Ultrafiltration for Purification and Treatment of Water Streams in Swimming Pool Circuits
Full text
Figure
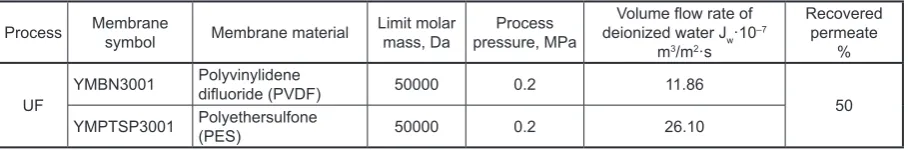
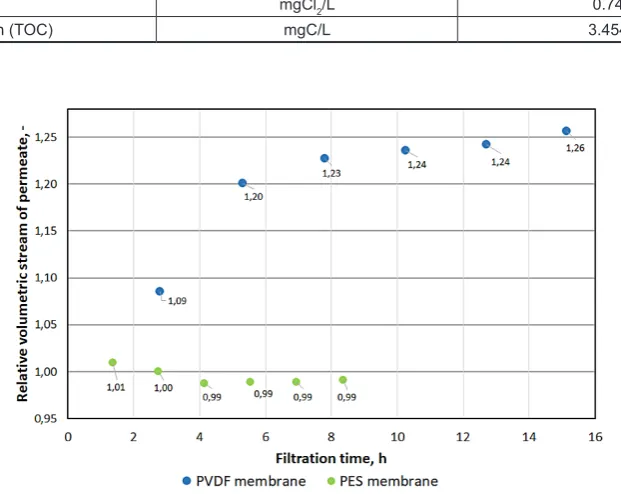
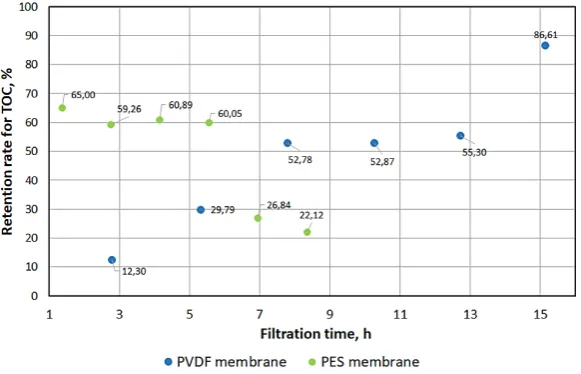
Related documents
[r]
The effort to open up markets for international trade in services results in the necessity of subordinating short- term national interests to a certain extent to long-term
The results suggest that personal economic well-being was significant in determining vote choice, with lower income voters marginally more likely to cast their vote for Kerry..
The undercarriage is a patented Liebherr mobile harbour crane chassis where the number of individually mounted sets of four wheels each can be easily adapted to comply with
[r]
a.. If you are given a molecule of CO 2 it means that there is 1 carbon atom bonded to 2 oxygen atoms.. The mass of the reactants in a chemical reaction must be equal to the mass
understanding, the partners do have plans to work together again in the future and the partnership was evaluated. SPINE began as a partnership between Cambridgeshire and Suffolk
Collected saliva was used for the assessment of salivary amylase activity using a colorimetric amylase assay kit (Abcam, Cambridge, UK) according to the manufacturer’s