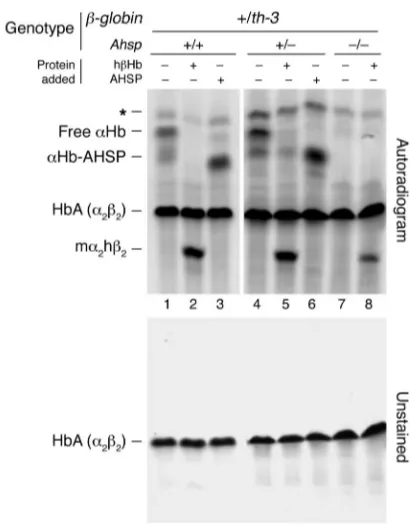
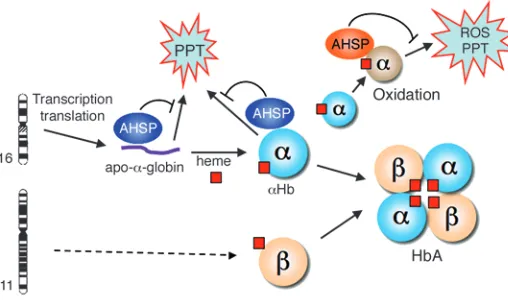
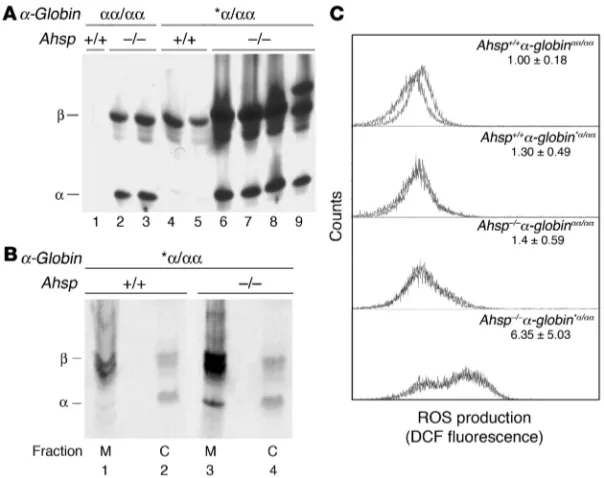
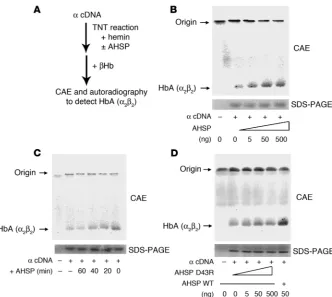
An erythroid chaperone that facilitates folding of α globin subunits for hemoglobin synthesis
Full text
Figure


Related documents
Therefore, in this thesis the diversity, abundance and activity of specific groups of chemolithoautotrophic microorganisms involved in the nitrogen and sulfur cycling, such
Exposure to fish cues led to reduced growth and increased metabolic rates but had little effect on 33.. the body %P content
In the newfound oligotrophic state of the lake with oxic bottom waters, terrestrial organic carbon provides an important substrate for microbes, which provides a source of carbon
The Danish Reform Strategy · October 2005 3 Fiscal sustainability requires, in particular, moderate growth in real public consump- tion of ½ per cent per year in the period
As presented above, the Department of Public Safety (State Police) and Department of Motor Vehicles share in the inspection of commercial vehicles and the issuance of
Determining the total work duration for a task involves knowledge of the quantity of work required for the task and the production rate for the specific crew
The chaperone protein SecB binds to the nascent polypeptide chain to prevent premature folding which would make transport across the plasma
The previous discussion clarifies why I mainly suggested using the loss distribution approach with comonotonic losses or (better) with the canonical aggregation model via