Infantile Hemangiomas: An Update on Pathogenesis
and Therapy
abstract
Infantile hemangiomas (IHs) are the most common vascular tumors of childhood, affecting ∼5% of all infants. Although most lesions pro-liferate and then involute with minimal consequence, a significant minority can be disfiguring, functionally significant, or, rarely, life-threatening. Recent discoveries concerning hemangioma pathogenesis provide both an improved understanding and more optimal approach to workup and management. Important detrimental associations can be seen with IH, such as significant structural anomalies associated with segmental IH. Standards of care have dramatically changed evaluation and management of hemangiomas. The goal of timely rec-ognition and therapy is to minimize or eliminate long-term sequelae. New modalities, such as oral propranolol, provide the caregiver with better therapeutic options, which can prevent or minimize medical risk or scarring, but the side effect profile and risk-benefit ratio of such interventions must always be evaluated before instituting therapy. Pediatrics2013;131:99–108
AUTHORS:Tina S. Chen, MD, Lawrence F. Eichenfield, MD, and Sheila Fallon Friedlander, MD
Pediatric Dermatology, Rady Children’s Hospital San Diego, San Diego, California
KEY WORD
hemangioma
ABBREVIATIONS
EPC—endothelial progenitor cell IH—infantile hemangioma PDL—pulsed dye laser
PHACE—Posterior fossa brain abnormalities, Hemangiomas, Ar-terial malformations, Coarctation of the aorta and other cardiac defects, Eye abnormalities
VEGF—vascular endothelial growth factor www.pediatrics.org/cgi/doi/10.1542/peds.2012-1128
doi:10.1542/peds.2012-1128
Accepted for publication Aug 29, 2012
Address correspondence to Tina S. Chen, MD, 8010 Frost St, Suite 602, San Diego, CA 92122. E-mail: tina.chen@gmail.com PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2013 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:Drs Friedlander and Eichenfield have served as investigators for Pierre-Fabre, without compensation; and Dr Chen has indicated she has nofinancial relationships relevant to this article to disclose.
Infantile hemangiomas (IHs) are the
most common pediatric vascular
tumors, affecting ∼5% of all infants born in the United States.1 A recent
prospective Australian study of new-borns noted an incidence of 2.6% by 6 weeks of age,2and an American study
of similar design found that 4.5% of infants developed IH, all of which were present by 3 months of age.3 IHs are
benign tumors that are usually not present at birth but instead are noted within thefirst few weeks of life. Pre-cursor lesions are common but often subtle;findings may include telangiec-tasias, pallor, a bruiselike appearance, and, rarely, ulceration. IHs typically have an initial proliferative phase, with rapid growth of the tumor in thefirst several months of life. This is followed by an involution stage, with slow, spontane-ous resolution spanning years. After involution of the vascular component, a residual fibrofatty mass often per-sists.
Although many of these lesions resolve spontaneously without concern, a sig-nificant proportion lead to function-threatening and cosmetically disfiguring consequences. For functionally signi-ficant or potentially deforming lesions, timely intervention is important to minimize the possibility of a poor out-come and permanent scarring. Many important and management-altering discoveries have occurred regarding IH in the past decade. The following highlights the most important of these findings.
PATHOPHYSIOLOGY
IHs are vascular tumors that involve the proliferation of benign endotheliallike cells that possess histochemical markers (GLUT-1, Lewis Yantigen, FcyRII, and merosin); these markers are also present on placental blood vessels.4The
immunohistochemical profile differ-entiates IH from other vascular birth-marks or tumors.
The pathophysiology associated with the unique natural history of these lesions, with initial rapid proliferation followed by gradual involution and re-gression, has not been completely elucidated. One etiologic hypothesis speculates that cells are “embolized” from the placenta.5 Another suggests
that IHs result from somatic mutations in a gene mediating endothelial cell proliferation.6Recent data suggest an
endothelial progenitor cell as the source of origin of the tumors.7–10 It
has been speculated that hypoxia, ei-ther systemically (eg, due to placental insufficiency) or in a specific“niche” area of poorly perfused tissue5,11
stimulates endothelial progenitor cells to proliferate inappropriately. The fol-lowing summarizes evidence for these various theories (see Table 1).
The placental theory is attractive be-cause it would explain the programmed life cycle of IH. Subsequent to North’s discoveries regarding the histochemi-cal similarities of IH and placenta,4
Barnes et al noted that placenta and IH have high levels of genetic similarity when compared with other vascular tumors and normal structures.12Waner
et al noted that IH tend to develop along embryonic fusion lines of the facial placodes.13 Piecing these 2 seemingly
disparate facts together, Mihm et al suggested that IH might represent“ be-nign metastases”originating from the placenta or other cells that proliferate in areas of low oxygen tension, such as the“end artery, vascular dead end” sites occurring in embryonic fusion planes.5Pittman et al were unable to
detect the presence of maternal-fetal chimerism in IH tissue, but this does not rule out the possibility of the placental origin of IH tissue because the placenta is predominantly fetal in origin.14
It has also been hypothesized that im-mature endothelial cells and pericytes, which coexist in the late stages of fetal
development, perhaps maintain per-sistent proliferative properties for a period of time postnatally, leading to the development of IH.15However, Boye
et al demonstrated the clonality of IH cells, making it less likely that a dispa-rate group of cells serve as the source of this tumor.16
Hypoxia has been proposed as a driving factor for the pathogenesis of vascular proliferation in general. IH proliferation may be a homeostatic attempt to nor-malize hypoxic tissue. Epidemiologic
TABLE 1 Pathogenesis of IH: Hypotheses and Supporting Data
Placental embolization Histochemical
GLUT1+, LeY+, FcyRII +, Merosin+4 Genetic analyses
Transcriptome similarities between placental and IH tissue12 Life history
Rapid proliferation, followed by stabilization an involution
Metastatic niche theory
IH cluster at embryonic fusion placode sites13 IH precursor cell comes from the placenta as
a“benign metastasis”5
Somatic mutation or hyperreactivity of an endothelial-type cell
Incidence of IH generally sporadic Clonality of IH cells16
Mutation of VEGR2 receptors in IH tissue19 Endothelial progenitor cells (EPC) play an
etiologic role
Circulating endothelial progenitor cells increased in IH infants7
EPCs present in proliferative but not involuting phase of IH growth8
Human IH EPCs injected into immunodeficient mice recapitulate IH life cycle and express GLUT110
IH growth is mediated by angiogenic peptides Upregulated VEGF2 signaling in IH19 Insulin-like growth factor (angiogenic)
upregulated in IH21
Hypoxia stimulates the release of EPCs; EPCs then hone to hypoxic sites
Epidemiology: hypoxia-associated factors (low birth weight, advanced maternal age) overrepresented in IH population23 IH occur in“end artery, vascular“dead-end”
embryonic fusion plane sites5,13 IH associated with retinopathy of
prematurity17
GLUT1 is a sensor for hypoxia and present on IH cells18
findings support this hypothesis, given that factors that are thought to be linked to hypoxia, such as low birth weight and advanced maternal age, are over-represented in IH populations.11Another
supportivefinding is the association of IH with retinopathy of prematurity, a condition known to be linked to ische-mia.17 GLUT-1, present on IH tissue, is
a facilitative glucose transporter that is an important sensor for hypoxia.18
The growth of IH likely involves angio-genic peptides, such as vascular en-dothelial growth factor (VEGF) and basic fibroblast growth factor, which induce proliferation of blood vessels. Receptors for these growth factors are also crucial in endothelial cell regulation, and a misbalance of VEGF-receptor-1 expression with conse-quent hyperactivity of VEGF-receptor-2 function has been noted in IH tissue.19
The suppressive effect of glucocorti-coids may be mediated through VEGF-A.20
Additionally, insulinlike growth factor-2, which stimulates angiogenesis, is up-regulated in proliferating but not in-voluting IH.21
Endothelial progenitor cells (EPCs) are vascular stem cells with the capacity to contribute to postnatal vascular de-velopment. There is now compelling evidence that these EPCs play an etio-logic role in the development of IH. A subset of progenitor cells isolated from IH tissues, which possess the surface markers CD34+ CD133+, are of partic-ular interest. These EPCs have been shown to differentiate into endothelial cells in vitro22 and are increased
15-fold in IH compared with controls.7
Cultured EPCs from patients with IH stain positively for known hemangioma markers GLUT1, CD32, and merosin. Several mediators of EPC trafficking and vasculogenesis, such as VEGF-A and hypoxia inducible factor-1 alpha (a transcription factor that regulates the formation of new blood vessels by EPCs), were found to be elevated in
blood and IH specimens taken from children with proliferating IH.9
A major breakthrough occurred when Khan et al were able to successfully inject CD133+ EPCs from human hem-angioma tissue into immunodeficient mice. These mice then developed GLUT1 vascular tumors, which recapitulated the development of human IH, providing investigators with the first viable IH animal model.10These studies highlight
the importance of CD133+ EPCs in the pathophysiology of IH and provide a means of testing putative therapies in this animal model.
EPIDEMIOLOGY AND DIAGNOSIS
Traditionally, misuse of the term“ hem-angioma” to describe other vascular lesions has impeded the collection of accurate demographic data. In par-ticular, misdiagnosis of port wine stains, venous and arterial malforma-tions, and vascular tumors such as tufted angiomas, affected the accuracy of many studies performed in the past. In 2007, a large, multicenter, prospective study was conducted by pediatric dermatologists skilled in distinguishing vascular lesions, 1058 children with IH were identified. The tumors were more commonly seen in patients who were female, white (non-Hispanic), premature, of low birth weight, a product of multiple gestation, or born to mothers with advanced maternal age.23 Placenta previa and
preeclampsia were also found to be more common.
Two less common types of“hemangioma mimickers,” congenital hemangiomas, occasionally confuse practitioners but do not possess the classic attributes of IH. Both noninvoluting congenital hemangiomas and rapidly involuting congenital hemangiomas are“fully for-med”at birth or can even be involuting or ulcerating. They may possess telan-giectases and a rim of pallor. In contrast to IH, they lack GLUT1 surface markers,
and thus histochemical evaluation is often useful in distinguishing between these vascular lesions.24 Rapidly
invo-luting congenital hemangiomas lesions rapidly involute, often within the first year, whereas noninvoluting congenital hemangiomas persist for a prolonged period.
Hemangiomas are now classified into 3 primary subtypes: segmental, focal (see Fig 1), and indeterminate. Waner et al found that focal hemangiomas were 3 times more common than dif-fuse or segmental hemangiomas on the face.13 The segmental subtype is
associated with a higher risk of com-plications, functional compromise, de-formity, and ulceration, as well as a greater need for therapy.25Other IH
that are regarded as being higher risk and may have a greater need for therapy are outlined in Table 2.
One of the most important complica-tions associated with segmental IH is PHACE syndrome. A novel neurocuta-neous syndrome associated with facial hemangiomas described by Frieden et al in 1996, the acronym PHACE refers to posterior fossa brain abnormalities, hemangiomas, arterial malformations, coarctation of the aorta and other cardiac defects, as well as eye abnor-malities.26 Although less well known,
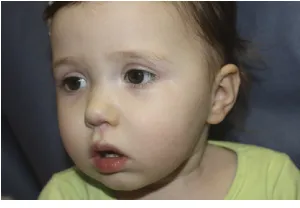
this syndrome may be more common that Sturge-Weber syndrome (facial port-wine stain with associated glau-coma and neurologic anomalies). In a multicenter study of children with large facial IH (see Fig 2), 31% met diag-nostic criteria for PHACE syndrome.27,28
FIGURE 1
Criteria for the diagnosis of this dis-order were delineated by using a stan-dard consensus method with review of published data and a multidisciplinary team of experts.29,30 Current criteria
are listed in Table 2, as are those for possible PHACE (see Table 3). The syn-drome has a 9:1 female-to-male pre-dominance.27 PHACES syndrome also
includes sternal malformation and supraumbilical raphe.31
Ninety-eight percent of patients with PHACE syndrome have a large segmental IH present on the face or head. Rarely, the disorder can occur in association with IH located elsewhere or even in the ab-sence of IH; in such circumstances, consensus criteria would term this
“probable PHACE syndrome.”28,31,32,33
Brain and cerebral vascular anomalies are the most common extracutaneous features of PHACE syndrome;
neuro-logic and cognitive impairments remain the greatest source of morbidity in these patients.34 Central nervous
sys-tem arterial anomalies are the most common vascular abnormalities seen in PHACE. Cerebrovascular accidents in IH patients with PHACE syndrome have been reported, with no previous spe-cific anomaly; these patients tend to stabilize neurologically over time.35In
addition, structural abnormalities in-volving the posterior fossa and cere-bellum have also been associated with PHACE syndrome.
A number of cardiac abnormalities can occur in this syndrome, the most common of which is coarctation of the aorta. The overall incidence of eye ab-normalities is small and includes microphthalmia and optic nerve hypo-plasia.29,36,37
Facial hemangiomas that are$5 cm in diameter should prompt evaluation for PHACE syndrome including MRI and magnetic resonance arteriogram of the brain, cardiovascular imaging, and an ophthalmologic examination. The vessels of the upper chest and neck should also be evaluated.
Large, segmental IH in the anogenital region also carry a risk for associated underlying anomalies (see Fig 3). Sev-eral pneumonics have been coined (PELVIS, LUMBAR, SACRAL) to emphasize major features, which include a lum-bosacral or perineal IH in association with spinal cord, anogenital, and renal anomalies.38–40
Infants with multiple classic focal IH may have extracutaneous involvement
(see Fig 4). Although significant mor-tality was historically attributed to multifocal hemangiomas with organ involvement, termed“diffuse neonatal hemangiomatosis,” it is now appreci-ated that multifocal hemangiomas of-ten do not involve extracutaneous sites and that medical consequences are variable when they do.41The commonly
involved extracutaneous site is the liver, and hepatic ultrasound should be performed in patients with $5 cuta-neous IH.42 Consumptive
hypothyroid-ism has been associated with IH and other large vascular lesions of the liver. This is a result of excessive iodothyr-onine deiodinase expression, and such patients require monitoring and ag-gressive thyroid hormone supplemen-tation.43
MANAGEMENT
Although most IHs proliferate and in-volute without functional impairment, a significant minority requires some form of intervention.44It is important to
consider the psychological as well as medical impact of IH, particularly when located on the face. Many central facial lesions leave residual scars or struc-tural deformities, which may have life-long effects. In the past, treatment options for IH were limited and their potential side effects considerable. Al-though most IHs do not pose significant risks, and careful observation is still the appropriate management option for many lesions, the introduction of rela-tively safer topical and systemic agents now allows earlier and easier inter-vention in appropriate cases (Table 4). However, a Cochrane analysis of inter-ventions for IH noted that a lack of well-designed clinical trials and the absence of US Food and Drug Administration– approved medications for IH limits the ability to clearly identify the single best treatment option.25
Until recently, intralesional and systemic corticosteroids were the mainstay of TABLE 2 High-Risk IH Lesions
Location Type Growth Phase
Periorificiala(eyes, nose, mouth) Segmentalb Maximal proliferation phase (usually 3–6 mo)
Central Facial Multiple
Lumbosacralb
Rapidly proliferatingb Genitalb,c
aFunction-threatening. bHigh risk for ulceration.
cRisk of associated structural anomalies.
FIGURE 2
therapy for problematic IH. Intralesional injection can be used for localized lesions. Prednisone, administered orally at doses of 1 to 3 mg/kg per day, is an effective therapy for the majority of patients.45In a quantitative systematic
literature review, Bennett et al found that systemic corticosteroids had a 84% response rate with 36% rebound in infants with problematic IH.46However,
corticosteroid therapy has significant side effects, including increased risk for systemic infection, hypertension, increased appetite, stomach irritation, growth suppression, and cardiomyop-athy. In addition, some IHs are resistant to corticosteroid therapy. On the other hand, corticosteroids remain useful in certain situations, particu-larly in those who cannot tolerate other therapeutic options.
Other systemic therapeutic options have included interferon and vincristine.47
Adverse effects have limited the utility of both of these drugs. In particular, reports of serious side effects with in-terferon, including blood abnormalities and spastic diplegia in up to 20% of patients are cause for concern.48
Simi-larly, vincristine, a vinca alkaloid used
widely in cancer chemotherapy, is effi -cacious for life-threatening IH but also has limited use due to the strong vesi-cant qualities of the drug, with need for central line access for chronic admin-istration as well as potential peripheral mixed sensory-motor neurotoxicity.49Of
note, some of the data for interferon and vincristine may be complicated by the misdiagnosis of Kaposiform hem-angioendotheliomas or other vascular tumors as IH.
In recent years, propranolol therapy has become increasingly more useful in the management of IHs that require intervention. Leaute-Labreze et alfirst fortuitously discovered the efficacy of
b-blockers for the treatment of IH in 2008.50An infant developed
cardiomy-opathy after systemic corticosteroid therapy for a large facial IH. When propranolol therapy was initiated for this complication, a remarkable fl at-tening and fading of the IH occurred. The investigators subsequently docu-mented the drug’s efficacy as a fi rst-line, as well as second-line (after steroid use), therapy. All 11 patients were noted to have softening and change in color from red to purple of their IHs within 24 hours of starting propranolol. Subsequently,.170 reports and stud-ies have substantiated the usefulness of this drug.51–53 A prospective study
comparing propranol to placebo docu-mented drug efficacy.54 In a recent
multicenter retrospective chart review, propranolol therapy was found to be more clinically efficacious and cost-effective than oral corticosteroids in the treatment of IH.55 In addition,
propranolol therapy led to fewer sur-gical interventions and had fewer side effects compared with oral cortico-steroids. Most recently, a response rate of 98% was noted in a systemic review of 41 studies of.1200 children treated with propranolol. The mean dose was 2.1 mg/kg per day, and mean treatment duration was 6.4 months. Serious side TABLE 3 Anomalies Reported in PHACE Syndrome
Category Abnormality
Structural brain Posterior fossa: Dandy-Walker complex, cerebellar hypoplasia/atrophy, subependymal and arachnoid cysts
Hypoplasia or agenesis of cerebrum, corpus callosum, septum pellucidum, vermis
Polymicrogyria: microcephaly, heterotopia, absent pituitary or partially empty sella turcica
Cerebral vascular Dysplasia of the large cerebral arteries
Absence or moderate to severe hypoplasia of the large cerebral arteries Aberrant origin or course of the large cerebral arteries
Saccular aneurysms
Persistent embryonic arteries (predominantly trigeminal) Pial enhancement
Cerebral sinus malformations Sinus pericranii
Dural arteriovenous malformations/pial malformations Intracranial hemangioma
Arterial stenosis or occlusion with or without Moyamoya collaterals Absent foramen lacerum
Acute arterial stroke
Reprinted with permission from Metry DW, Heyer G, Hess C, et al. Consensus statement on diagnostic criteria for PHACE syndrome.Pediatrics. 2009;124(5):1447-1456.
FIGURE 3
Segmental IH in the sacral region.
FIGURE 4
effects were rare, occurring in,1% of patients.56 Unfortunately, direct
com-paritive studies of these 2 agents have not been performed.
Propranolol is a systemic nonselective
b-blocker that has been used for dec-ades in pediatric patients with cardio-vascular disease with standard dosing of 0.5 to 4mg/kg per day. Currently, many uncertainties exist regarding the appropriate and optimal use in chil-dren with IH, including optimal dosing, frequency of dosing, duration of ther-apy, age of therapy initiation, and timing and method of tapering to minimize the chance of rebound. An ongoing
in-ternational multicenter study may shed significant light on these issues.57
Theories regarding propranolol’s mechanism of action include an
ini-tial capillary vasoconstrictive effect, suppression/blockade of growth fac-tors with induction of apoptosis of endothelial cells,51,58 and blockade of
GLUT1 receptors.59 CD34+ endothelial
progenitor cells in IH express factors in-fluencing the renin-angiotensin system. Because the renin-angiotensin system can stimulate angiogenesis, Itinteang et al suggested that propranol’s in-hibitory effect on the renin-angiotensin
system might account for propranolol-induced involution.60
Although systemic propranolol is clearly efficacious, rare side effects, a few of which may be life-threatening, are cause for concern. These include symptomatic hypoglycemia, hypoten-sion, bronchial hyperreactivity, seizure, restless sleep, constipation, and cold extremities.59 Hypoglycemia has been
the most commonly noted serious ef-fect. Careful monitoring, particularly of medically fragile infants (eg small, premature, failure to thrive) is re-quired. Those patients with PHACE syn-drome should be evaluated for cerebral vascular anomalies because poor ce-rebral perfusion secondary to abnor-mal vessels can place them at higher risk for hypotensive stroke. Epinephrine injections for emergency treatment of allergic reactions may be ineffective in children on chronicb-blocker therapy. Therefore, the risk-benefit ratio must be considered when deciding to use pro-pranolol in children with a history of anaphylactic allergic reactions that might require epinephrine therapy. Collaborative care is often optimal for appropriate propranolol treatment of IH, including a pediatrician, dermatolo-gist, ophthalmolodermatolo-gist, cardiolodermatolo-gist, and other specialists, if needed.
Topical agents are appropriate therapy in some situations (eg, small, thin lesions), and their use is sometimes driven by parental anxieties or the de-sire for active therapy. The risks of ad-verse effects are less with topical rather than systemic agents, but there is lim-ited data on efficacy. Topical clobetasol has been used with some success, particularly in small periorbital lesions. However, concerns regarding atrophy, glaucoma, and cataracts exist with this drug. Investigators have also consid-ered the utility of topical, rather than systemic, b-blockers. Timolol maleate is a nonselective topicalb-blocker that has been approved for the treatment of TABLE 4 Management Options for IH
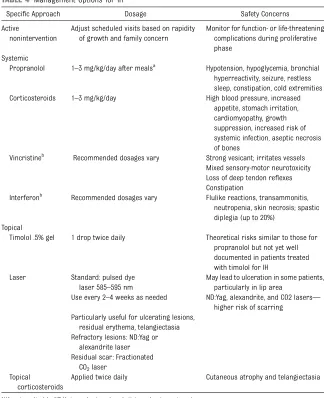
Specific Approach Dosage Safety Concerns
Active
nonintervention
Adjust scheduled visits based on rapidity of growth and family concern
Monitor for function- or life-threatening complications during proliferative phase
Systemic
Propranolol 1–3 mg/kg/day after mealsa
Hypotension, hypoglycemia, bronchial hyperreactivity, seizure, restless sleep, constipation, cold extremities Corticosteroids 1–3 mg/kg/day High blood pressure, increased
appetite, stomach irritation, cardiomyopathy, growth suppression, increased risk of systemic infection, aseptic necrosis of bones
Vincristineb Recommended dosages vary Strong vesicant; irritates vessels Mixed sensory-motor neurotoxicity Loss of deep tendon reflexes Constipation
Interferonb Recommended dosages vary Flulike reactions, transammonitis, neutropenia, skin necrosis; spastic diplegia (up to 20%)
Topical
Timolol .5% gel 1 drop twice daily Theoretical risks similar to those for propranolol but not yet well documented in patients treated with timolol for IH
Laser Standard: pulsed dye laser 585–595 nm
May lead to ulceration in some patients, particularly in lip area
Use every 2–4 weeks as needed ND:Yag, alexandrite, and CO2 lasers— higher risk of scarring
Particularly useful for ulcerating lesions, residual erythema, telangiectasia Refractory lesions: ND:Yag or
alexandrite laser Residual scar: Fractionated
CO2laser Topical
corticosteroids
Applied twice daily Cutaneous atrophy and telangiectasia
N/A, not applicable; ND:Yag, neodymium-doped yttrium aluminum garnet. aDosing guidelines have not been fully elucidated.
ocular glaucoma and hypertension in children and infants by the Food and Drug Administration.61The
ophthalmo-logic literature suggests that the side effects of timolol might be similar to those found in oral propranolol; how-ever, in studies of ophthalmic prepara-tions of timolol used topically for the treatment of IH, adverse effects repor-ted to date are limirepor-ted to a single epi-sode of severe sleep disturbance. Nonetheless, experts urge caution with the drug and recommend using no more than 1 drop twice a day to af-fected lesions.62
Recent studies showing efficacy of this topical agent for IH are promising, but most published works consist of indi-vidual cases or small pilot studies.63–67
The largest study to date found that the greatest indicators of optimal thera-peutic response were increased
con-centration and duration of drug
treatment, as well as superficial nature of the lesion.67
Other therapeutic options have been considered, but risk-benefit issues have thus far limited extensive investigations into these therapies. Angiogenesis inhibitors are theoretically a natural choice for the treatment of hemangio-mas. Sirolimus (also known as rapa-mycin), an inhibitor of mTOR, negatively affects cell proliferation and metabo-lism as well as angiogenesis. In vivo and in vitro studies have demonstrated suppression of IH growth in a mouse model. The drug appears to limit stem cell replicative capabilities and acts in a mechanism distinct from that of corti-costeroids.68Although this is a
poten-tially attractive therapeutic option, possible risks related to inhibition of angiogenesis, particularly in a growing organism raise concerns. At this time, it is most appropriate to restrict use to clinical trials until better safety data are available.
Procedural and multimodal therapies have been used to treat IH, but
con-troversy exists regarding their appro-priate role, and few evidence-based, controlled studies are available that evaluate pediatric laser therapy. Batta et al published a controlled trial in which the author noted no significant difference in outcome when pulsed dye laser (PDL; 585–595 nm) therapy was used in the treatment of IH; however, the suboptimalfluences used, lack of appropriate cooling during treatment, and positive evidence that laser-treated lesions healed faster weak-ened the validity of their assessment.69
Most experts believe that PDL therapy can diminish pain and hasten healing in ulcerating lesions, particularly in those located in the perineal area that have not responded to topical or sys-temic therapeutic measures. Treat-ment can also decrease redness and residual telangiectasias.
The ideal form of laser therapy is in a state of evolution, but PDL is the most commonly used modality. This therapy can safely diminish and sometimes eliminate superficial lesions with min-imal requirements for anesthesia and only rare scarring. It penetrates to a depth of ∼1 mm and is therefore most useful for superficial lesions or ulcers. Longer wavelength alexandrite or neodymium-doped yttrium alumi-num garnet laser therapy is some-times used for recalcitrant lesions but carries a higher risk for scarring. Fractionated CO2 laser holds promise for diminishing textural changes and scars that can develop in affected children. Multimodal therapy, using systemic agents in conjunction with ju-dicious use of laser or other procedural therapy, can optimize therapy for se-lected patients (see Figs 5, 6, and 7). Some experts use PDL as adjunctive or
“mop-up”therapy to treat residual tel-angiectasia or erythema, whereas others believe in early utilization when lesions areflatter and more amenable to therapy.70
Occasionally, surgical excision is the optimal therapeutic intervention. Ap-propriate lesions are often large pe-dunculated IH located in a site where a surgical scar will be less noticeable. In some instances, surgical intervention is inevitable because permanent, baggy residual scars develop. In such in-stances, health care providers may opt to remove the lesion early in its life cycle, given that a surgical intervention and scar are inevitable.
FIGURE 5
Segmental IH before propranolol and PDL ther-apy.
FIGURE 6
Segmental IH during propranolol and PDL ther-apy.
FIGURE 7
SUMMARY
Recent discoveries have led to an im-proved understanding of the patho-genesis and clinical behavior of IH. The best scientific evidence to date sup-ports the hypothesis that IHs originate from a subset of endothelial progenitor cells (CD133+) that are stimulated and proliferate under hypoxic con-ditions. These cells theoretically“hone” to areas of relative hypoxia, such as embryonic fusion planes. Perturbation of angiogenic factors may also play a role in the inappropriate prolif-eration of these cells. The clinician must be aware of potential anomalies that can occur in association with large segmental IH. Facial lesions raise
concern for PHACES, whereas lumbo-sacral and perineal lesions should raise suspicion for associated spinal cord, renal, and genital anomalies. When diagnosis is uncertain and in patients with high-risk lesions (Table 2), referral to a multidisciplinary vas-cular anomaly center with experienced subspecialists is optimal for patient care.
Newer treatment options for IH may well pose less risk for the patient, allowing the practitioner to intervene in a relatively safe, and more timely manner. Propranolol is now first-line therapy for many practitioners, and it is hoped that future studies will confirm its efficacy and safety. Timolol, a topical
b-blocker, may have a particular role in the treatment of superficial lesions. Pulsed dye and other laser modalities may be useful as adjunctive or“ mop-up” therapy. Other antiangiogenic agents may prove to be more effective in the future. However, it is important to proceed cautiously when imple-menting new therapies and evaluate appropriately for both short and long-term safety issues.71 The risk-benefit
ratio of any therapy must be scruti-nized, keeping in mind that“watchful waiting”may often be appropriate, but timely intervention is sometimes cru-cial in minimizing long-term sequelae such as functional deformity or per-manent scars.
REFERENCES
1. Kilcline C, Frieden IJ. Infantile hemangio-mas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168–173
2. Dickison P, Christou E, Wargon O. A pro-spective study of infantile hemangiomas with a focus on incidence and risk factors.
Pediatr Dermatol. 2011;28(6):663–669 3. Kanada KN, Merin MR, Munden A, Friedlander
SF. A prospective study of cutaneousfindings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomencla-ture.J Pediatr. 2012;161(2):240–245 4. North PE, Waner M, Mizeracki A, Mihm MC
Jr. GLUT1: a newly discovered immunohis-tochemical marker for juvenile hemangio-mas.Hum Pathol. 2000;31(1):11–22 5. Mihm MC Jr, Nelson JS. Hypothesis: the
metastatic niche theory can elucidate in-fantile hemangioma development.J Cutan Pathol. 2010;37(suppl 1):83–87
6. Walter JW, North PE, Waner M, et al. So-matic mutation of vascular endothelial growth factor receptors in juvenile
hem-angioma. Genes Chromosomes Cancer.
2002;33(3):295–303
7. Kleinman ME, Tepper OM, Capla JM, et al. Increased circulating AC133+ CD34+ endo-thelial progenitor cells in children with hemangioma.Lymphat Res Biol. 2003;1(4): 301–307
8. Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile
hemangioma. Blood. 2004;103(4):1373– 1375
9. Kleinman ME, Greives MR, Churgin SS, et al. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma.Arterioscler Thromb Vasc Biol. 2007;27(12):2664–2670
10. Khan ZA, Boscolo E, Picard A, et al. Multi-potential stem cells recapitulate human infantile hemangioma in immunodeficient mice.J Clin Invest. 2008;118(7):2592–2599 11. Drolet BA, Frieden IJ. Characteristics of
in-fantile hemangiomas as clues to patho-genesis: does hypoxia connect the dots?
Arch Dermatol. 2010;146(11):1295–1299 12. Barnés CM, Huang S, Kaipainen A, et al.
Evidence by molecular profiling for a pla-cental origin of infantile hemangioma.Proc Natl Acad Sci U S A. 2005;102(52):19097– 19102
13. Waner M, North PE, Scherer KA, Frieden IJ, Waner A, Mihm MC Jr. The nonrandom distribution of facial hemangiomas. Arch Dermatol. 2003;139(7):869–875
14. Pittman KM, Losken HW, Kleinman ME, et al.
No evidence for maternal-fetal
micro-chimerism in infantile hemangioma: a molec-ular genetic investigation.J Invest Dermatol. 2006;126(11):2533–2538
15. Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellu-lar markers that distinguish the phases of hemangioma during infancy and childhood.
J Clin Invest. 1994;93(6):2357–2364
16. Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered be-havior of endothelial cells from
hemangio-mas.J Clin Invest. 2001;107(6):745–752 17. Praveen V, Vidavalur R, Rosenkrantz TS,
Hussain N. Infantile hemangiomas and reti-nopathy of prematurity: possible association.
Pediatrics. 2009;123(3). Available at: www. pediatrics.org/cgi/content/full/123/3/e484
18. Mobasheri A, Richardson S, Mobasheri R, Shakibaei M, Hoyland JA. Hypoxia inducible factor-1 and facilitative glucose trans-porters GLUT1 and GLUT3: putative molec-ular components of the oxygen and glucose sensing apparatus in articular chondrocytes.
Histol Histopathol. 2005;20(4):1327–1338 19. Jinnin M, Medici D, Park L, et al.
Sup-pressed NFAT-dependent VEGFR1 expres-sion and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14 (11):1236–1246
20. Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362(11): 1005–1013
21. Ritter MR, Dorrell MI, Edmonds J, Fried-lander SF, FriedFried-lander M. Insulin-like growth factor 2 and potential regulators of hem-angioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci USA. 2002;99(11):7455–7460 22. Peichev M, Naiyer AJ, Pereira D, et al.
circulating human CD34(+) cells identifies a population of functional endothelial pre-cursors.Blood. 2000;95(3):952–958 23. Haggstrom AN, Drolet BA, Baselga E, et al;
Hemangioma Investigator Group. Prospective study of infantile hemangiomas: demo-graphic, prenatal, and perinatal character-istics.J Pediatr. 2007;150(3):291–294 24. Enjolras O, Mulliken JB, Boon LM, Wassef M,
Kozakewich HP, Burrows PE. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107(7):1647–1654
25. Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangio-mas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887
26. Frieden IJ, Reese V, Cohen D. PHACE syn-drome. The association of posterior fossa brain malformations, hemangiomas, arte-rial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities.
Arch Dermatol. 1996;132(3):307–311 27. Haggstrom AN, Garzon MC, Baselga E, et al.
Risk for PHACE syndrome in infants with large facial hemangiomas.Pediatrics. 2010; 126(2). Available at: www.pediatrics.org/ cgi/content/full/126/2/e418
28. Metry DW, Haggstrom AN, Drolet BA, et al. A prospective study of PHACE syndrome in infantile hemangiomas: demographic fea-tures, clinicalfindings, and complications.
Am J Med Genet A. 2006;140(9):975–986 29. Metry DW, Heyer G, Hess C, et al; PHACE
Syndrome Research Conference. Consen-sus statement on diagnostic criteria for PHACe syndrome. Pediatrics. 2009;124(5): 1447–1456
30. Metry DW, Garzon MC, Drolet BA, et al. PHACE syndrome: current knowledge, fu-ture directions.Pediatr Dermatol. 2009;26 (4):381–398
31. Chan YC, Eichenfield LF, Malchiodi J, Fried-lander SF. Small facial haemangioma and supraumbilical raphe—a forme fruste of PHACES syndrome?Br J Dermatol. 2005;153 (5):1053–1057
32. Torer B, Gulcan H, Kilicdag H, Derbent M. PHACES syndrome with small, late-onset hemangiomas.Eur J Pediatr. 2007;166(12): 1293–1295
33. Haggstrom AN, Lammer EJ, Schneider RA, Marcucio R, Frieden IJ. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial de-velopment. Pediatrics. 2006;117(3):698– 703
34. Metry DW, Dowd CF, Barkovich AJ, Frieden IJ. The many faces of PHACE syndrome.J Pediatr. 2001;139(1):117–123
35. Drolet BA, Dohil M, Golomb MR, et al. Early stroke and cerebral vasculopathy in chil-dren with facial hemangiomas and PHACE association. Pediatrics. 2006;117(3):959– 964
36. Cartwright MS, Hickling WH, Roach ES. Is-chemic stroke in an adolescent with arte-rial tortuosity syndrome.Neurology. 2006; 67(2):360–361
37. Layde PM, Dooley K, Erickson JD, Edmonds LD. Is there an epidemic of ventricular septal defects in the U.S.A.?Lancet. 1980;1 (8165):407–408
38. Girard C, Bigorre M, Guillot B, Bessis D. PELVIS syndrome.Arch Dermatol. 2006;142 (7):884–888
39. Iacobas I, Burrows PE, Frieden IJ, et al. LUMBAR: association between cutaneous infantile hemangiomas of the lower body
and regional congenital anomalies. J
Pediatr. 2010;157(5):795–801, e1–e7 40. Drolet B, Garzon M. SACRAL syndrome.
Dermatology. 2007;215(4):360–, author re-ply 360–361
41. Glick ZR, Frieden IJ, Garzon MC, Mully TW, Drolet BA. Diffuse neonatal hemangioma-tosis: An evidence-based review of case reports in the literature.J Am Acad Der-matol. 2012; [epub ahead of print]
42. Horii KA, Drolet BA, Frieden IJ, et al; Hem-angioma Investigator Group. Prospective study of the frequency of hepatic heman-giomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011;28(3):245–253
43. Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodo-thyronine deiodinase in infantile heman-giomas.N Engl J Med. 2000;343(3):185–189 44. Leonardi-Bee J, Batta K, O’Brien C, Bath-Hextall FJ. Interventions for infantile hae-mangiomas (strawberry birthmarks) of the skin. Cochrane Database Syst Rev. 2011; (5):CD006545
45. Bennett ML, Fleischer AB Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is ef-fective for cutaneous hemangiomas: an evidence-based evaluation.Arch Dermatol. 2001;137(9):1208–1213
46. Rössler J, Wehl G, Niemeyer CM. Evaluating
systemic prednisone therapy for
pro-liferating haemangioma in infancy. Eur J Pediatr. 2008;167(7):813–815
47. Ezekowitz RA, Mulliken JB, Folkman J. In-terferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326(22):1456–1463
48. Dubois J, Hershon L, Carmant L, Bélanger S, Leclerc JM, David M. Toxicity profile of in-terferon alfa-2b in children: A prospective evaluation.J Pediatr. 1999;135(6):782–785
49. Pérez-Valle S, Peinador M, Herraiz P, Saénz P, Montoliu G, Vento M. Vincristine, an effi -cacious alternative for diffuse neonatal haemangiomatosis. Acta Paediatr. 2010;99 (2):311–315
50. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649– 2651
51. Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangio-mas: follow-up report.Pediatrics. 2009;124 (3). Available at: www.pediatrics.org/cgi/ content/full/124/3/e423
52. Buckmiller L, Dyamenahalli U, Richter GT. Propranolol for airway hemangiomas: case report of novel treatment. Laryngoscope. 2009;119(10):2051–2054
53. Marsciani A, Pericoli R, Alaggio R, Brisigotti M, Vergine G. Massive response of severe infantile hepatic hemangioma to prop-anolol. Pediatr Blood Cancer. 2010;54(1): 176
54. Hogeling M, Adams S, Wargon O. A ran-domized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011; 128(2). Available at: www.pediatrics.org/ cgi/content/full/128/2/e259
55. Price CJ, Lattouf C, Baum B, et al. Pro-pranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147(12): 1371–1376
56. Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol for infantile hemangiomas: A meta analysis. Poster at The Society for Pediatric Dermatology annual meeting; July 2012; Monterey, CA
57. A randomized, controlled, multidose, multi-centre, adaptive phase II/III study in infants with proliferating infantile hemangiomas re-quiring systemic therapy to compare four regimens of propranolol (1 or 3 mg/kg/day for 3 or 6 months) to placebo (double blind). Ongoing (active) but not recruiting. Clinical trials.gov identifier: NCT01056341. Available at: http://clinicaltrials.gov/show/NCT01056341. Accessed November 7, 2012
58. Manunza F, Syed S, Laguda B, et al. Pro-pranolol for complicated infantile hae-mangiomas: a case series of 30 infants.Br J Dermatol. 2010;162(2):466–468
59. Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26(5):610–614
propranolol-induced accelerated involution.
J Plast Reconstr Aesthet Surg. 2011;64(6): 759–765
61. Coppens G, Stalmans I, Zeyen T, Casteels I. The safety and efficacy of glaucoma medi-cation in the pediatric population.J Pediatr Ophthalmol Strabismus. 2009;46(1):12–18 62. McMahon P, Oza V, Frieden IJ. Topical
ti-molol for infantile hemangiomas: putting a note of caution in“cautiously optimistic.”
Pediatr Dermatol. 2012;29(1):127–130 63. Ni N, Guo S, Langer P. Current concepts in
the management of periocular infantile (capillary) hemangioma. Curr Opin Oph-thalmol. 2011;22(5):419–425
64. Khunger N, Pahwa M. Dramatic response to topical timolol lotion of a large hemifacial
infantile haemangioma associated with PHACE syndrome.Br J Dermatol. 2011;164 (4):886–888
65. Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study.Arch Dermatol. 2010;146(5):564–565 66. Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010; 128(2):255–258
67. Chakkittakandiyil A, Phillips R, Frieden IJ, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangio-mas: a retrospective, multicenter, cohort study.Pediatr Dermatol. 2012;29(1):28–31 68. Greenberger S, Yuan S, Walsh LA, et al.
Rapamycin suppresses self-renewal and
vasculogenic potential of stem cells iso-lated from infantile hemangioma.J Invest Dermatol. 2011;131(12):2467–2476 69. Batta K, Goodyear HM, Moss C, Williams HC,
Hiller L, Waters R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangio-mas: results of a 1-year analysis. Lancet. 2002;360(9332):521–527
70. Admani S, Krakowski AC, Nelson JS,
Eichenfield LF, Friedlander SF. Beneficial effects of early pulsed dye laser therapy in patients with infantile hemangiomas. Der-matol Surg. 2012;38(10):1739–1740 71. Frieden IJ. Infantile hemangioma research:
looking backward and forward. J Invest Dermatol. 2011;131(12):2345–2348
HEAD BANGING:A few weeks ago, I saw two girls collide while trying to‘head’ a ball during a high school soccer game. One fell to the ground holding her hand to her face. I immediately thought that she had fractured her nose. However, the longer she stayed on the ground the more worried I became. Eventually, she got up and unsteadily made her way to the sideline. A few questions confirmed my suspicions: she had a concussion. It did not seem much of a knock so I was a bit surprised, especially since on my farm, I often see our ram hitting everything with his head and emerging unfazed. According to an article inThe New York Times (Science: October 1, 2012), animals that repetitively bang their heads (e.g. woodpeckers and antlered mammals) have developed specific adaptations to prevent brain damage. Their brains tend to be a bit smaller and have a smooth surface compared to the richly folded and textured human brain. Also, the skull tends to be quite thick and there is littlefluid between the brain and the skull, with the result that the brain does not jostle back and forth after impact—a key mechanism of injury in humans. Lastly, the animals minimize side-to-side torsion on their brains by banging their heads only along a single plane. Gannets, large seabirds that dive forfish, have an even more difficult problem. They dive from 100 feet—hitting the water at 60 mph—and continue chasingfish underwater, using their wings to propel them. A supersized skull would be heavy. Instead, the skull is narrow, laced with air pockets along the face, and designed to displace the energy of impact to the side. The human skull, while remarkable, is not designed to withstand the same sort of impact that rams, woodpeckers, and gannets routinely withstand. The implications to me are clear: always wear a bike helmet and be a bit careful whipping your head around for headers in a soccer game.
DOI: 10.1542/peds.2012-1128 originally published online December 24, 2012;
2013;131;99
Pediatrics
Services
Updated Information &
http://pediatrics.aappublications.org/content/131/1/99 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/131/1/99#BIBL This article cites 68 articles, 14 of which you can access for free at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2012-1128 originally published online December 24, 2012;
2013;131;99
Pediatrics
Tina S. Chen, Lawrence F. Eichenfield and Sheila Fallon Friedlander
Infantile Hemangiomas: An Update on Pathogenesis and Therapy
http://pediatrics.aappublications.org/content/131/1/99
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.