R E S E A R C H A R T I C L E
Open Access
Predictive factors of neurologic
deterioration in patients with spontaneous
cerebellar hemorrhage: a retrospective
analysis
Yu-Ni Ho
1†, Shih-Yuan Hsu
2†, Yu-Tsai Lin
3, Fu-Chang Cheng
1, Yu-Jun Lin
2,5, Nai-Wen Tsai
4, Cheng-Hsien Lu
4,5and
Hung-Chen Wang
2*Abstract
Background:Cerebellar hemorrhage is a potentially life-threatening condition and neurologic deterioration during hospitalization could lead to severe disability and poor outcome. Finds out the factors influencing neurologic deterioration during hospitalization is essential for clinical decision-making.
Methods:One hundred fifty-five consecutive patients who suffered a first spontaneous cerebellar hemorrhage (SCH) were evaluated in this 10-year retrospective study. This study aimed to identify potential clinical, radiological and clinical scales risk factors for neurologic deterioration during hospitalization and outcome at discharge. Results:Neurologic deterioration during hospitalization developed in 17.4% (27/155) of the patient cohort.
Obliteration of basal cistern (p≦0.001) and hydrocephalus (p≦0.001) on initial brain computed tomography (CT), median Glasgow Coma Scale (GCS) score at presentation (p≦0.001) and median intracerebral hemorrhage (ICH) score (P≦0.001) on admission were significant factors associated with neurologic deterioration. Stepwise logistic regression analysis showed that patients with obliteration of basal cistern on initial brain CT scan had an odds ratio (OR) of 9.17 (p= 0.002; 95% confidence interval (CI): 0.026 to 0.455) adjusted risk of neurologic deterioration compared with those without obliteration of basal cistern. An increase of 1 point in the ICH score on admission would increase the neurologic deterioration rate by 83.2% (p= 0.010; 95% CI: 1.153 to 2.912). The ROC curves showed that the AUC for ICH score on presentation was 0.719 (p= 0.000; 95% CI: 0.613–0.826) and the cutoff value was 2.5 (sensitivity 80.5% and specificity 73.7%).
Conclusion:Patients had obliteration of basal cistern on initial brain CT and ICH score greater or equal to 3 at admission implies a greater danger of neurologic deterioration during hospitalization. Cautious clinical assessments and repeated brain images study are mandatory for those high-risk patients to prevent neurologic deterioration during hospitalization.
Keywords:Neurologic deterioration, ICH score, Spontaneous cerebellar hemorrhage, Risk factors
© The Author(s). 2019Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
* Correspondence:m82whc@gmail.com
†Yu-Ni Ho and Shih-Yuan Hsu contributed equally to this work.
2Departments of Neurosurgery, Kaohsiung Chang Gung Memorial Hospital,
Chang Gung University College of Medicine, 123, Ta Pei Road, Niao Sung, Kaohsiung, Taiwan
Background
Spontaneous cerebellar hemorrhage (SCH) account for represent 5 to 13% of all cases of spontaneous intracere-bral hemorrhage and about 15% of cerebellar strokes [1– 6]. It is often due to hypertension and the reported mor-tality rate within 6 months can reach 50%, and more than 60% of surviving patients had moderate or severe disability [6–8]. The prognostic risk factors of outcome in patients with SCH including hyperglycemia and platelet count at ad-mission, a larger hematoma volumes or diameter, a lower Glasgow Coma Scale (GCS) at admission, and imaging findings that reveal the initial presence of hydrocephalus, intraventricular hemorrhage (IVH), the appearance of the fourth ventricle, or basal cistern obliteration have been re-ported in several studies [2,6,8–12].
Because of its unique neurological location near the brainstem, neurologic deterioration usually results from brain stem compression due to the direct mass effect of the haematoma and/ or the development of hydroceph-alus [13]. However, here is still uncertainty about which risk factors significantly influence neurologic deterior-ation during hospitalizdeterior-ation in patients with SCH.
This study aimed to identify potential clinical, radio-logical and clinical scales risk factors to predict neuro-logic deterioration during hospitalization and outcome at discharge in patients with SCH.
Methods
Study design
This is a single-centre retrospective study. Medical re-cords were retrospectively reviewed using pre-existing standardized evaluation forms as well as brain computed tomography (CT) findings for patients with SCH admit-ted to the Department of Neurology or Neurosurgery in our tertiary academic centre from January 2005 to April 2015. The study approved by the Institutional Review Board (IRB)/Ethics Committee (Institutional Review Board numbers: 104-0985B).
Clinical assessment
On admission, a detailed physical examination, the rou-tine laboratory testing, and brain imaging were evaluated for all patients. The initial neurologic state was evaluated by the Glasgow Coma Scale (GCS). Then systolic blood pressure, diastolic blood pressure, heart rate, and body temperature were recorded immediately before brain computed tomography (CT) scanning. After CT scan, the ICH score was calculated by the all parameters [14]. The ICH score includes the following items: Glasgow Coma Scale score, age, infratentorial origin of ICH, cere-bellar hemorrhage (CH) volume, and presence of IVH. The ICH Score was the sum of individual points assigned as follows: GCS score 3 to 4 (=2 points), 5 to 12 (=1), 13 to 15 (=0); age > =80 years yes (=1), no (=0);
infratentorial origin yes (=1), no (=0); CH volume > =30 cm(3) (=1), < 30 cm(3) (=0); and intraventricular hemorrhage yes (=1), no (=0). The maximal score is 5 and the lowest score is 0.
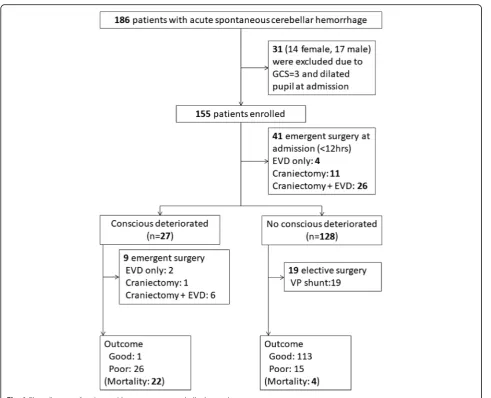
Acute SCH was diagnosed by the clinical history and brain CT. Patients were excluded if they had: 1) non-spontaneous cerebellar hemorrhage, such as traumatic cerebellar hemorrhage; 2) SCH caused by a primary or secondary brain tumor, cavernomas, arteriovenous malfor-mations or aneurysms, or hemorrhagic transformation of a cerebellar infarct; and 3) preexisting neurological condi-tions with various neurological deficits (such as stroke, head trauma, and hypoxic encephalopathy). From January 2005 to April 2015, a total of 186 patients with acute SCH were admitted to our hospital. This study is to evaluate neurologic deterioration during hospitalization, therefore, GCS =3 and no brain stem reflex on presentation was ex-cluded. Finally, a total of 155 patients were enrolled in the studies (Fig.1).
The SCH volumes were calculated the area occupied by the hyperdense hematoma with the ABC/2 formula in the initial brain CT. In the formula, A indicated the largest diameter of hemorrhage by CT, B indicated the largest diameter perpendicular to A, and C indicated the CT slice thickness multiplied by the number of CT slices of the hemorrhage [10,15].
In the study, we determined the presence of hydroceph-alus retrospectively by the dilated temporal horns of the ventricle. The image feature is consistent with obstructive hydrocephalus. [13, 16, 17]. The image feature of brain stem compression was judged by basal cistern obliteration in the brain CT [18, 19]. The fourth ventricle appearance was divided into 3 grades, according to Kirollos et al. study [1], as following: Grade 1, the 4th ventricle is in normal size and configuration, and located in the midline (if intraven-tricular hemorrhage is present, the cerebrospinal fluid is visible in the 4th ventricle); Grade 2, the 4th ventricle was partially compressed, distorted or shifting to the contralat-eral side (in cases of unilatcontralat-eral hematomas); Grade 3, the 4th ventricle is completely obliteration with shift distorting the brainstem anteriorly and obliteration of the prepontine space (even if partial compression of the 4th ventricle).
In the study, the neurosurgical interventions for man-agement SCH were performed with three types: external ventricular drainage (EVD) only, midline suboccpital craniectomy for hematoma evacuation, and suboccpital craniotomy plus EVD.
The patients were divided into two groups according to the discharge outcome: 1) The good outcome group with independent performance of daily activities (Glas-gow Outcome scale (GOS) score = 4 or 5). 2) The poor outcome group with disability of daily living, vegetative states, or death (GOS score = 1, 2, or 3) [10]..
Statistical analysis
The descriptive data were showed as both median and inter-quartile range (IQR). Categorical variables were accessed by the Chi-square test or Fisher’s exact test. The Mann-Whitney U test was used for the continuous vari-ables analysis. The Spearman rank test was applied for cor-relation analysis for the cor-relationship between age, GCS and laboratory data. Statistical significance was set atp< 0.05.
We used stepwise logistic regression analysis for evalu-ating the association between significant variables and pa-tients with neurologic deterioration during hospitalization and outcome. ROC curve was generated to estimate an
optimal cut-off value for ICH score on admission, and the area under ROC curve was measured. All statistical ana-lyses were conducted using the SPSS software (IBM SPSS statistic version 22.0).
Results
From January 2005 to April 2015, a total of 186 patients with SCH were admitted to our hospital. Thirty-one pa-tients whose GCS was 3 and without pupil reflex at presentation were excluded. A total of 155 patients fi-nally were enrolled in this study (Fig.1).
Baseline characteristics and clinical features
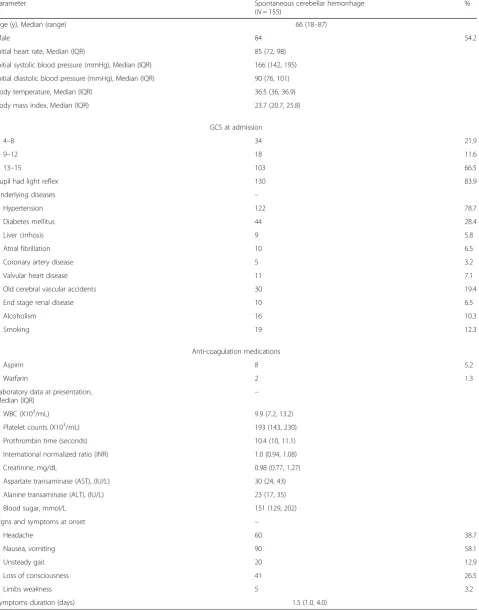
Table 1Characteristics of patients with spontaneous cerebellar hemorrhage
Parameter Spontaneous cerebellar hemorrhage (N= 155)
% Age (y), Median (range) 66 (18–87)
Male 84 54.2
Initial heart rate, Median (IQR) 85 (72, 98) Initial systolic blood pressure (mmHg), Median (IQR) 166 (142, 195) Initial diastolic blood pressure (mmHg), Median (IQR) 90 (76, 101) Body temperature, Median (IQR) 36.5 (36, 36.9) Body mass index, Median (IQR) 23.7 (20.7, 25.8)
GCS at admission
4–8 34 21.9
9–12 18 11.6
13–15 103 66.5
Pupil had light reflex 130 83.9
Underlying diseases –
Hypertension 122 78.7
Diabetes mellitus 44 28.4
Liver cirrhosis 9 5.8
Atrial fibrillation 10 6.5
Coronary artery disease 5 3.2
Valvular heart disease 11 7.1
Old cerebral vascular accidents 30 19.4
End stage renal disease 10 6.5
Alcoholism 16 10.3
Smoking 19 12.3
Anti-coagulation medications
Aspirin 8 5.2
Warfarin 2 1.3
Laboratory data at presentation,
Median (IQR) –
WBC (X103/mL) 9.9 (7.2, 13.2)
Platelet counts (X103/mL) 193 (143, 230) Prothrombin time (seconds) 10.4 (10, 11.1) International normalized ratio (INR) 1.0 (0.94, 1.08) Creatinine, mg/dL 0.98 (0.77, 1.27) Aspartate transaminase (AST), (IU/L) 30 (24, 43) Alanine transaminase (ALT), (IU/L) 23 (17, 35) Blood sugar, mmol/L 151 (129, 202) Signs and symptoms at onset –
Headache 60 38.7
Nausea, vomiting 90 58.1
Unsteady gait 20 12.9
Loss of consciousness 41 26.5
Limbs weakness 5 3.2
Symptoms duration (days) 1.5 (1.0, 4.0)
mean initial heat rate on arrive the hospital was 85 bpm (IQR 72-98 bpm) and the mean systolic arterial blood pres-sure on arrival in the hospital was 166 mmHg (IQR 142-195 mmHg). 26.5% (41/155) patients showed loss of conscious-ness at initial presentation. Otherwise, signs of SCH and in-creased intracranial pressure (e.g., unsteady gaits, nausea or vomiting) were presented at 90 patients (58.1%). The men symptoms duration was 1.5 (1.0–4.0) days.
Neuroradiological findings
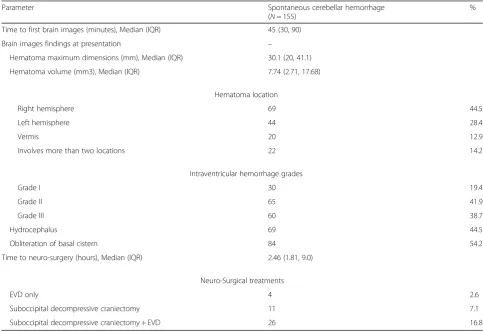
Neuroradiological characteristics and neuro-surgical treat-ment at presentation were listed in Table 2. The median interval between hospital admission and the first brain CT was 45 min (IOR: 30–90 min). The median maximum hematoma dimension was 30.1 mm(IOR: 20–41.1 mm), corresponding to a median volume of 7.74 cm3 (IQR: 2.71–17.68 cm3
) Hydrocephalus in initial image was pre-sented in 69 patients (44.5%). The appearance of 4th ven-tricle compression was grade I in 30 (19.4%), grade II in 65 (41.9%), and grade III in 60 (38.7%) study sample pa-tients. Eighty-four patients (54.2%) exhibited obliteration of the basal cistern.
Forty-one patients (26.5%) received neurosurgical inter-vention, including 11 patients (7.1%) with suboccpital
decompressive craniectomy, 4 patients (2.6%) with EVD only, and 26 patients (16.8%)with both suboccpital decom-pressive craniectomy and EVD. There were 10 patients underwent the re-operative craniectomy. The median time between symptoms and surgery was 2.46 h (IQR: 1.81–9.0 h). The median time of neurologic deterioration after ad-mission was 12.5 h (IQR: 6–72 h).
Factors predict of neurologic deterioration during hospitalization and the outcome
Twenty-six (26/155, 16.8%) patients died during hospitalization. Twenty-three died of the hemorrhage. Three patients died of unrelated causes, one was aspir-ation pneumonia, one was massive GI bleeding, and the other one was uncontrolled liver cirrhosis. Factors pre-dict of neurologic deterioration during hospitalization and the outcome in patients with SCH were listed in Table 3. In total 27 patients (17.4%) with SCH had neurologic deterioration during the hospitalization, 15 patients had spontaneous decrease in GCS motor score of 2 points or more from the previous examination, one had further loss of pupillary reactivity, three had devel-opment of pupillary asymmetry greater than 1 mm, five had both spontaneous decrease in GCS motor score of 2
Table 2Radiological characteristics at presentation and neuro-surgical treatments
Parameter Spontaneous cerebellar hemorrhage (N= 155)
% Time to first brain images (minutes), Median (IQR) 45 (30, 90)
Brain images findings at presentation –
Hematoma maximum dimensions (mm), Median (IQR) 30.1 (20, 41.1) Hematoma volume (mm3), Median (IQR) 7.74 (2.71, 17.68)
Hematoma location
Right hemisphere 69 44.5
Left hemisphere 44 28.4
Vermis 20 12.9
Involves more than two locations 22 14.2 Intraventricular hemorrhage grades
Grade I 30 19.4
Grade II 65 41.9
Grade III 60 38.7
Hydrocephalus 69 44.5
Obliteration of basal cistern 84 54.2 Time to neuro-surgery (hours), Median (IQR) 2.46 (1.81, 9.0)
Neuro-Surgical treatments
EVD only 4 2.6
Suboccipital decompressive craniectomy 11 7.1 Suboccipital decompressive craniectomy + EVD 26 16.8
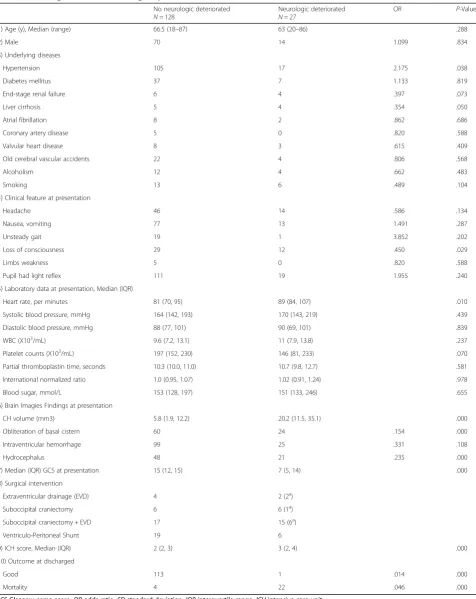
points and further loss of pupillary reactivity, and three had both spontaneous decrease in GCS motor score of 2 points and development of pupillary asymmetry greater than 1 mm. Statistical analysis revealed significant asso-ciations of clinical feature and laboratory data at presen-tation are underlying disease with hypertension (p = 0.038), liver cirrhosis (p= 0.005), the initial presentation with loss of consciousness (p= 0.029), and elevated heart rate (p = 0.010). Statistical analysis revealed significant associations at initial neuro-image findings are median CH volume (p≦0.001), the presentation of obliteration of basal cistern (p≦0.001) and hydrocephalus (p≦0.001). But there is no significant statistical difference for pa-tients with presentation of intraventricular hemorrhage in initial CT scan(P= 0.108). GCS and ICH score at presentation showed significant statistical difference be-tween the two groups (both p≦0.001).
The functional outcome at discharged, the neurologic deterioration group showed poor outcome compared to the no neurologic deterioration group. Only 1 of 27 pa-tients in neurologic deterioration group had good out-come, compared to 113 of 128 patients in no neurologic deterioration group (p≦0.001). Of these 27 patients had neurologic deterioration during hospitalization, 22 died during hospitalization. Of the five patients survived, only one had good outcome (GOS =5) with minor neurologic deficit (persistent headache, dizziness, and unsteady gait), two were vegetative state (GOS =2) and the other two were severe disability state (GOS =3) at discharged. Moreover, the SCH patients with neurologic deterior-ation during hospitalizdeterior-ation revealed a specific high mor-tality rate during hospitalization. 22 of 27(81.4%) patients died during hospitalization in the neurologic de-terioration group, compared to 4 of 128 (3.1%) patients died during hospitalization in no neurologic deterior-ation groups (P≦0.001).
Stepwise logistic regression analysis identified obliteran of basal cistern at initial brain CT and the higher ICH score as independent risk factors for neurologic deterior-ation during hospitalizdeterior-ation. (P= 0.002 andP= 0.010, re-spectively) The adjusted risk of SCH patients with neurologic deterioration during hospitalization with oblit-eran of basal cistern at initial brain CT scan had odds ratio (OR) of 9.17 (95% confidence interval (CI): 0.026 to 0.455) compared with those without obliteran of basal cistern at initial brain CT scan. Furthermore, an increase of 1 point in ICH score on admission would increase the risks of neurologic deterioration during hospitalization rate by 83.2% (p= 0.010; 95% CI: 1.153 to 2.912).
To determine the relationship between ICH score on the initial presentation and risks of neurologic deterior-ation during hospitalizdeterior-ation, the ROC curves were gen-erated. The AUC for ICH score on presentation was 0.719 (p≦0.001, 95% CI: 0.613–0.826). The cutoff value
of ICH score on presentation was 2.5 (sensitivity 80.5% and specificity 73.7%).
Discussion
In our study, 17.4% patients with SCH had neurologic deterioration during hospitalization and most of these patients (26/27, 96.3%) had poor outcome. Arterial hypertension, initial loss of consciousness, the presenta-tion of obliteran of basal cistern, hydrocephalus and CH volume in the initial brain CT, lower GCS and higher ICH score are statistically significant in patients with risks of neurologic deterioration. Although those factors are statistically significant in patients with neurologic de-terioration, only obliteran of basal cistern at initial brain CT and the higher ICH score as independent risk factors (p= 0.002 andp= 0.010, respectively).
Several studies described the risks factors associated with neurologic deterioration in patients with spontan-eous intracerebral hemorrhage. Flemming et.al [15] followed 61 patients with spontaneous supratentorial in-tracerebral hemorrhage and they demonstrated that the large volume lobar hematoma with consciousness dis-turbance (GCS < 14) and midline shift on CT are at risk for further deterioration. One recent meta-analysis [21] study mentioned the risks associated with early neuro-logic deterioration are hematoma volume, glucose con-centration, fibrinogen concentration, and d-dimer concentration at hospital admission. Those studies were focused on supratentorial intracerebral hemorrhage. Many studies showed that initial impaired consciousness is a risk factor for poor outcome in patients with acute SCH [14,22–24], however, there are limited studies spe-cific focused on the risks of neurologic deterioration in patients with SCH or infratentorial hemorrhage.
In one previous study including 76 patients with SCH, the preoperative GCS and IVH were not prognostic factor for the patients’outcome [13]. However, the GCS at admis-sion was the risk factor for neurologic deterioration during hospitalization in our study. The difference between the two studies is the endpoint is different. Tsitsopoulos et.al study is to predict patient’s outcome, but our study is fo-cused on neurological deterioration during hospitalization. However, most patients had neurological deterioration dur-ing hospitalization in our study had poor outcome.
Table 3Neurologic deterioration during hospitalization and outcome No neurologic deteriorated N= 128
Neurologic deteriorated N= 27
OR P-Value
(1) Age (y), Median (range) 66.5 (18–87) 63 (20–86) .288
(2) Male 70 14 1.099 .834
(3) Underlying diseases
Hypertension 105 17 2.175 .038
Diabetes mellitus 37 7 1.133 .819
End-stage renal failure 6 4 .397 .073
Liver cirrhosis 5 4 .354 .050
Atrial fibrillation 8 2 .862 .686
Coronary artery disease 5 0 .820 .588
Valvular heart disease 8 3 .615 .409
Old cerebral vascular accidents 22 4 .806 .568
Alcoholism 12 4 .662 .483
Smoking 13 6 .489 .104
(4) Clinical feature at presentation
Headache 46 14 .586 .134
Nausea, vomiting 77 13 1.491 .287
Unsteady gait 19 1 3.852 .202
Loss of consciousness 29 12 .450 .029
Limbs weakness 5 0 .820 .588
Pupil had light reflex 111 19 1.955 .240
(5) Laboratory data at presentation, Median (IQR)
Heart rate, per minutes 81 (70, 95) 89 (84, 107) .010
Systolic blood pressure, mmHg 164 (142, 193) 170 (143, 219) .439
Diastolic blood pressure, mmHg 88 (77, 101) 90 (69, 101) .839
WBC (X103/mL) 9.6 (7.2, 13.1) 11 (7.9, 13.8) .237
Platelet counts (X103
/mL) 197 (152, 230) 146 (81, 233) .070
Partial thromboplastin time, seconds 10.3 (10.0, 11.0) 10.7 (9.8, 12.7) .581
International normalized ratio 1.0 (0.95, 1.07) 1.02 (0.91, 1.24) .978
Blood sugar, mmol/L 153 (128, 197) 151 (133, 246) .655
(6) Brain Imagies Findings at presentation
CH volume (mm3) 5.8 (1.9, 12.2) 20.2 (11.5, 35.1) .000
Obliteration of basal cistern 60 24 .154 .000
Intraventricular hemorrhage 99 25 .331 .108
Hydrocephalus 48 21 .235 .000
(7) Median (IQR) GCS at presentation 15 (12, 15) 7 (5, 14) .000
(8) Surgical intervention
Extraventricular drainage (EVD) 4 2 (2a)
Suboccipital craniectomy 6 6 (1a
)
Suboccipital craniectomy + EVD 17 15 (6a)
Ventriculo-Peritoneal Shunt 19 6
(9) ICH score, Median (IQR) 2 (2, 3) 3 (2, 4) .000
(10) Outcome at discharged
Good 113 1 .014 .000
Mortality 4 22 .046 .000
GCSGlasgow coma score, ORodds ratio, SDstandard deviation, IQRinterquartile range, ICUintensive care unit, CHcerebellar hemorrhage
(a
): emergent surgery while conscious deteriorated during hospitalization
of mortality in patients with SCH in this study. St. Louis et al. [25] followed 72 SCH patients and mentioned that acute hydrocephalus is the radiologic feature for predict-ing neurologic deterioration in patient with SCH. Our study also demonstrated the same result. In addition, the obliteration of basal cistern seen at initial brain CT may directly compress brainstem and cause neurologic de-terioration. Previous studies also showed that the im-paired conscious state seen in cerebellar hemorrhage can largely be attributed to the development of hydro-cephalus, resulting in intracranial hypertension and/or direct brain stem compression [13,24]. The both image feature imply the increase of posterior fossa intracranial pressure and impairment of the CSF circulation due to the cerebellar hematoma mass effect.
The ICH score was first introduced by Hemphill et al. [26] in 2001 and was a simple and reliable scale for pre-dicting the 30-day mortality in patients with spontan-eous intracerebral hemorrhage. The ICH score includes the following items: Glasgow Coma Scale score, age, infratentorial origin of ICH, CH volume, and presence of intraventricular hemorrhage. The maximal score is 5 and the lowest score is 0. Therefore, the ICH score is more representative than each of the above single fac-tors. Several studies described the role of ICH score for predicting the outcome in patients with spontaneous intracranial hemorrhage [27–29]. In our study, an crease of 1 point in ICH score on admission would in-crease the risks of neurologic deterioration during hospitalization rate by 83.2%. ROC curve revealed the cutoff value of ICH score on presentation was 2.5 (sensi-tivity 80.5% and specificity 73.7%). Base on our study, because of risks of neurologic deterioration and high possibility of poor outcome, we suggest early cautious assessments for neurosurgical intervention for those SCH patients whose ICH score are greater or equal to 3.
In our study, the median time of neurologic deterior-ation after admission is 12.5 h. Of the 27 patients had neurological deterioration during hospitalization, only one had good outcome. For those high risk patients, cautious clinical assessment and repeated neuro-image study within the first 12 h after admission should be considered.
There are several limitations in our study. First, it is a retrospective review. Therefore, the decision for treatment of acute SCH, such as surgery, was individualized in each case, taking into account patient comorbidities. Second, neurologic deterioration can occur in both the acute stage and later stages during treatment. The findings may under-estimate the “true” frequency of late neurologic deterior-ation in those patients who had been discharged. Thus, there is continued uncertainty in assessing the incidence of neurologic deterioration after SCH in non-selected patients. Lastly, this is a single hospital, small patient sample study.
However, after excluding secondary cerebellar hemorrhage caused by trauma, tumors, cavernomas, arteriovenous mal-formations or aneurysms, hemorrhagic transformation of a cerebellar infarct, patients with “pure” acute SCH are not easy to collect. It needs prospective, multi-center research to clarify the causes of neurologic deterioration and out-come in future.
Conclusions
In our study, 17.4% patients with SCH had neurologic de-terioration during hospitalization and most of these pa-tients (26/27, 96.3%) had poor outcome. Obliteration of basal cistern and hydrocephalus in the initial brain CT, lower GCS scale and higher ICH score on presentation implies a greater danger of neurologic deterioration dur-ing hospitalization. The median time of neurologic deteri-oration after admission was 12.5 h. Cautious clinical assessment, repeated neuro-image study within the first 12 h after admission and possibility of early neurosurgical intervention needed to be recognized by clinical physi-cians for high-risk patients, especially in those with ICH score greater or equal to 3, in order to early management the presence of neurologic deterioration in SCH patients.
Abbreviations
CGS:Glasgow Coma Scale; CSF: Cerebrospinal fluid; CT: Computed tomography; EVD: External ventricular drainage; GOS: Glasgow Outcome scale; ICH score: Intracerebral hemorrhage score; IVH: Intraventricular hemorrhage; SCH: Spontaneous cerebellar hemorrhage
Acknowledgements
Not applicable.
Funding
None to report.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Authors’contributions
Conceptualization: HCW; Data collection: YNH, SYH, and FCC; Data Analysis: LYT, YJL, and NWT; Writing - original draft: YNH and SYH; Writing - review& editing: LYT, NWT, CHL and HCW. All authors read and approved the final manuscript.
Authors’information
Not applicable.
Ethics approval and consent to participate
This study was permitted by the Institutional Review Board (IRB)/Ethics Committee of Kaohsiung Chang Gung Memorial Hospital (Institutional Review Board numbers: 104-0985B). Written informed consent was sought from all participants, in cases suffered severe disability and were not able to give informed consent, this was obtained from their legally authorized repre-sentatives as required by the IRB.
Consent for publication
Not applicable.
Competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author details
1Departments of Emergency Medicine, Kaohsiung Chang Gung Memorial
Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan.
2
Departments of Neurosurgery, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, 123, Ta Pei Road, Niao Sung, Kaohsiung, Taiwan.3Departments of Otolaryngology, Kaohsiung Chang Gung
Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan.4Departments of Neurology Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan.
5Department of Biological Science, National Sun Yat-Sen University,
Kaohsiung, Taiwan.
Received: 20 September 2018 Accepted: 18 April 2019
References
1. Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurgery. 2001;49(6):1378–86; discussion 86-7.
2. Hsu SY, Chang HH, Shih FY, Lin YJ, Lin WC, Cheng BC, et al. Risk factors for Ventriculoperitoneal shunt dependency in patients with spontaneous cerebellar hemorrhage. World Neurosurg. 2017;105:63–8.
3. Mathew P, Teasdale G, Bannan A, Oluoch-Olunya D. Neurosurgical management of cerebellar haematoma and infarct. J Neurol Neurosurg Psychiatry. 1995;59(3):287–92.
4. Kobayashi S, Sato A, Kageyama Y, Nakamura H, Watanabe Y, Yamaura A. Treatment of hypertensive cerebellar hemorrhage--surgical or conservative management? Neurosurgery. 1994;34(2):246–50 discussion 50-1.
5. Lui TN, Fairholm DJ, Shu TF, Chang CN, Lee ST, Chen HR. Surgical treatment of spontaneous cerebellar hemorrhage. Surg Neurol. 1985;23(6):555–8. 6. Chang CY, Lin CY, Chen LC, Sun CH, Li TY, Tsai TH, et al. The predictor of
mortality within six-months in patients with spontaneous cerebellar hemorrhage: a retrospective study. PLoS One. 2015;10(7):e0132975. 7. Datar S, Rabinstein AA. Cerebellar hemorrhage. Neurol Clin. 2014;32(4):993–1007. 8. Al Safatli D, Guenther A, McLean AL, Waschke A, Kalff R, Ewald C.
Prediction of 30-day mortality in spontaneous cerebellar hemorrhage. Surg Neurol Int. 2017;8:282.
9. Tao C, Hu X, Wang J, You C. Effect of admission hyperglycemia on 6-month functional outcome in patients with spontaneous cerebellar hemorrhage. Med Sci Monit. 2017;23:1200–7.
10. Lin CY, Chang CY, Sun CH, Li TY, Chen LC, Chang ST, et al. Platelet count and early outcome in patients with spontaneous cerebellar hemorrhage: a retrospective study. PLoS One. 2015;10(3):e0119109.
11. van Loon J, Van Calenbergh F, Goffin J, Plets C. Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir. 1993;122(3–4):187–93. 12. Witsch J, Neugebauer H, Zweckberger K, Juttler E. Primary cerebellar
haemorrhage: complications, treatment and outcome. Clin Neurol Neurosurg. 2013;115(7):863–9.
13. Tsitsopoulos PP, Tobieson L, Enblad P, Marklund N. Prognostic factors and long-term outcome following surgical treatment of 76 patients with spontaneous cerebellar haematoma. Acta Neurochir. 2012;154(7):1189–95. 14. Doukas A, Maslehaty H, Barth H, Hedderich J, Petridis AK, Mehdorn HM.
A novel simple measure correlates to the outcome in 57 patients with intracerebellar hematomas. Results of a retrospective analysis. Surg Neurol Int. 2015;6:176.
15. Flemming KD, Wijdicks EF, St Louis EK, Li H. Predicting deterioration in patients with lobar haemorrhages. J Neurol Neurosurg Psychiatry. 1999; 66(5):600–5.
16. Greenberg J, Skubick D, Shenkin H. Acute hydrocephalus in cerebellar infarct and hemorrhage. Neurology. 1979;29(3):409–13.
17. Wang YM, Lin YJ, Chuang MJ, Lee TH, Tsai NW, Cheng BC, et al. Predictors and outcomes of shunt-dependent hydrocephalus in patients with aneurysmal sub-arachnoid hemorrhage. BMC Surg. 2012;12:12. 18. Mezzadri JJ, Otero JM, Ottino CA. Management of 50 spontaneous
cerebellar haemorrhages. Importance of obstructive hydrocephalus. Acta Neurochir. 1993;122(1–2):39–44.
19. Taneda M, Hayakawa T, Mogami H. Primary cerebellar hemorrhage. Quadrigeminal cistern obliteration on CT scans as a predictor of outcome. J Neurosurg. 1987;67(4):545–52.
20. Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The executive Committee of the International Selfotel Trial. J Neurosurg. 2000;92(1):1–6.
21. Specogna AV, Turin TC, Patten SB, Hill MD. Factors associated with early deterioration after spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96743.
22. Dammann P, Asgari S, Bassiouni H, Gasser T, Panagiotopoulos V, Gizewski ER, et al. Spontaneous cerebellar hemorrhage--experience with 57 surgically treated patients and review of the literature. Neurosurg Rev. 2011;34(1):77–86. 23. Han J, Lee HK, Cho TG, Moon JG, Kim CH. Management and outcome of
spontaneous cerebellar hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2015;17(3):185–93.
24. Pong V, Chan KH, Chong BH, Lui WM, Leung GK, Tse HF, et al. Long-term outcome and prognostic factors after spontaneous cerebellar hemorrhage. Cerebellum. 2012;11(4):939–45.
25. St Louis EK, Wijdicks EF, Li H. Predicting neurologic deterioration in patients with cerebellar hematomas. Neurology. 1998;51(5):1364–9.
26. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–7.
27. Appelboom G, Hwang BY, Bruce SS, Piazza MA, Kellner CP, Meyers PM, et al. Predicting outcome after arteriovenous malformation-associated intracerebral hemorrhage with the original ICH score. World Neurosurg. 2012;78(6):646–50.
28. Hemphill JC, 3rd, Farrant M, Neill TA, Jr. Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73(14):1088–1094. 29. Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. A novel risk score to