, 8, and 11) and bendamustine (50–100 mg⁄m2, days 1 and 8). In this non-responding patient population, the combination resulted in an ORR of 86% (57% PR; 29% MR) (81). Further clinical trials are needed to establish the role of bendamustine alone and in combination with other tar-geted agents in the treatment of relapsed⁄refractory MM.
Targeted inhibition of heat shock protein
Novel treatment approaches targeting diverse pathways complementary to those targeted by conventional and
newer approved agents show promise for inducing myeloma cell cytotoxicity and downregulating signaling pathways that induce myeloma growth, survival, and therapeutic resistance. Heat shock proteins (e.g., HSP27, HSP90) are potential therapeutic targets because expres-sion of these Bcl-2-like proteins interferes with the mito-chondrial stress response and activation of proapoptotic signaling (e.g., activation of Bax, caspase-3) that can result in the development of drug resistance (82, 83). For example, overexpression of HSP27 correlated with resis-tance to dexamethasone in myeloma cells, whereas block-ade of HSP27 restored sensitivity to bortezomib (84, 85). Tanespimycin
Tanespimycin, an inhibitor of HSP90, has shown activity in combination with bortezomib in MM patients (Table 1) (86, 87). In a phase I⁄II study in 72 pretreated MM patients (74% had received prior bortezomib ther-apy and 69% had received prior lenalidomide), tanespi-mycin [340 mg⁄m2 intravenously (IV), days 1, 4, 8, and 11 of each 21-d cycle] in combination with bortezomib (0.7–1.3 mg⁄m2 IV, days 1, 4, 8, and 11) inhibited HSP90 and proteasome activity and showed antitumor activity based on modified EBMT criteria. The ORR (defined as MR or better) was 48% in bortezomib-naive patients, 22% in bortezomib-pretreated patients, and 13% in bortezomib-refractory patients, with a median response duration of 12 months. There were no reports of grade 3–5 peripheral neuropathy (87). In another study in 22 heavily pretreated MM patients (96% had received prior thalidomide therapy), the combination of
tanespimycin (50, 175, or 340 mg⁄m2, days 1, 4, 8, and 11 of each 21-d cycle) with bortezomib (1.3 mg⁄m2, days 1, 4, 8, and 11) demonstrated clinical activity, with 14% of patients achieving MR or better. The most common grade 3–5 AEs were hematologic in nature, and one patient experienced grade 3 peripheral neuropathy (86).
Histone deacetylase inhibitors
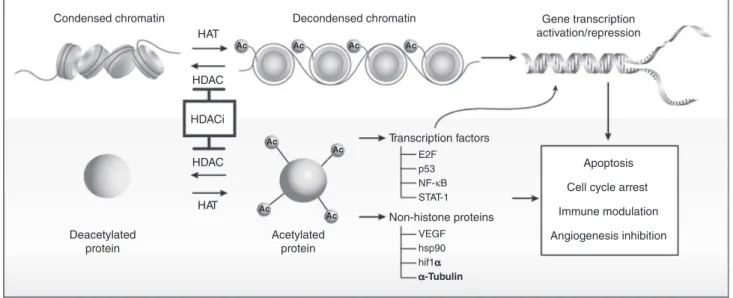
Histone deacetylase inhibitors are another new class of molecules that show promise as a complementary approach for the treatment of relapsed⁄refractory MM, and a number of phase I and II studies have recently been conducted with these agents. HDAC inhibition pro-motes acetylation of histone and non-histone proteins (Fig. 1). Histone acetylation affects higher-order DNA⁄chromatin structure, and HDAC inhibition leads to increased transcription of genes that have been down-regulated by histone acetylation (88). Therefore, inhibi-tion of HDAC affects epigenetic mechanisms that help restore or increase expression of genes that may play a critical role in the control of tumor growth and survival. As is the case with PIs, HDAC inhibitors may help restore or increase the expression of proapoptotic pro-teins in tumors.
Non-histone proteins are also regulated by acetylation, with evidence for non-histone-mediated effects on tumor cell growth. Transcription factor acetylation disrupts control of cell cycle transit and apoptosis in cancer. Direct acetylation of p53 affects its growth-regulatory and proapoptotic functions. Treatment of a variety of
Ac Ac Ac Ac Ac Ac Ac Ac
Decondensed chromatin Gene transcription
activation/repression Transcription factors Non-histone proteins E2F p53 NF-κB STAT-1 VEGF hsp90 hif1αα α-Tubulin Apoptosis Cell cycle arrest Immune modulation Angiogenesis inhibition Condensed chromatin Deacetylated protein Acetylated protein HAT HAT HDAC HDACi HDAC
Figure 1Effects of histone deacetylase (HDAC) inhibitors on histone protein acetylation and chromatin structure, acetylation of transcription fac-tors resulting in changes in gene expression, and acetylation of other non-histone proteins leading to diverse biologic effects underlying the patho-genesis and treatment of multiple myeloma. Reprinted with permission. Paik PK, Krug LM. HDAC inhibitors in malignant pleural mesothelioma: preclinical rationale and clinical trials.J Thorac Oncol2010; 5: 275–279.
tumor cells with HDAC inhibitors resulted in hyperacet-ylation of p53 and induction of p21⁄Waf⁄Cip1–mediated cell cycle arrest, increased expression of proapoptotic proteins (e.g., cytochrome c, BAX, Bid, activated cas-pase), and downregulated expression of antiapoptotic proteins (e.g., Bcl-2) (89–91).
In addition, increased acetylation of HSP90 can dis-rupt its chaperone function, resulting in decreased intra-cellular levels of progrowth and antiapoptotic proteins (e.g., Akt), possibly through enhanced proteasomal deg-radation of proteins (92, 93). HDAC6 links acetylation of HSP90 with aggresome formation and the accumula-tion of ubiquinated proteins (94, 95).
Panobinostat
Panobinostat (LBH589) is an oral HDAC inhibitor (96) that is currently being investigated alone (97) and in combination with lenalidomide and dexamethasone (98), melphalan (99), or bortezomib for the treatment of patients with relapsed or relapsed⁄refractory MM (100, 101).
In a phase II study, single-agent panobinostat showed clinical activity with a durable VGPR and MR (based on EBMT criteria) in 2 of 38 patients with heavily pretreat-ed (including bortezomib, lenalidomide, and thalidomide) refractory MM, and there were no reports of significant thromboembolic events (97).
Panobinostat is also being investigated as a component of combination regimens for the treatment of relapsed or refractory MM (summarized in Table 1). In a phase I study, panobinostat in combination with melphalan demonstrated clinical activity with an ORR of 33% (one immunofixation-positive CR, three PR) in 12 patients with relapsed or refractory MM previously treated with melphalan (99). The most common grade 3–5 AEs were reversible neutropenia (n= 6) and thrombocytopenia (n= 6) (99).
Combination therapy with panobinostat and bortezo-mib is also being investigated in patients with advanced MM. In a phase IB study in 29 heavily pretreated patients (55% had received prior bortezomib therapy), treatment with panobinostat combined with bortezomib resulted in at least a PR in 14 of 28 evaluable patients (50%), including four patients with immunofixation-negative CR (101). Importantly, an objective response (PR + MR) was observed in 6 of 10 (60%) evaluable patients who were refractory to previous bortezomib therapy (101). The most common grade 3–5 AEs were thrombocytopenia (n= 25), neutropenia (n= 18), and anemia (n= 6) (101).
Romidepsin
Romidepsin, an HDAC inhibitor administered as a 4-h infusion, is approved for the treatment of patients with
relapsed or refractory cutaneous T-cell lymphoma who have received at least 1 prior systemic therapy (102). In patients with relapsed or refractory MM, romidepsin is currently being evaluated in combination with bortezo-mib (103, 104) or with bortezobortezo-mib and dexamethasone (105).
In a phase I study in relapsed or refractory MM patients (N= 25; 37% had received prior vincristine, 50% received prior thalidomide, and 25% received prior bortezomib therapy), treatment with romidepsin at the maximum tolerated dose (MTD) of 10 mg⁄m2 in combi-nation with bortezomib resulted in an ORR of 71% (based on EBMT criteria), including one CR, three PR, and one MR among seven evaluable patients (103). No grade 3 or 4 non-hematologic AEs or dose-limiting toxic-ities were reported (103).
In a phase I⁄II study, romidepsin was shown to have clinical activity in combination with bortezomib and dexamethasone in patients with relapsed or refractory MM (Table 1) (105). No dose-limiting toxicities were reported among seven evaluable patients who completed at least two cycles (range, 1–8) of treatment with romi-depsin 8 or 10 mg⁄m2 once weekly; however, grade 3 fatigue (n= 2), peripheral neuropathy (n= 1), neutro-penia (n= 1), and sepsis (n= 2) were reported in this small cohort of patients (105). Importantly, 12 of 18 evaluable patients (67%) experienced at least a PR (four CR⁄near CR, four VGPR, four PR); five additional patients (28%) achieved an MR (105). Of the seven patients receiving long-term maintenance therapy with romidepsin (10 mg⁄m2 on days 1 and 8 of every 28-d cycle), four experienced disease progression, including three who had progressed on a previous bortezomib maintenance regimen.
Vorinostat
Vorinostat is an oral HDAC inhibitor that was approved in the United States in 2006 for the treatment of patients with cutaneous T-cell lymphoma who have progressive, persistent, or recurrent disease on or following 2 systemic therapies (106–108).
In patients with relapsed⁄refractory MM, vorinostat is currently being investigated in combination with bortezo-mib (109–111), in combination with lenalidomide and dexamethasone (112), or in combination with PLD and bortezomib (113). In a phase I study in 23 heavily pre-treated patients (100% had received prior thalidomide; 83% had received prior bortezomib), vorinostat demon-strated clinical activity at the MTD of 400 mg daily on days 4–11 in combination with bortezomib 1.3 mg⁄m2on days 1, 4, 8, and 11 of each 21-d cycle, with 55% of patients achieving PR or better (109). The most frequent grade‡3 AEs were reversible myelosuppression and fati-gue; 1 patient had grade 3 peripheral neuropathy.
In another phase I study, the addition of vorinostat 200–400 mg daily on days 4 to 11 to PLD 30 mg⁄m2 on day 4 and bortezomib 1.3 mg⁄m2on days 1, 4, 8, and 11 in 21-d cycles showed clinical activity in six of seven evaluable patients (1 CR, 1 VGPR, and 4 PR) based on International Myeloma Working Group criteria (114) and was generally well tolerated. No dose-limiting toxici-ties, serious AEs, or deaths were reported, although some neurologic (grade 3 sensory neuropathy in two of nine patients) and hematologic toxicities (grade ‡3 neutrope-nia, lymphopeneutrope-nia, and thrombocytopenia in 2, 3, and two patients, respectively) were identified in this small cohort of patients (113). Another study (summarized in Table 1) showed that extended treatment (‡12 cycles) with vorinostat 200 mg twice daily or 400 mg once daily in combination with bortezomib was well tolerated (one patient had grade 4 neutropenia, and five patients had grade 3 treatment-related AEs). Long-term clinical activ-ity was observed, with five PR, two MR, and two SD among nine evaluable patients. The duration of PR ran-ged from 147 to 609 d (110).
The safety and tolerability of vorinostat has been well documented in patients with hematologic malignancies and those with solid tumors. In an analysis of 341 patients who had received vorinostat as monotherapy, the most common grade 3–4 drug-related adverse events were fatigue (12% of patients) and thrombocytopenia (11%). In an analysis of 157 patients who had received vorinostat in combination, the most common grade 3–4 drug-related adverse event was fatigue (13% of patients). Furthermore, an analysis in more than 1845 vorinostat-treated patients revealed that the rate of thromboembolic events related to treatment was <2.6% (115).
Ongoing clinical studies include a pivotal phase IIB study and a randomized, blinded, phase III study to determine the ORR, TTP, PFS, OS, and tolerability of vorinostat in combination with IV bortezomib.
Conclusions
The development and application of targeted therapies, such as bortezomib and lenalidomide, have improved treatment outcomes in patients with relapsed⁄refractory MM. Emerging studies suggest that new combination therapies targeting complementary signaling pathways may further improve prognosis in treating this advanced form of MM. Completion of the numerous ongoing clini-cal investigations should determine which if any of these newly emerging therapies are viable treatment options for patients with relapsed⁄refractory MM. Regardless of outcome, the clinical study results will further improve our understanding of the biology of MM and the role for targeted therapies in providing durable disease con-trol and symptomatic relief in MM patients.
Disclosures
Dr. Dimopoulos has received honoraria from Merck, Sharp, and Dohme, a subsidiary of Merck & Co., Inc., Celgene Corporation, and Centocor Ortho Biotech. Dr. Anderson has served as an advisor for Celgene Corporation, Novartis Oncology, Millenium Pharma-ceuticals, Inc., Onyx PharmaPharma-ceuticals, and Nereus Phar-maceuticals. Dr. San-Miguel has served as an advisor for Celgene Corporation, Novartis Oncology, Millenium Pharmaceuticals, Inc., and Janssen-Cilag.
Acknowledgements
The authors wish to thank Craig Albright, PhD, for writing and editorial assistance during the development of this manuscript. Dr. Albright is employed by Com-plete Healthcare Communications, Inc., a medical com-munications company under contract with Merck, Sharp, and Dohme, a subsidiary of Merck & Co., Inc.
References
1. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008.Eur J Cancer2010;46:765–81.
2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009.CA Cancer J Clin2009;59: 225–49.
3. NCCN.Clinical Practice Guidelines in Oncology: Multiple Myeloma v.2.2010. Fort Washington, PA: National Com-prehensive Cancer Network, 2009.
4. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med2003;348: 1875–83.
5. Fermand JP, Katsahian S, Divine M,et al.High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a ran-domized control trial from the Group Myelome-Auto-greffe.J Clin Oncol2005;23:9227–33.
6. Singhal S, Mehta J, Desikan R,et al.Antitumor activity of thalidomide in refractory multiple myeloma.N Engl J Med1999;341:1565–71.
7. Kumar S, Gertz MA, Dispenzieri A,et al.Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma.Mayo Clin Proc
2003;78:34–9.
8. Dimopoulos M, Spencer A, Attal M,et al.Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma.N Engl J Med2007;357:2123–32.
9. Weber DM, Chen C, Niesvizky R,et al.Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America.N Engl J Med2007;357:2133–42.
10. Richardson PG, Barlogie B, Berenson J,et al.A phase 2 study of bortezomib in relapsed, refractory myeloma.N Engl J Med2003;348:2609–17.
11. Orlowski RZ, Nagler A, Sonneveld P,et al.Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression.J Clin Oncol2007;25:3892– 901.
12. Gerecke C, Knop S, Topp MS, Liebisch P, Vollmuth C, Platzbecker U, Ma¨der U, Einsele H, Bargou RC. Lena-lidomide, Adriamycin and dexamethasone (RAD) in relapsed and refractory multiple myeloma: final results from a phase I⁄II trial of ‘‘Deutsche Studiengruppe Mul-tiples Myelom’’ [ASH abstract].Blood2008;112:2782. 13. Knop S, Gerecke C, Liebisch P, Topp MS, Hess G, Platzbecker U, Frohnert S, Einsele H, Bargou R. The efficacy and toxicity of the RAD regimen (Revlimid, Adriamycin, dexamethasone) in relapsed and refractory multiple myeloma-a phase I⁄II trial of ‘‘Deutsche Studi-engruppe Mutiples Myelom’’ [ASH abstract].Blood
2007;110:2716.
14. Reece DE, Masih-Khan E, Khan A, Dean S, Anglin P, Chen C, Kukreti V, Mikhael JR, Trudel S. Phase I-II trial of oral cyclophosphamide, prednisone and lenalido-mide (Revlimid) (CPR) for the treatment of patients with relapsed and refractory multiple myeloma [ASH abstract].Blood2009;114:1874.
15. Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma.Leukemia
2008;22:231–9.
16. Vescio RA, Cao J, Hong CH, Lee JC, Wu CH, Der Dani-elian M, Wu V, Newman R, Lichtenstein AK, Berenson JR. Myeloma Ig heavy chain V region sequences reveal prior antigenic selection and marked somatic mutation but no intraclonal diversity.J Immunol1995;155:2487–97. 17. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging,
risk stratification and response assessment of multiple myeloma.Leukemia2009;23:3–9.
18. Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma.Nat Rev Cancer2002;2:927–37.
19. Uchiyama H, Barut BA, Chauhan D, Cannistra SA, Anderson KC. Characterization of adhesion molecules on human myeloma cell lines.Blood1992;80:2306–14. 20. Hideshima T, Chauhan D, Schlossman R, Richardson P,
Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: thera-peutic applications.Oncogene2001;20:4519–27.
21. Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma.Bone2008;42: 1007–13.
22. Oyajobi BO. Multiple myeloma⁄hypercalcemia.Arthritis Res Ther2007;9:S4.
23. Mileshkin L, Honemann D, Gambell P,et al.Patients with multiple myeloma treated with thalidomide: evaluation of clinical parameters, cytokines,angiogenic markers, mast cells and marrow CD57+ cytotoxic T cells as predictors of outcome.Haematologica
2007;92:1075–82.
24. Avet-Loiseau H, Attal M, Moreau P,et al.Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome.
Blood2007;109:3489–95.
25. Cavo M, Testoni N, Terragna C, et al. Up-front thalidomide-dexamethasone (THAL) and double autolo-gous transplant (Double TX) for multiple myeloma: comparison with double TX without added thalidomide and prognostic implications of chromosome 13 deletion and translocation t(4;14) [abstract]. Blood2006;108: 3081.
26. Avet Loiseau H, Soulier J, Fermand J-P,et al.Impact of chromosomal abnormalities del(13), t(4;14), and del(17p) and prior treatment on outcomes in patients with relapsed or refractory multiple myeloma treated with lenalidomide [ASH annual meeting abstract].Blood
2008;112:3685.
27. Palumbo A, Gay F, Bringhen S,et al.Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma.Ann Oncol2008;19:1160–5.
28. Reece D, Song KW, Fu T,et al.Influence of cytogenet-ics in patients with relapsed or refractory multiple mye-loma treated with lenalidomide plus dexamethasone: adverse effect of deletion 17p13.Blood2009;114:522–5. 29. Dimopoulos M, Kastritis E, Christoulas D, Migkuo M,
Gavriatopoulou M, Iakovaki M, Roussou M, Efstathiou E, Terpos E. Treatment of patients with relapsed⁄ refrac-tory multiple myeloma (MM) with lenalidomide and dexamethasone with or without bortezomib: prospective evaluation of the impact of cytogenetic abnormalities [ASH abstract].Blood2009;114:958.
30. San Miguel JF. Relapse⁄refractory myeloma patient: potential treatment guidelines.J Clin Oncol2009;27: 5676–7.
31. Anderson H, Scarffe JH, Ranson M, Young R, Wieringa GS, Morgenstern GR, Fitzsimmons L, Ryder D. VAD chemotherapy as remission induction for multiple mye-loma.Br J Cancer1995;71:326–30.
32. Browman GP, Belch A, Skillings J, Wilson K, Bergsagel D, Johnston D, Pater JL. Modified adriamycin-vincris-tine-dexamethasone (m-VAD) in primary refractory and relapsed plasma cell myeloma: an NCI (Canada) pilot study. The National Cancer Institute of Canada Clinical Trials Group.Br J Haematol1992;82:555–9.
33. Gertz MA, Kalish LA, Kyle RA, Hahn RG, Tormey DC, Oken MM. Phase III study comparing vincristine, doxorubicin (Adriamycin), and dexamethasone (VAD) chemotherapy with VAD plus recombinant interferon alfa-2 in refractory or relapsed multiple myeloma. An
Eastern Cooperative Oncology Group study.Am J Clin Oncol1995;18:475–80.
34. Lokhorst HM, Meuwissen OJ, Bast EJ, Dekker AW. VAD chemotherapy for refractory multiple myeloma.Br J Haematol1989;71:25–30.
35. Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma.Blood
1986;67:1298–301.
36. Barlogie B, Alexanian R, Smallwood L, Cheson B, Dixon D, Dicke K, Cabanillas F. Prognostic factors with high-dose melphalan for refractory multiple myeloma.
Blood1988;72:2015–9.
37. Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients.Blood
2008;111:2977–83.
38. Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy.AAPS J2005;7:E14– 9.
39. Palumbo A, Facon T, Sonneveld P,et al.Thalidomide for treatment of multiple myeloma: 10 years later.Blood
2008;111:3968–77.
40. Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N, Eleftherakis-Papaiakovou E, Tsionos K, Croucher P, Dimopoulos MA. The combina-tion of bortezomib, melphalan, dexamethasone and inter-mittent thalidomide is an effective regimen for
relapsed⁄refractory myeloma and is associated with improvement of abnormal bone metabolism and angio-genesis.Leukemia2008;22:2247–56.
41. Palumbo A, Ambrosini MT, Benevolo G,et al. Bortezo-mib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma.Blood2007;109:2767–72.
42. Zangari M, Barlogie B, Burns M, Bolejack V, Hollmig K, van Rhee F, Pineda-Roman M, Elice F, Tricot G. Velcade (V)-thalidomide(T)-dexamethasone (D) for advanced and refractory multiple myeloma (MM): long-term follow-up of phase I-II trial UARK 2001-37: supe-rior outcome in patients with normal cytogenetics and no prior T [ASH annual meeting abstract].Blood
2005;106:2552.
43. Anderson KC, Jagannath S, Jakubowiak A, Lonial S, Raje N, Alsina M, Ghobrial I, Knight R, Esseltine D, Richardson P. Lenalidomide, bortezomib, and dexameth-asone in relapsed⁄refractory multiple myeloma (MM): encouraging outcomes and tolerability in a phase II study [ASCO abstract].J Clin Oncol2009;27:8536. 44. Agura E, Niesvizky R, Matous J, Munshi N, Hussein M,
Parameswaran RV, Tarantolo S, Whiting NC, Drachman JG, Zonder JA. Dacetuzumab (SGN-40), lenalidomide, and weekly dexamethasone in relapsed or refractory mul-tiple myeloma: mulmul-tiple responses observed in a phase Ib study [ASH abstract].Blood2009;114:2870.
45. Delforge M, Facon T, Bravo M-L, Dimopoulos M. Le-nalidomide plus dexamethasone has similar tolerability
and efficacy in treatment of relapsed⁄refractory multiple myeloma patients with or without history of peripheral neuropathy [ASH abstract].Blood2009;114:3873. 46. Facon T, Leleu X, Stewart AK,et al.Dasatinib in
com-bination with lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma: preliminary results of a phase I study [ASH abstract].
Blood2009;114:1876.
47. Raje N, Richardson P, Hari PN,et al.An open-label phase I study of the safety and efficacy of RAD001 in combination with lenalidomide in the treatment of patients with relapsed and relapsed⁄refractory multiple myeloma [ASH abstract].Blood2009;114:3856. 48. Chauhan D, Bianchi G, Anderson KC. Targeting the
UPS as therapy in multiple myeloma.BMC Biochem
2008;9:S1.
49. Strauss SJ, Higginbottom K, Juliger S, Maharaj L, Allen P, Schenkein D, Lister TA, Joel SP. The protea-some inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catas-trophe in B-cell lymphoma cell lines. Cancer Res
2007;67:2783–90.
50. Richardson PG, Sonneveld P, Schuster MW,et al. Bort-ezomib or high-dose dexamethasone for relapsed multiple myeloma.N Engl J Med2005;352:2487–98.
51. Colado E, Mateos MV, Moreno MJ,et al.VAMP⁄ Tha-CyDex: Velcade(bortezomib), adriamycin, melphalan, and prednisone alternating with thalidomide, cyclophos-phamide and dexamethasone as a salvage regiment in relapsed multiple myeloma patients [ASH abstract].
Blood2008;112:3694.
52. Safra T. Cardiac safety of liposomal anthracyclines.
Oncologist2003;8(Suppl. 2):17–24.
53. Nagler A, Hajek R, Sonneveld P, Spencer A, Blade J, Robak T, Mundle S, Zhuang S, harousseau J, Orlowski R. Doxil + velcade in previously treated myeloma w⁄prior SCT.Haematologica2007;92:161.
54. Sonneveld P, Hajek R, Nagler A, Spencer A, Blade J, Robak T, Zhuang SH, Harousseau JL, Orlowski RZ. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide⁄lenalidomide therapy.Cancer2008;112: 1529–37.
55. Spencer A, Hajek R, Nagler A, Sonneveld P, Blade J, Robak T, Mundle S, Zhuang S, Harousseau J, Orlowski R. Doxil + velcade in previously treated high risk mye-loma.Haematologica2007;92:162.
56. San Miguel J, Hajek R, Nagler A,et al.Doxil + velcade in previously treated‡65y myeloma pts.Haematologica
2007;92:159.
57. Anderson G, Gries M, Kurihara N,et al.Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1.Blood2006;107:3098–105. 58. Verhelle D, Corral LG, Wong K,et al.Lenalidomide
cells while expanding normal CD34+ progenitor cells.
Cancer Res2007;67:746–55.
59. Richardson P, Siegel D, Baz R,et al.A phase 1⁄2 multi-center, randomized, open label escalation study to deter-mine the maximum tolerated dose, safety, and efficacy of pomalidomide alone or in combination with low-dose dexamethasone in patients with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide and bortezomib [ASH abstract].
Blood2009;114:301.
60. Lacy MQ, Hayman SR, Gertz MA,et al.Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma.J Clin Oncol2009;27:5008– 14.
61. Kuhn DJ, Chen Q, Voorhees PM,et al.Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma.Blood2007;110:3281–90.
62. Badros AZ, Vij R, Martin T, Zonder JA, Woo T, Wang Z, Lee S, Wong A, Niesvizky R. Phase I study of carfilzomib in patients (pts) with relapsed and refractory multiple myeloma (MM) and varying degrees of renal insufficiency [ASH abstract].Blood
2009;114:3877.
63. Phase 2 study of carfilzomib in relapsed and refractory multiple myeloma (NCT00511238). Available at: http:// clinicaltrials.gov/ct2/show/NCT00511238. Accessed March 10, 2010.
64. Phase 2 study of carfilzomib in relapsed multiple mye-loma (NCT00530816). Available at: http://clinicaltrials. gov/ct2/show/NCT00530816. Accessed March 10, 2010. 65. Williamson MJ, Blank JL, Bruzzese FJ,et al.
Compari-son of biochemical and biological effects of ML858 (sali-nosporamide A) and bortezomib.Mol Cancer Ther
2006;5:3052–61.
66. Richardson P, Hofmeister C, Jakubowiak A,et al.Phase 1 clinical trial of the novel structure proteasome inhibitor NPI-0052 in patients with relapsed and relapsed⁄ refrac-tory multiple myeloma (MM) [ASH abstract].Blood
2009;114:431.
67. Hideshima T, Catley L, Yasui H,et al.Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multi-ple myeloma cells.Blood2006;107:4053–62.
68. Mitsiades CS, Mitsiades N, Poulaki V,et al.Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1⁄Akt signaling in human multiple myeloma cells: therapeutic implications. Onco-gene2002;21:5673–83.
69. Jakubowiak A, Richardson P, Zimmerman TM,et al.
Phase I results of perifosine (KRX-0401) in combination with lenalidomide and dexamethasone in patients with relapse or refractory multiple myeloma (mm) [ASH abstract].Blood2008;112:3691.
70. Richardson P, Lonial S, Jakubowiak A,et al. Multi-cen-ter phase II study of perifosine (KRX-0401) alone and in
combination with dexamethasone (dex) for patients with relapsed or relapsed⁄refractory multiple myeloma: prom-ising activity as combination therapy with manageable toxicity [ASH abstract].Blood2007;110:1164.
71. Richardson P, Wolf JL, Jakubowiak A,et al.Perifosine in combination with bortezomib and dexamethasone extends progression-free survival and overall survival in relapsed⁄refractory multiple myeloma patients previously treated with bortezomib: updated phase I⁄II trial results [ASH abstract].Blood2009;114:1869.
72. Tai YT, Dillon M, Song W,et al.Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu.Blood2008;112: 1329–37.
73. Hsi ED, Steinle R, Balasa B,et al.CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma.Clin Cancer Res2008;14:2775–84.
74. van Rhee F, Szmania SM, Dillon M,et al. Combinato-rial efficacy of anti-CS1 monoclonal antibody elot-uzumab (HuLuc63) and bortezomib against multiple myeloma.Mol Cancer Ther2009;8:2616–24.
75. Jakubowiak A, Benson DM Jr, Bensinger W,et al. Elot-uzumab in combination with bortezomib in patients with relapsed⁄refractory multiple myeloma: a phase I study.
J Clin Oncol2010;28:8003.
76. Lonial S, Vij R, Harousseau J, T F, Moreau P, Leleu X, Westland C, Singhal A, Jagannath S, Multiple Myeloma Research Consortium. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma: a phase I⁄II study.J Clin Oncol2010;28:8020.
77. Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliott G, Niemeyer CC. Bendamustine (Tre-anda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylat-ing agents.Clin Cancer Res2008;14:309–17.
78. Michael M, Bruns I, Bolke E, Zohren F, Czibere A, Sa-faian NN, Neumann F, Haas R, Kobbe G, Fenk R. Ben-damustine in patients with relapsed or refractory multiple myeloma.Eur J Med Res2010;15:13–9.
79. Knop S, Straka C, Haen M, Schwedes R, Hebart H, Ein-sele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemother-apy.Haematologica2005;90:1287–8.
80. Ponisch W, Rozanski M, Goldschmidt H,et al. Com-bined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a Phase I clinical trial.Br J Haematol
2008;143:191–200.
81. Fenk R, Michael M, Zohren F, Graef T, Czibere A, Bruns I, Neumann F, Fenk B, Haas R, Kobbe G. Esca-lation therapy with bortezomib, dexamethasone and ben-damustine for patients with relapsed or refractory multiple myeloma.Leuk Lymphoma2007;48:2345–51.
82. Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activa-tion and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism.J Biol Chem2008;283:12305–13. 83. Ciocca DR, Calderwood SK. Heat shock proteins in
can-cer: diagnostic, prognostic, predictive, and treatment implications.Cell Stress Chaperones2005;10:86–103. 84. Chauhan D, Li G, Hideshima T,et al.Hsp27 inhibits
release of mitochondrial protein Smac in multiple mye-loma cells and confers dexamethasone resistance.Blood
2003;102:3379–86.
85. Chauhan D, Li G, Shringarpure R, Podar K, Ohtake Y, Hideshima T, Anderson KC. Blockade of Hsp27 over-comes bortezomib⁄proteasome inhibitor PS-341 resis-tance in lymphoma cells.Cancer Res2003;63:6174–7. 86. Badros AZ, Richardson PG, Albitar M, Jagannath S,
Tarantolo S, Wolf JL, Messina M, Berman D, Anderson KC. Tanespimycin + bortezomib in relapsed⁄refractory myeloma patients: results from the Time-2 study [ASH abstract].Blood2009;114:1871.
87. Richardson P, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Albitar M, Kopit J, Berman D, Ander-son KC. Tanespimycin + bortezomib demonstrates safety, activity, and effective target inhibition in relapsed⁄refractory myeloma patients: updated results of a phase 1⁄2 study [ASH abstract].Blood2009;114:2890. 88. Kim TY, Bang YJ, Robertson KD. Histone deacetylase inhibitors for cancer therapy.Epigenetics2006;1:14–23. 89. Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR,
Tainton KM, Kofler R, Smyth MJ, Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and pro-duction of reactive oxygen species.Proc Natl Acad Sci USA2001;98:10833–8.
90. Condorelli F, Gnemmi I, Vallario A, Genazzani AA, Canonico PL. Inhibitors of histone deacetylase (HDAC) restore the p53 pathway in neuroblastoma cells.Br J Pharmacol2008;153:657–8.
91. Peart MJ, Tainton KM, Ruefli AA, Dear AE, Sedelies KA, O’Reilly LA, Waterhouse NJ, Trapani JA, John-stone RW. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors.Cancer Res2003;63:4460– 71.
92. Bali P, Pranpat M, Bradner J,et al.Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone func-tion of heat shock protein 90: a novel basis for antileuke-mia activity of histone deacetylase inhibitors.J Biol Chem2005;280:26729–34.
93. Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy.J Clin Oncol2009;27:5459–68.
94. Boyault C, Zhang Y, Fritah S,et al.HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates.Genes Dev2007;21:2172–81. 95. Richon VM, Garcia-Vargas J, Hardwick JS.
Develop-ment of vorinostat: current applications and future
perspectives for cancer therapy.Cancer Lett
2009;280:201–10.
96. Khan N, Jeffers M, Kumar S,et al.Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors.Biochem J2008;409:581–9. 97. Wolf JL, Siegel D, Matous J,et al.A phase II study of
oral panobinostat (LBH589) in adult patients with advanced refractory multiple myeloma [ASH abstract].
Blood2008;112:2774.
98. Spencer A, Taylor KM, Lonial S, Mateos MV, Jalalud-din M, RHazell K, Bourquelot PM, San Miguel JF. Pan-obinostat plus lenalidomide and dexamethasone phase I trial in multiple myeloma (MM) [ASCO abstract].J Clin Oncol2009;27:8542.
99. Berenson JR, Yellin O, Boccia RV, Nassir Y, Rothstein S, Swift R. A phase I study of oral melphalan combined with LBH589 for patients with relapsed or refractory multiple myeloma (MM) [ASH abstract].Blood
2009;114:1855.
100. Siegel D, Sezer O, San Miguel J, Mateos MV, Prosser I, Cavo M, Jalaluddin M, Hazell K, Bourquelot PM, Anderson KC. A phase IB, multicenter, open-label, dose-escalation study of oral panobinostat (LBH589) and I.V. bortezomib in patients with relapsed multiple myeloma [ASH abstract].Blood2008;112:2781.
101. San Miguel J, Sezer O, Siegel D,et al.A phase IB, multi-center, open-label dose-escalation study of oral panobinostat (LBH589) and I.V. bortezomib in patients with relapsed multiple myeloma [ASH abstract].Blood
2009;114:3852.
102. ISTODAX(romidepsin).Full Prescribing Infor-mation. Cambridge, MA: Gloucester Pharmaceuticals, Inc, 2009.
103. Prince M, Quach H, Neeson P,et al.Safety and efficacy of the combination of bortezomib with the deacetylase inhibitor romidepsin in patients with relapsed or refrac-tory multiple myeloma: preliminary results of a phase I trial [ASH abstract].Blood2007;110:1167.
104. Berenson JR, Yellin O, Mapes R, Eades B, Abaya CD, Strayer A, Nix D, Swift RA. A phase II study of a 1-hour infusion of romidepsin combined with bortezomib for multiple myeloma (MM) patients with relapsed or refractory disease [ASCO abstract].J Clin Oncol
2009;27:e19508.
105. Harrison SJ, Quach H, Yuen K,et al.High response rates with the combination of bortezomib, dexametha-sone and the pan-histone deacetylase inhibitor romidep-sin in patients with relapsed or refractory multiple myeloma in a phase I⁄II clinical trial [ASH abstract].
Blood2008;112:3698.
106. Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncolo-gist2007;12:1247–52.
107. Duvic M, Talpur R, Ni X,et al.Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for
refractory cutaneous T-cell lymphoma (CTCL).Blood
2007;109:31–9.
108. Olsen EA, Kim YH, Kuzel TM,et al.Phase IIb multi-center trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lym-phoma.J Clin Oncol2007;25:3109–15.
109. Badros A, Burger AM, Philip S,et al.Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma.Clin Cancer Res
2009;15:5250–7.
110. Jagannath S, Weber D, Sobecks R, Schiller GJ, Lupinac-ci L, Allen J, Rizvi S. The combination of vorinostat and bortezomib provides long-term responses in patients with relapsed or refractory multiple myeloma [ASH abstract].
Blood2009;114:3886.
111. Siegel D, Jagannath S, Lonial S, Dimopoulos MA, Graef T, Pietrangelo D, Lupinacci L, Reiser D, Rizvi S, Ander-son KC. Update on the phase IIb, open-label study of vorinostat in combination with bortezomib in patients with relapsed and refractory multiple myeloma [ASH abstract].Blood2009;114:3890.
112. Siegel D, Weber DM, Mitsiades C,et al.Combined vori-nostat, lenalidomide and dexamethasone therapy in patients with relapsed or refractory multiple myeloma: a phase I study [ASH abstract].Blood2009;114:305. 113. Voorhees PM, Gasparetto C, Richards KL,et al.
Vori-nostat in combination with pegylated liposomal doxoru-bicin and bortezomib for patients with
relapsed⁄refractory multiple myeloma: results of a phase I study [ASH abstract].Blood2009;114:306.
114. Durie BG, Harousseau JL, Miguel JS,et al.International uniform response criteria for multiple myeloma. Leuke-mia2006;20:1467–73.
115. Siegel D, Munster PN, Rubin EH,et al.The combined safety and tolerability profile of vorinostat-based therapy for solid or hematologic malignancies [ASH abstract].
Blood2009;114:1710.
116. Lacy MQ, Gertz MA, Hayman SR, et al. Pomalido-mide (CC4047) plus low dose dexamethasone (Pom⁄ -dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) [ASH abstract].
Blood 2009;114:429.
117. Niesvizky R, Wang L, Orlowski RZ, Bensinger W, Alsi-na M, Gabrail N, Gutirrez A, Kunkel L, Kauffman M, (MMRC) TMMRC. Phase 1b multicenter dose escala-tion study of carfilzomib plus lenalidomide and low dose dexamethasone (CRd) in relapsed and refractory multiple myeloma (MM) [ASH abstract].Blood2009;114:304. 118. Siegel D, Wang L, Orlowski RZ,et al.PX-171-004, an
ongoing open-label, phase II study of single-agent carfilzomib (CFZ) in patients with relapsed or refractory myeloma (MM): updated results from the bortezomib-treated cohort [ASH abstract].Blood2009;114:303. 119. Wang L, Siegel D, Kaufman JL,et al.Updated results
of bortezomib-naive patients in PX-171-004, an ongoing open-label, phase II study of single-agent carfilzomib (CFZ) in patients with relapsed or refractory myeloma (MM) [ASH abstract].Blood2009;114:302.