AP Chemistry
Chapter 5: Gases
Objective: Students will review the following topics from Chemistry: pressure; Boyle’s, Charles’,
A gas
Uniformly fills any container.
Mixes completely with any other gas
Pressure
force/unit area
SI unit = Newton/meter2 = 1 Pascal (Pa)
1 standard atmosphere = 1 atm
Figure 5.1a:
Figure 5.1b:
As the can cools, the
water vapor condenses, lowering the gas
See video at
Figure 5.2: A torricellian
barometer. The tube, completely
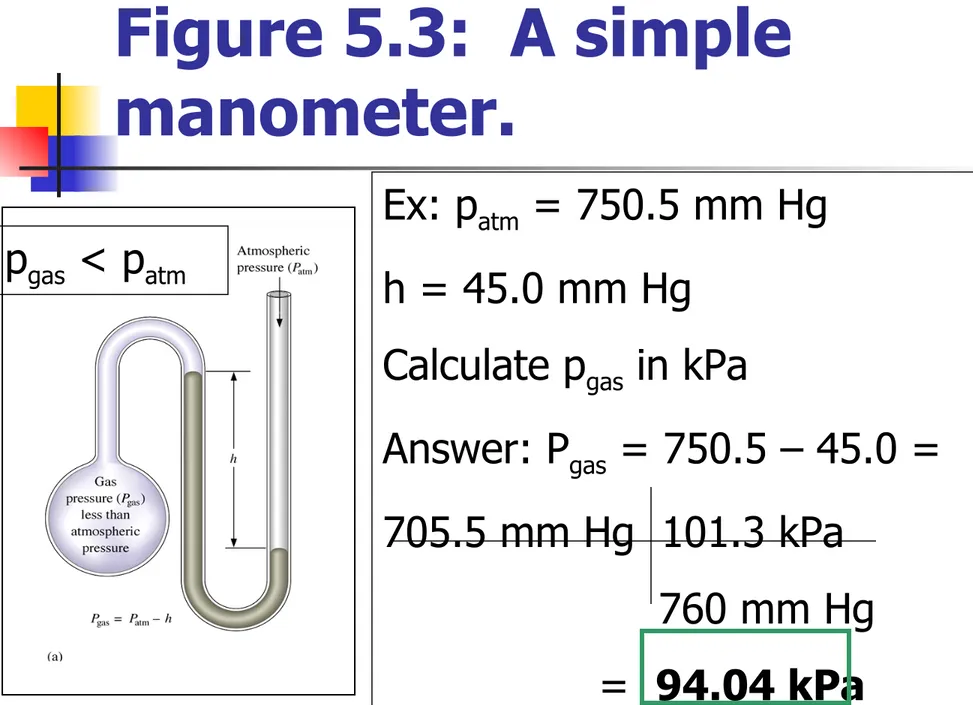
Figure 5.3: A simple
manometer.
Ex: patm = 750.5 mm Hg
h = 45.0 mm Hg
Calculate pgas in kPa
Answer: Pgas = 750.5 – 45.0 =
705.5 mm Hg 101.3 kPa 760 mm Hg
= 94.04 kPa
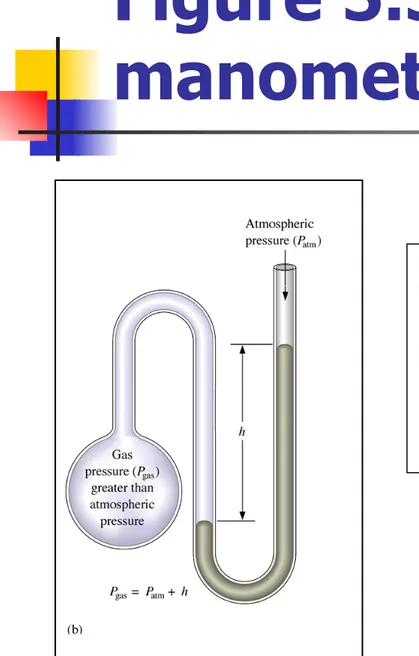
Figure 5.3: A simple
manometer.
Boyle’s law
pressure volume = constant
(T = constant)
P1V1 = P2V2 (T = constant)
Robert Boyle
1627 – 1691
Father of modern chemistry
Instrumental in founding the Royal
Society
Developed the vacuum pump
First to publish the details of his work,
including unsuccessful experiments
First use of the term “chemical
Figure 5.4:
Figure 5.5:
Plotting Boyle’s data. (a) A plot of P versus V
shows that the volume
doubles as the pressure is halved.
(b) A plot of V versus 1/P
gives a straight line. The slope of this line equals the value of the constant
Charles’ law
The volume of a gas is directly
proportional to temperature, and extrapolates to zero at 0 Kelvin.
V
T
V
T
P
1 1 2 2Jacques Charles
(1746-1823)
Worked for the Bureau of Finances Met Ben Franklin in Paris 1779
Self-taught physicist, began giving public lectures named a resident member of the Académie des
Sciences 1795
Amateur balloonist, ascending in a hydrogen
balloon to an altitude of 10,000 ft. and redesigned hot air balloon.
His work with gases resulted in the forming of
Combined gas law
P
1
V
1= P
2V
2Avogadro’s law
Figure 5.10: Equal volumes at equal P and T…
Therefore they contain _____ numbers of
molecules.
Ideal gas law
Ideal gas law
P
V
=
n
R
T
R = proportionality constant = 8.31 L kPa molK
P = pressure in kPa
V = volume in liters
n = moles
T = temperature in Kelvins
Ex. What volume is occupied by 50.0 g of nitrogen (N2) at 100.0 kPa and 20.°C?
PV = nRT V = nRT
P
= 50.0 g N2
= 43.3 L
mol N2
28.0 g N2
8.31 L kPa mol K
293 K
To find molar mass of an
unknown gas based on density:
M = mRT = DRT
Standard Temperature and
Pressure
“STP”
P = 1 atmosphere
T = 0C
Problem 1
20.0 g 44.9 L = 11.2 L
Problem 2
PV = nRT P = nRT/V
P = 0.010 x 10-3 g mol 8.31 L kPa 296 K
28.0 g mol K 5.0 L = 1.76 x 10-4 kPa 1 atm
End of first lesson on gases
Dalton’s law of partial
pressures
For a mixture of gases in a container,
Figure 5.12: The partial pressure of each gas in a mixture of gases in a
container depends on the
First, divide each mass by molar mass to get moles.
15.0 g halothane/197.38 g/mol = 0.0760 mol halothane 23.5 g O2 / 32.00 g/mol = 0.7344 mol O2
Add up to get total moles, then divide each number by the total to get mole fraction.
Total moles = 0.0760 + 0.7344 = 0.8104 moles Then multiply mole fractions by the total pressure:
Figure 5.13: The production of oxygen by thermal decomposition of KClO3. The MnO2 is mixed with the KClO3 to speed up the
Ex: A CFC has an empirical formula of
CHF2. To find the molecular formula, you must find the molar mass. A 0.100 g
sample of the compound exerts a pressure of 70.5 mmHg in a 256 mL container at
M = DRT= P
0.100 g 8.31 L kPa 295.5 K 760 mm Hg
0.256 L mol K 70.5 mm Hg 101.3 kPa
M = 102.1 g/mol
MM/efm = 102.1/51.0 = 2
Molecular formula = 2(CHF2) = C2H2F4
Ex: A CFC has an empirical formula of CHF2. To find the molecular formula, you must find the molar mass. A 0.100 g sample of the
Ex: Calculate the density of dry air at 15.0 C and 1.00 atm, if its average molar mass is 28.96 g/mol.
M = DRT/P
D = MP = 28.96 g 101.3 kPa mol K RT mol 288 K 8.31 L kPa
327 mL O2; 19 °C; Ptotal = 735 torr; pH2O = 16.5 mm Hg
n = PV/RT
= (735 – 16.5 mm Hg) 101.3 kPa 0.327 L mol K 760 mm Hg 292 K 8.31 L kPa = 0.0129 mol O2
0.0129 mol O2 2 mol KClO3 122. 55 g KClO3
3 mol O2 mol KClO3
= 1.05 g 100% 1.56 g
Kinetic Molecular Theory
(KMT) of gases
1. Volume of individual particles is zero.
2. Collisions of particles with container walls cause pressure exerted by gas.
3. Particles exert no forces on each other.
The meaning of temperature
Kelvin temperature is an index of the random motions of gas particles (higher T means
greater motion.)
( K E )
3
2
a v g
R T
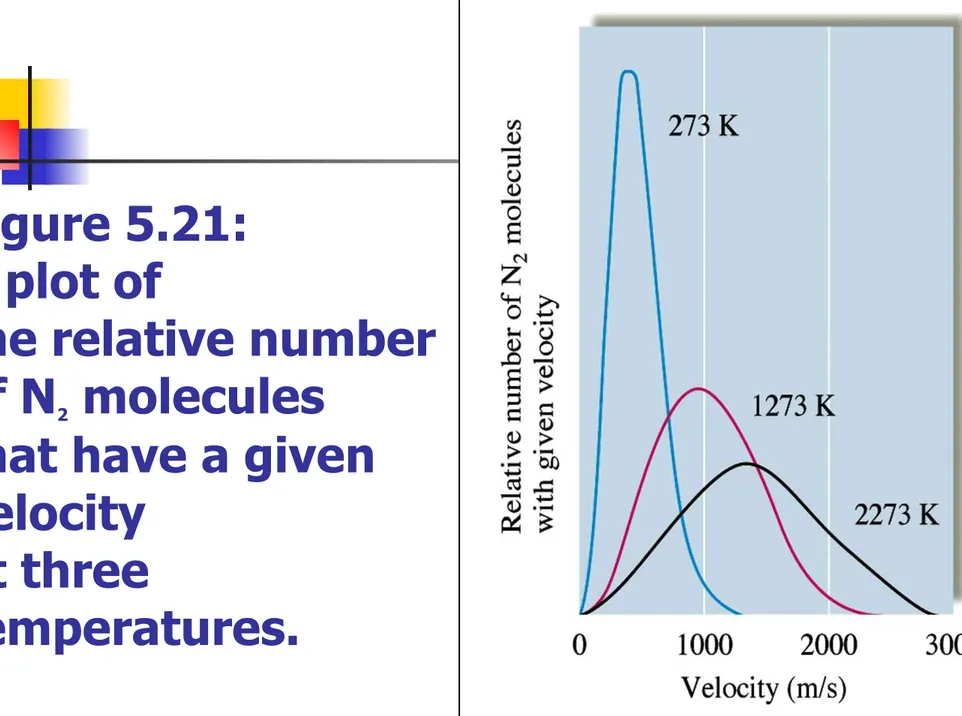
Figure 5.21: A plot of
the relative number of N2 molecules
that have a given velocity
at three
Root-mean-square velocity
2 3
8.3145 in Kelvin
= mass of one mole of gas particles in kg
rms
RT
u u
M
R J mol K
T M
The symbol means the average of the squares
of the particle velocities.
The square root of is called the root mean
square velocity and is symbolized by u rms.
2
u
2
u
CB p. 18
Diffusion vs. effusion
Diffusion: describes the mixing of gases. The rate of diffusion is the rate of gas
mixing.
Effusion: the process in which a gas
escapes through a small hole
R a t e o f e f f u s i o n f o r g a s 1
R a t e o f e f f u s i o n f o r g a s 2
2
1
M
Ex: Tetrafluoroethylene, C2F4, effuses through a barrier at a rate of 4.6 x 10-6
mol/h. An unknown gas, consisting of only boron and hydrogen, effuses at the rate of 5.8 x 10-6 mol/h under the same
vBH? = √MC2F4
vC2F4 √ MBH?
5.8 … = √(100.02) 4.6 … √ x
Square both sides.
X = 100.02 x 4.62/5.82 = 62.9 g/mol
Ex: Tetrafluoroethylene, C2F4, effuses through a barrier at a rate of 4.6 x 10-6 mol/h. An unknown
gas, consisting of only boron and hydrogen, effuses at the rate of 5.8 x 10-6 mol/h under the same
Figure 5.23: Relative molecular
Figure 5.24: (top) When HCl(g) and NH3(g) meet
in the tube, a white ring of NH4Cl(s) forms.
(bottom) A demonstration of the relative diffusion
Real gases
We must correct ideal gas behavior for
gases at high pressure (smaller volume) and low temperature (when attractive forces become important).
Only a qualitative understanding of this
Real gases
[ P o b s a ( /n V ) 2 ] V n b n R T
corrected pressure
corrected pressure corrected volumecorrected volume
P
Pidealideal VVidealideal