D. Tampieri1 R. Moumdjian D. Melanson R. Ethier
Received October 19. 1990; revision requested November 30. 1990; revision received February 1, 1991; accepted February 4, 1991.
' All authors: Department of Diagnostic Radiol-ogy, Montreal Neurological Hospital and Institute, 4801 University St., Montreal, Quebec, Canada H3A 284. Address reprint requests to D. Tampieri.
0195-6108/91/1204-07 49
© American Society of Neuroradiology
Intracerebral Gangliogliomas in
Patients with Partial Complex
Seizures:
CT and MR Imaging Findings
749
The clinical and radiologic findings in 19 patients with partial complex seizures and surgically proved intracerebral gangliogliomas were reviewed to characterize the radio-logic features of these lesions. The CT and MR findings were not specific. On CT the gangliogliomas can be hypodense with no enhancement and they often have calcifica-tions. On MR these tumors have a wide variety of signals. In five of our cases the tumor had a high-intensity signal with a cystlike component on proton density- and T2 -weighted images. In five cases the lesion had an inhomogeneously intense signal on proton density-weighted images and high signal intensity on T2-weighted images. The tumor had high-intensity signal on both proton density- and T2-weighted images in four patients. Finally, in two cases the MR findings were normal.
We recommend MR as the examination of choice for patients with partial complex seizures because it allows an artifact-free evaluation of the temporal region. However, CT should also be performed in order to recognize calcifications that may be missed on the MR examination.
AJNR 12:749-755, July/August 1991; AJR 157: October 1991
Gangliogliomas are rare, slow-growing, relatively benign neoplasms consisting
of a mixture of adult ganglion cells and glial tumor cells. They are often responsible
for a prolonged clinical course, characterized primarily by seizures. To better define
the imaging characteristics of these tumors, we reviewed the clinical and radiologic
findings in 19 patients presenting with partial complex seizures who were subse -quently found to have surgically proved gangliogliomas.
Materials and Methods
From January 1987 to August 1990, 19 patients, 11 men and eight women, 7 to 44 years
old (mean age, 21 years), were referred to our institute for intractable partial complex seizures
(PCoS). PCoS are one of the features found in temporal lobe epilepsy. They often, but not always, start with motor arrest typically followed by oroalimentary automatism lasting less
than 1 min, which in turn is followed by post-ictal confusion and amnesia [1). These patients
received a complete clinical and radiologic work-up and underwent surgery for removal of intracerebral space-occupying lesions histologically proved to be gangliogliomas. EEG fin d-ings, skull radiographs, and CT scans were available for all patients. CT examinations were performed with an EMI scanner 1 01 0 and a GE 9800 Quick scanner. In eight cases the CT scans were obtained without contrast enhancement; in six cases the examinations were performed after injection of contrast medium. Five patients had both plain and enhanced CT examinations. Eighteen patients were studied by MR imaging; in one patient (case 4) the examination was stopped because the boy had a seizure. In five cases, the MR examinations
were performed with a Philips Gyroscan 0.5-T unit, and T1-weighted, 500{30/2 (TRJTE/
excitations), proton density-weighted, and T2-weighted (1500{30,60/2) images were o b-tained. In 13 cases, a 1.5-T unit was used, and T1-weighted (500/30/2), proton densit
750 TAMPIERI ET AL. AJNR:12, July/August 1991
TABLE 1: Clinical and Electroencephalographic Findings
Case Age Type of Age at Free Interval Frequency of EEG
Tumor Location
Sex Onset
No. (years) Seizures
(years) (years) Attacks Focus
1 7 M PCoS + left fms 11 months 6 4/day R temp Rtemp
2 11 F PCoS + 1 gen 3 8 2-3fweek R posterior R posterotemp
temp/
occ
3 12 M PCoS 2 10 L temp L temp
4 12 M PCoS 3 months 11 6fyear L temp L temp
5 14 M PCoS + da 11 3 L temp L temp
6 15 F PCoS + gen 5 10 Frequent L temp L temp
7 16 F PCoS 8 8 8-10/month R+L L temp
temp
8" 17 M PCoS 14 3 L temp L frontoopercular
9 17 F PCoS 6 11 1fweek R temp R temp
10 18 M PCoS 12 6 10/month R temp R temp
11" 21 M PCoS 2 19 10fmonth L temp L temp
12 25 M PCoS + gen 9 16 (PCoS)/8 12fmonth L temp L temp
(gen)
13 25 F PCoS 12 13 R temp R temp
14 27 M PCoS + gen 10 17 4fyr (gen) R temp R temp
15 27 F PCoS + abs 16 11 3/month (abs) R abn R temp
16 32 M PCoS + gen 21 11 2fyear R abn R frontoopercular
17 33 F PCoS 13 20 2fday L abn L temp
18 34 F PCoS + gen 8 26 3/day Rtemp R temp
19 44 M PCoS + gen 31 13 1fmonth R temp Rtemp
Note.-PCoS = partial complex seizures, fms = focal motor seizures, gen = generalized seizures, da = drop attacks, abs =absence episodes, R = right, L =
left, temp = temporal lobe, occ = occipital lobe, abn = abnormality. • Previous partial resection in another center.
• Previous partial corticectomy and partial amygdalo-hippocampectomy.
were available only in seven cases. None of the MR studies were contrast enhanced, since this study was carried out before the clinical use of gadopentetate dimeglumine in Canada.
Results
Clinical and EEG Findings (Table 1)
The familial and medical histories were unremarkable in 16 patients in our series. One patient (case 5) was born 3 weeks post-term without any evidence of perinatal sequela. Another patient (case 18) was suffering from Rosenthal syndrome caused by deficiency of factor XI. A 33-year-old woman (case 17) developed PCoS after an episode reported as encephalitis atage13.
All the patients were referred for intractable PCoS. In seven cases, these episodes were associated at an older age with tonic-clonic seizures and secondary generalization. Case 1 presented with PCoS and left focal motor seizures. In one patient (case 5) the PCoS were associated with drop attacks,
and in another (case 15) with absences, characterized by short episodes of loss of contact without automatism.
The age of onset of seizure disorders ranged from 3 months to 31 years. The frequency of the attacks, when specified,
ranged from four attacks per day to two per year at the time of admission.
All the patients had a prolonged clinical course with an
important free interval between the onset of the seizures and the radiologic diagnosis. This time interval ranged from 3 to 26 years (mean, 11 V2 years).
The EEGs showed epileptiform abnormality in all the cases in our series.
In seven patients the EEG reports described an abnormal discharge with a right temporal focus, and in seven cases the focus was found in the left temporal region. In case 7 the abnormal discharge was from both temporal lobes, and in case 2 the abnormality was from the right posterior tempo-rooccipital area. In only three patients (cases 15, 16, and 17) did the EEG demonstrate an abnormal discharge lateralized to one cerebral hemisphere with no evidence of focal anom-alies.
Radiologic Findings (Table 2)
Skull Radiographs. In no case did the plain films reveal any evidence of raised intracranial pressure or mass effect with displacement of the calcified pineal gland. In four patients flocular calcifications were visualized in the middle cranial fossa on the same side of the lesion. In cases 8 and 11, the plain films documented the previous craniotomy.
CT Scans. The CT findings were available in all cases. In 15 patients it was positive: four lesions (cases 1, 4, 15, 16) were hypodense (Figs. 1 and 2); two lesions (cases 7 and 8) were ring-enhancing (Fig. 3), and in six patients (cases 2, 3, 6, 9, 10, 13) the examination demonstrated only calcifications (Fig. 4). Two CT studies (cases 14 and 18) showed asymmetry of the temporal horn (Fig. 5), the larger being on the tumoral side, and in case 12 the CT study demonstrated only a prominent left hippocampus. In three cases (5, 17, 19) the examination was negative, and, finally, in case 11 there was only evidence of the previous surgery.
[image:2.613.55.565.91.314.2]AJNR:12. July/August 1991 INTRACEREBRAL GANGLIOGLIOMAS 751
TABLE 2: Radiologic Findings
CT MR
Case Age
No. (years) Sex Skull Proton Location
Plain Enhanced T1
Density T2
1 7 M Neg Hypodense jis + cystlike jis + cystlike R temp
2 11 F Neg Calcifications No enh R posterotemp
3 12 M Calcifications Calcifications No enh lnh is + cyst- lnh is + cyst- L temp
like like
4 12 M Neg Hypodense L temp
5 14 M Neg Neg lis lnh is jis L temp
6 15 F Neg Calcifications No enh jis jis L temp
7 16 F Neg Ring enh lso is jis + cystlike jis + cystlike L temp
8' 17 M Prev. crani- Ring enh + jis + cystlike jis + cystlike L frontoopercular
otomy
calcifica-tion
9 17 F Calcification Calcification No enh ISO iS jis +small jis +small R temp
void sig void sig
10 18 M Calcifications Calcifications lnh is jis R temp
11" 21 M Prev. crani- Prev. surgery L temp
otomy Prev. surgery
12 25 M Neg Prominent lso is jis jis L temp
hippocam-pus
13 25 F Calcifications Calcifications lnh is+ void jis +void R temp
sig sig
14 27 M Neg Asymmetric Asymmetric tem- Neg Neg Rtemp
temporal poral horn R > L
horn R > L
15 27 F Neg Hypodense Hypo is + cystlike jis + cystlike jis + cystlike Rtemp
16 32 M Hypodense No enh lnh is jis R frontoopercular
17 33 F Neg Neg Neg Neg L temp
18 34 F Neg Asymmetric lis lnh is jis R temp
temporal horn R > L
19 44 M Neg Neg jis jis R temp
Note.-neg = negative examination, - = examination not done, no enh = no enhancement after injection, inh is = inhomogeneously intense signal, iso is = isointense signal, hypo is = hypointense signal, jis = high-intensity signal, Vs = low-intensity signal, void sig = void signal, R = right, L = left, temp = temporal lobe.
' Previous partial resection in another center.
"Previous partial corticectomy and partial amygdalo-hippocampectomy.
(88%). One MR examination (case 17) was negative; one (case 14) showed the right temporal horn to be larger than the left (Fig. 5); and one (case 11) demonstrated prior surgery but no evidence of residual tumor.
The gangliogliomas demonstrated a wide variety of signals. In five patients (cases 1, 3, 7, 8, 15) the lesion had a signal similar to CSF on proton density-weighted images and higher than CSF on T2-weighted images (Figs. 1-3). In five cases the tumor had an inhomogenously intense signal on proton density-weighted images that became hyperintense on the T2-weighted images (cases 5, 10, 13, 16, 18). Finally, the lesion had a high-intensity signal on both proton density- and T2-weighted images in four patients (cases 6, 9, 12, 19).
MR detected calcifications in only two patients (cases 9 and 13) (Fig. 4). T1-weighted images were available in only seven patients: they showed the lesions to be isointense in three cases, hypointense in two cases, and isointense with CSF in one case (Fig. 2).
Discussion
The first description of gangliogliomas was given by Cour-ville in 1930 [2]. The term ganglioglioma clearly defines the structure of these uncommon tumors as a mixture of nerve
cells and glial elements. They constitute just 0.4% of all CNS tumors [3, 4]. This percentage has been reported to be as high as 4.3% [5] and 7.6% [6] in studies that factor out the adult population, reflecting the fact that this tumor is more common among children. The tumors do not have any sex predilection, but some authors report a predominance in males [2, 7]. Gangliogliomas can occur in any number of locations in the CNS, and can involve both brain and spinal cord; however, the most common locations in the adult pop-ulation seem to be the temporal lobe and the floor of the third ventricle [3, 8]. In the present series we included only lesions causing PCoS, and for this reason a preference for the temporal lobe, particularly the hippocampal gyrus, was found. In childhood, gangliogliomas seem to occur more frequently in the medulla and spinal cord [9] and the differential diagnosis
with the more benign gangliocytoma has to be considered.
This latter tumor, rarely supratentorial [1 0, 11] and purely neuronal without a glial component, is an extremely rare
entity, accounting for 0.1% of intracranial tumors [12] with a
slight male predilection [13], and it cannot be radiologically
differentiated from ganglioglioma.
The clinical course suggests that gangliogliomas are
slow-growing neoplasms, and a malignant transformation is very
uncommon. When it happens it is usually the glial element
[image:3.612.56.564.93.386.2]752 TAMPIERI ET AL. AJNR:12, July/August 1991
c
D
appears to be exceptional [14]. Some authors have also raised the possibility that gangliogliomas are hamartomatous lesions since they have been found in temporal lobes of patients with a long history of seizures [15] and because collections of heterotopic gray matter similar to ganglioglioma can cause seizures [16]. Nowadays, the literature seems to agree that gangliogliomas are true neoplasms and their re-moval has to be attempted [5, 17].
The EEG findings usually suggest the focal abnormality that must be confirmed by the imaging findings. Radiologi-cally, gangliogliomas do not have a specific appearance. Calcifications can be visualized on a skull radiograph in 1 0%
of patients [18]. Although the skull radiograph is a superfluous examination, it was performed in all our patients and we observed four cases (21 %) with flocular calcifications in the middle cranial fossa.
CT findings also are nonspecific. Gangliogliomas are usually hypodense [19] with calcifications [20]. In our series CT scans were positive in 12 patients (63%); six of them (32%) dem
-Fig. 1.-Case 1.
A and B, Unenhanced CT scans show two
small, poorly defined areas of hypodensity
(ar-rowheads).
C, Axial proton density-weighted (2100/30/1) MR image better defines the lesion, which ap-pears as two low-intensity cystlike areas
sur-rounded by a high-intensity signal in right tem-poromesial region (arrowheads).
D, Coronal T2-weighted (2100/80/1) MR im
-age of tumor shows increased signal intensity. Cystlike component is surrounded by
high-inten-sity signal, which involves the cortex and adja-cent white matter (arrowhead).
onstrated only abnormal calcifications, and the other six showed a wide variety of findings. In no case in our series did
CT demonstrate edema or mass effect.
In a small series [21] of four patients studied with MR imaging, two lesions had a cystic appearance while the other two displayed an increased signal on T1-weighted images and a decreased signal on T2-weighted images. The authors did not try to explain these findings. In a larger series of 14 patients reported by Castillo et al. [22] there were four cystic lesions (low signal on T1-weighted images and high signal on T2-weighted images) and 1 0 solid masses that demonstrated high-intensity signal on T2-weighted images. The authors did not mention whether the cystic nature of the lesions observed on MR was confirmed later at surgery.
[image:4.612.57.396.76.486.2]AJNR:12, July/August 1991 INTRACEREBRAL GANGLIOGLIOMAS 753
A
B
C
Fig. 2.-Case 15.
A, Enhanced CT scan shows well-defined hypodense nonenhancing lesion (arrowhead).
8 and C, T1-weighted (500/30/1) (8) and T2-weighted (2100/80/1) (C) MR images show cystlike tumor (arrowhead) in right inferotemporal gyrus surrounded by some edema.
A
B
c
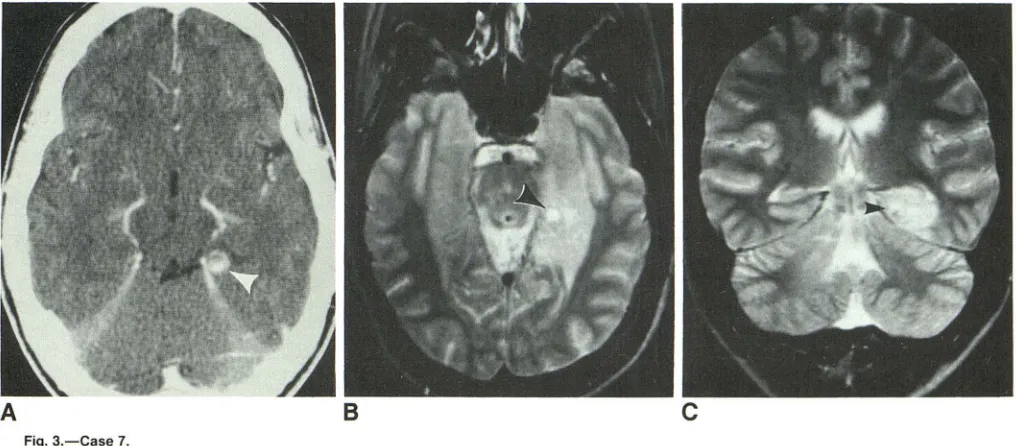
Fig. 3.-Case 7.
A, Enhanced CT scan shows ring-enhancing lesion (arrowhead) in left posterior parahippocampal region.
8 and C, Axial (8) and coronal (C) T2-weighted (2100/80/1) MR images enable complete evaluation of the tumor, which involves the left hippocampus and parahippocampus gyri. The ganglioglioma has a high-intensity signal and a central cystlike nodule (arrowheads), which corresponds to the ring
-enhancing lesion seen on the enhanced CT scan.
in five patients (29%) the lesions displayed inhomogeneous signal on proton density-weighted images and high-intensity signal on T2-weighted images. Finally, in four cases (24%) the tumors had high signals on both proton density- and T2-weighted images. In only two patients (12%) was MR able to detect calcifications (Fig. 4). We prefer to use the term cystlike appearance to describe the MR characteristic of a well-defined area with signal intensity similar to CSF on proton
density-weighted images and higher than CSF on T2-weighted images. This is because the content of the lesions is not fluid but solid; therefore, it cannot be drained but has
to be removed, as was the case in the patients in our series.
MR failed to show the tumor in two (12%) of the 17 cases.
Owing to their frequent attacks of seizures and their EEG
results, these patients eventually underwent temporal lobec
[image:5.612.52.558.75.300.2] [image:5.612.51.559.360.583.2]754
T AMPIERI ET AL. AJNR:12, July/August 1991c
D
lesion. The CT and MR findings in these patients demon-strated an asymmetry of the temporal horns in one patient (case 14) and were completely normal in the other (case 17).
In conclusion, gangliogliomas are uncommon lesions, but their presence has to be considered in patients with a long
Fig. 4.-Case 9.
A, Contrast-enhanced CT scan shows floccu-lar calcification (arrowhead) in right middle cra-nial fossa.
8, Coronal T2-weighted (2100/80/1) MR im-age confirms presence of calcification (arrow·
head) in right fusiform gyrus and shows a hy-perintense signal that involves the amygdala, hippocampus and parahippocampus gyri, and adjacent white matter.
Fig. 5.-Case 14.
A-D, Unenhanced CT scans (A and 8) and T1-weighted (500/30/1) MR image (C) show slight
asymmetry of temporal horn, with right (arrow-head) larger than left. T2-weighted (2100/80/1)
MR shows no evidence of the ganglioglioma discovered later at surgery in the right temporal
lobe.
[image:6.612.64.478.79.646.2]AJNR:12, July/August 1991 INTRACEREBRAL GANGLIOGLIOMAS 755
REFERENCES
1. Commission on Classification and Terminology of the International League
Against Epilepsy. Proposal for revised classification of epilepsies and
epileptic syndromes. Epilepsia 1989;30:389-399
2. Courville CB. Ganglioglioma: tumor of the central nervous system; review
of the literature and report of two cases. Arch Neurol Psychiatry
1930;24:439-491
3. Zulch KJ. Brain tumors: their biology and pathology, 2nd ed. New York:
Springer, 1965
4. Cox JO, Zimmerman HM, Haughton VM. Microcystic ganglioglioma treated by partial removal and radiation therapy. Cancer 1982;50:473-477
5. Sutton LN, Packer RJ, Rorke LB, et al. Cerebral gangliogliomas during childhood. Neurosurgery 1983;13: 124-128
6. Johannsson JH, Rekate HL, Roessmann U. Gangliomas: pathological and
clinical correlation. J Neurosurg 1981;54:48-63
7. Rommel T, Hamer J. Development of ganglioglioma in computed
tomog-raphy. Neuroradiology 1983;24:237-239
8. Zulch KJ. Progress in neurological surgery. Chicago: Yearbook, 1968 9. Albright L, Byrd RP. Ganglioglioma of the entire spinal cord. Child's Brain
1980;6:274-280
10. Dom R, Brucher JM. Hamartoblastoma (gangliocytome diffus) unilateral de I' ecorce cerebrale associe
a
une degenerescence soudanophile de Iasubstance blanche du cote oppose. Rev Neural (Paris) 1969;120: 307-318
11. Kawamoto K, Yamanouchi Y, Suwa J, Kurimoto T, Matsumura H.
Ultra-structural study of a cerebral gangliocytoma. Surg Neurol 1985;24: 541-549
12. Roski RA, Roessmann U, Spetzler RF, Kaufman B. Nulsen FE. Clinical and
pathological study of dysplastic gangliocytoma: a case report. J Neurosurg 1981;55:318-321
13. Altman NR. MR and CT characteristics of gangliocytoma: a rare cause of epilepsy in children. AJNR 1988;9: 917-921
14. Russel OS, Rubinstein LJ. Pathology of tumors of the nervous system.
London: Arnold, 1989:289-306
15. Cavanagh JB. On certain small tumors encountered in the temporal lobe.
Brain 1958;81 :389-405
16. Layton DO. Heterotopic cerebral gray matter as an epileptogenic focus. J Neuropathol Exp Neurol1962;21 :244-249
17. Garrido E, Becker LF, Hoffman HJ, et al. Gangliogliomas in children: a clinicopathological study. Child's Brain 1978;4:339-346
18. Katz MC, Kier EL, Schechter MM. The radiology of gangliogliomas and
ganglioneuromas of the central nervous system. Neuroradiology
1972;4:69-73
19. Zimmerman RA, Bilaniuk LT. Computed tomography of intracerebral
gan-gliogliomas. J Comput Assist Tomogr 1979;3:24-30
20. Dorne HL, O'Gorman AM, Melanson D. Computed tomography of intracra-nial gangliogliomas. AJNR 1986;7:281-285
21. Denierre B, Stinchnoth FA, Hori A, Spoerri 0. Intracerebral gangliogliomas.
J Neurosurg 1986;65: 177-182
22. Castillo M, Davis PC, Takei Y, Hoffman JC. Intracranial ganglioglioma: MR.