Recirculation of Chilean Copper Smelting Dust with High Arsenic Content
to the Smelting Process
Victor Montenegro
1;*, Hiroyuki Sano
2and Toshiharu Fujisawa
21
Department of Materials Science and Engineering, Graduate School of Engineering, Nagoya University, Nagoya 464-8603, Japan
2EcoTopia Science Institute, Nagoya University, Nagoya 464-8603, Japan
Most of the dust generated from the copper smelting process in Chili has been stored after the stabilization by hydrometallurgical process because it contains high concentration of arsenic. However, in recent years, the generation of dust has increased because of degrading the quality of concentrate. In addition, the environmental regulations become stricter. On the other hand, valuable metals such as copper and zinc are contained in the dust at high concentrations and it is desirable to recover them. In this study, the effect of recirculation of dust to the smelting process was examined in order not only to decrease the amount of dust but also to recover copper. The generation of dust and the distribution of arsenic, lead, zinc and so on among the matte, slag and gas phases were evaluated as a function of the amount of recirculated dust, partial pressure of oxygen and temperature. It was found that the direct return of dust to the smelting process was quite effective for the recovery of copper and the reduction of dust amount, but the acceptable amount of returnable dust was limited by the produced matte quality.
[doi:10.2320/matertrans.M-MRA2008817]
(Received March 29, 2007; Accepted May 22, 2008; Published August 25, 2008)
Keywords: copper smelting dust, dust recirculation, minor elements distribution, dust generation, dust reduction, arsenic
1. Introduction
Copper smelter dust production is a worldwide problem in copper pyrometallurgy. Copper smelting processes generate gaseous emissions that contain SO2, N2, O2, water vapor, heavy metals and other impurities. Dust is generated as a result of gas cleaning process, containing condensate matter and fine particles semi-melted of concentrate, which are transported with gas. The mass of the dust produced in matte smelting varies mainly from the type of smelting furnace. Generally dust contains Cu, Fe and other minor elements such as Mo, Pb, As, Cd, Sb, Zn, Bi and Se, but the form of the elements depends on the conditions and the operational parameters in the smelting process. Arsenic is the main impurity of concern in dust. In the smelting process, around 15% of total input of arsenic from concentrate is transferred to matte, 22% to the slag phase, but the main part is eliminated through the gas phase, creating the copper smelter dust.1)
In Chili, the dust generated during the smelting process is generally treated by hydrometallurgical methods for the stabilization because it contains a high concentration of arsenic.2)The final residue is stored in a special landfill. In recent years, the copper production has increased and the quality of copper ore has been degraded, and as a result, the amount of dust from the process has increased. In addition, the environmental regulations become stricter. From 2003 the maximum arsenic emissions into the atmosphere should not be exceeded 400 tons/year for copper smelter in northern Chili.3)For example, in the actual operation of Chuquicamata smelter around 2500 tons/day of concentrate are charges in a Flash furnace and 2500 tons per day in a Teniente converter. Assuming 300 workdays per year, 30000 and 6000 tons per year of dust are calculated to be produced in a Flash and Teniente converter, respectively. Such great amount of dust,
it is required to be reduced, but also it is desirable to recover copper and the other valuable elements. Actually, this dust is usually directed to hydrometallurgical process to recover the soluble copper and the final residue is stored, creating a big environmental problem.4)
In order to decrease the amount of dust and recover copper from it, recirculation of dust to smelting process can be thought one of the attractive methods. In this study, the effect of dust recirculation on the new dust generation was investigated by conducting the experiments to imitate the copper smelting process. The distributions of copper and the other elements among the gas, slag and matte were also investigated.
2. Experimental Methods
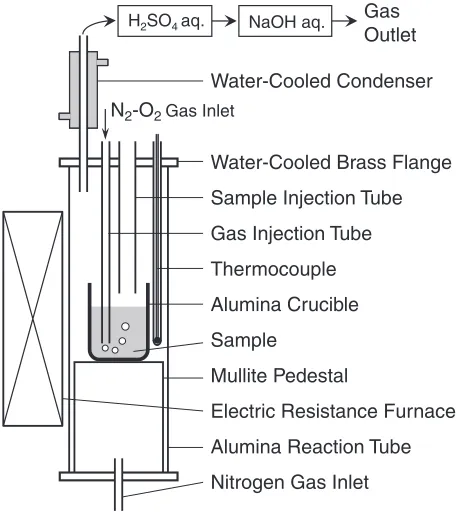
Industrial copper concentrates, matte, slag and flue dust from Chilean smelters were used as test materials. Table 1 shows the example of the chemical compositions of them. Dust recirculation tests in the smelting process were conducted in an electric furnace, as shown in Fig. 1. The furnace was heated up to a fixed temperature (1523–1573 K) under a continuous flow of nitrogen (3:3106Nm3s1). A Pt/Pt-Rh thermocouple in an alumina sheath was introduced into the furnace to monitor the temperature.
[image:1.595.306.548.710.776.2]Two types of experiments were conducted. In the first type, 13 g of slag, 10 g of matte of different grades and 20 g of charge (concentrate, dust and silica mixed in different ratios)
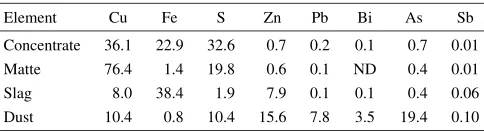
Table 1 Chemical composition of samples.
Element Cu Fe S Zn Pb Bi As Sb
Concentrate 36.1 22.9 32.6 0.7 0.2 0.1 0.7 0.01
Matte 76.4 1.4 19.8 0.6 0.1 ND 0.4 0.01
Slag 8.0 38.4 1.9 7.9 0.1 0.1 0.4 0.06
Dust 10.4 0.8 10.4 15.6 7.8 3.5 19.4 0.10
(Unit: mass%)
*Graduate Student, Nagoya University
were initially charged in an alumina crucible (12 mm I.D., 100 mm H.) and placed in an alumina reaction tube closed by a water-cooled brass flange. After melting and stabilizing of temperature, an alumina tube (4 mm I.D., 6 mm O.D.) was introduced into the molten sample on 5 mm from the bottom in the crucible to start the gas injection in the flow rate of 5:0106Nm3s1to simulate the smelting process.
In the second type of experiments, 13 g of slag, 10 g of matte were initially charged and placed in the reaction tube, after melting and stabilization of temperature, an alumina tube was introduced to start the gas injection. The mixture of concentrate, silica and dust was injected from top of the furnace into the crucible through an alumina tube. The mixture was formed into pellets to prevent the volatilization during the injection. The off-gas from the reaction tube was continuously passed through a water-cooled condenser, which collected the volatile matter, and then directed to solutions of 1 M H2SO4 and 1 M NaOH to remove harmful components and to neutralize the SO2.
When the gas injection was finished (9 ks), the sample was kept for 1.8 ks to promote the settling and phase separation. Then, the crucible was taken out of the furnace and quenched immediately by jetting helium gas. The condensed volatile matter, the slag and the matte phases were analyzed for their elemental content by inductively coupled plasma atomic emission spectrometry (ICP-AES).
The used mass ratio of matte, slag and the other charges in the test was the same as an industrial Teniente converter from Chilean smelter.5,6)The silica addition was adjusted so that the ratio Fe/SiO2in slag was constant at about 2.
To clarify the influence of temperature on the volatilization of impurities during the heating up stage, a preliminary test was conducted. The experimental result showed that volati-lization was negligible. Results showed that less than 1% of the total mass was evaporated in this stage.
3.1 Distribution of minor elements
Recirculation ratio of dust represents the amount of dust recirculated to the process, which is defined by the following equation:
Dust Recirculation (%)
¼[Mass of Recirculated Dust]=[Mass of Charge]
100 ð1Þ
where, [Mass of Charge] = [Mass of (Concentrate + Dust + Silica)].
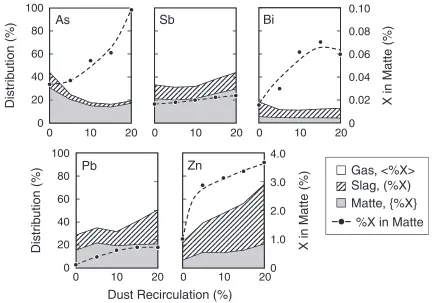
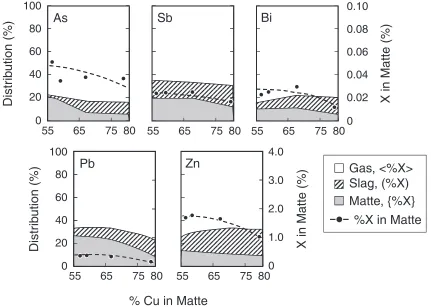
The distributions of As, Sb, Bi, Pb and Zn were investigated with varying the amount of the recirculation of dust to the smelting process with 21% of oxygen in injection gas at 1523 K. The following results correspond to the first type of experiments where the samples of matte, slag, concentrate and dust initially charged into the crucible. The obtained results are shown in Fig. 2. The termsh%Xi, (%X) and {%X} represents the fractional distribution of the element X among the gas, slag and matte phases, respec-tively. Closed circle represents the concentration of each element in matte. The concentration of As, Bi, and Zn in the matte increased remarkably with increasing of the recirculation ratio of dust.
The Sb and Pb in matte increased slightly. The amount of h%Asi andh%Bii increased andh%Sbi,h%Pbi andh%Zni decreased. With decreasing the distribution of Zn and Pb in gas, Zn and Pb were mainly transferred to the slag, because {%Pb} and {%Zn} were almost constant with the recircu-lation ratio of dust. Itagaki and Yazawa7,8) simulated the distribution of As, Sb, and Bi in smelting stage at 1573 K when the concentration of impurities increases in the charge. They showed that the concentration of impurities in matte increased with increasing the concentration of them in the charge, especially As which was strongly dependent on the initial concentration, and the volatilization of As is dependent on the concentration in charge and volatilizations of Sb and Bi were almost independent of the content in the charge. The present results were generally in agreement with their data. Pb and Zn were mainly transferred to the slag phase because the initial form of them in dust was oxide.
Table 2 shows an example of the chemical compositions of the final products. At all conditions, almost no change of the copper content in the slag was observed from the initial charge. In addition, the copper content in the dust was very low. Therefore, the copper in the recirculated dust could be effectively recovered into matte by the dust recirculation.
3.1.1 Effect of oxygen enrichment
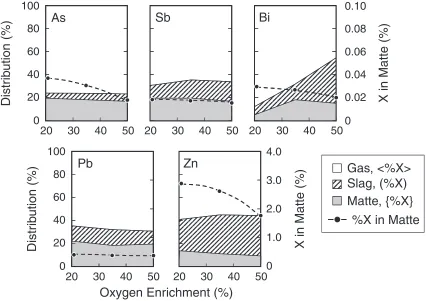
Figure 3 shows the effect of oxygen enrichment in the injection gas on the distribution of minor elements and the concentration of them in matte at 5% dust recirculation at 1523 K in the first type of experiments. The distribution in the slag (%X) increased with oxygen enrichment, and the distribution and concentration of each element in matte {%X} were suppressed. These characteristic behaviors were basically caused by the increase of slagging with increasing oxygen partial pressure.
3.1.2 Effect of temperature
Figure 4 shows the effect of temperature on the distribu-H2SO4 aq. NaOH aq.
Outlet
Water-Cooled Condenser
Water-Cooled Brass Flange
Sample Injection Tube
Gas Injection Tube
Thermocouple
Alumina Crucible
Sample
Mullite Pedestal
Electric Resistance Furnace
Alumina Reaction Tube
Nitrogen Gas Inlet N2-O2 Gas Inlet
[image:2.595.54.284.74.330.2]tion of minor elements at 10% of dust recirculation with 35% of oxygen in injection gas in the first type of experiments. Temperature has an effect mainly on the vapor pressure of volatile species, the vapor pressure of minor elements increases with temperature. This would be expected to lead higher elimination to the gas. As shown in Fig. 4, the concentration of minor elements in matte slightly decreased with increasing temperature but it had small effect on the distribution behavior.
3.1.3 Effect of matte grade
The matte grade is one of the most important operation parameters in the copper smelting process. The distribution of minor elements among the phases were examined by varying the matte grades at 1523 K. It was observed that the effect of matte grade showed the same tendency for all the recirculation ratios of dust in the first type of experiments. An example of the obtained results in 5% of dust recirculation with 21% of oxygen in injection gas is shown in Fig. 5. In general, the distribution of minor elements in gas phase slightly increases and that in matte phase decreases with
increasing of matte grade. The distribution of them in slag phase slightly increases with matte grade.
Yazawa and his co-workers have been intensively inves-tigated the distribution ratios of various elements during the copper smelting process.7–11) Figure 6 shows the effect of matte grade on the distribution ratios of As, Sb, Pb and Cu between slag and matte reported by Yazawaet al.9)It was not the main purpose of this study to measure the distribution ratio, but the present results were compared with their results and plotted in the same figure. The present experiments were conducted at 1523 K without copper precipitation. The partial pressure of SO2 prevailing the system was estimated to be about 0.2 atm by assuming that all oxygen injected was converted to SO2. Although Yazawa et al.9)conducted the experiments at 1573 K underPSO2¼0:1atm, such difference of temperature andPSO2might not give a significant effect on the distribution ratios. As the present results agreed fairly well with their results, it is estimated that the equilibrium between slag and matte was maintained during the present experiments.
3.2 Reduction of dust amount
In order not only to recover copper but also to decrease the amount of dust, it is necessary to clarify the effect of the recirculation on the total generation of new dust in the process. Based on the experimental results, dust generation for the different conditions was estimated according to the following equation as a function of the initial charge:
[image:3.595.82.514.72.375.2]DG¼WD=WC100 ð2Þ
Table 2 Chemical composition of the final products in 20% of dust
recirculation experiment at 1523 K with 21%O2.
Element Cu Fe S Zn Pb Bi As Sb
Matte 61.0 13.3 23.5 3.3 0.86 0.06 0.09 0.01
Slag 9.5 24.2 ND 4.3 0.47 0.03 0.03 0.02
Dust 0.5 ND 3.3 1.7 6.47 2.16 57.6 0.21
(Unit: mass%)
0
20
40
60
80
100
0
1.0
2.0
3.0
4.0
0
0.02
0.04
0.06
0.08
0.10
Distr
ib
ution (%)
Distr
ib
ution (%)
X in Matte (%)
X in Matte (%)
Dust Recirculation (%)
0
As
Bi
0
20
40
60
80
100
Pb
Zn
Gas, <%X>
Slag, (%X)
Matte, {%X}
%X in Matte
Sb
20
10
0
10
20
0
10
20
0
10
20
0
10
20
Fig. 2 Distribution of As, Sb, Bi, Pb and Zn among matte, slag and gas phases in relation to dust recirculation ratio. (1523 K, 21%O2,
[image:3.595.48.289.455.508.2]where,DGrepresents the dust generation ratio against to the injected mixture (concentrate, silica and dust),WD andWC represents the mass of the generated dust and the mixture, respectively. WD was calculated from an arsenic mass balance by the following equation:
WD¼
100AsT fmass%AsgWM ðmass%AsÞWS
hmass%Asi ð3Þ
where AsT represents the total arsenic mass, WM and WS represent the mass of matte and slag, respectively, {mass%As} and (mass%As) represent the arsenic content in matte and slag, respectively, and hmass%Asi represents
the arsenic content collected in a water-cooled condenser. The amount of the dust reduction was derived from the following relationship:
[Dust Reduction] ¼[Recirculated Dust]
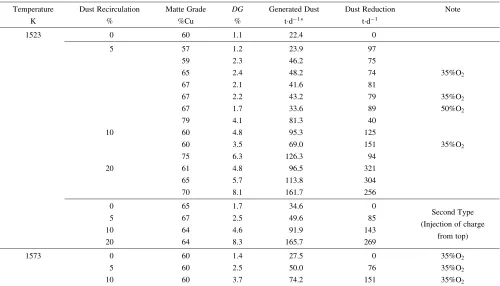
þ[Basic Generated Dust without Recirculation] [Generated Dust with Dust Recirculation]: ð4Þ Table 3 shows the calculated amounts of generated dust with dust recirculation and dust reduction by assuming 2000 tons charge per day in consideration of actual industrial operation. The amount of the generated dust without
0
20
40
60
80
0
1.0
2.0
3.0
4.0
0
0.02
0.04
0.06
0.08
0.10
Distr
ib
ution (%)
Distr
ib
ution (%)
X in Matte (%)
X in Matte (%)
Oxygen Enrichment (%)
As
Sb
Bi
Pb
Zn
0
20
40
60
80
100
20
30
Gas, <%X>
Slag, (%X)
Matte, {%X}
%X in Matte
50
40
20
30
40
50
20
30
40
50
20
30
40
50
20
30
40
50
Fig. 3 Distribution of As, Sb, Bi, Pb and Zn among matte, slag and gas phases against oxygen enrichment in blowing gas. (5% dust
recirculation, 1523 K, matte grade 67%Cu)
0 1.0 2.0 3.0 4.0
0 0.02 0.04 0.06 0.08 0.10
Distr
ib
ution (%)
X in Matte (%)
Temperature,
T
/
K
Gas,<%X>
Slag, (%X)
Matte, {%X}
%X in Matte
Pb
As
Sb
Zn
0 20 40 60 80 100
1523 15731523 1573 1523 15731523 1573
Fig. 4 Distribution of As, Sb, Pb and Zn among matte, slag and gas phases against temperature. (10% dust recirculation, 35%O2, matte
[image:4.595.86.512.74.375.2] [image:4.595.88.509.425.599.2]recirculation calculated from the first type of experiment was in good agreement with the actual operation data of the Teniente converter process at Chuquicamata smelter. On the other hand, the calculated data from the second type of experiment was lower than the actual operation data of the Flash furnace process. It was thought that almost all the injected mixture was directly dissolved into the sample melt
and not carried over to exhaust gas in this experiment differing from the actual operation.
It should be noted that generated dust increases with the increasing of recirculation and matte grade. This was mainly due to increases of concentration of impurities such as As, Bi and Sb, which were mainly eliminated by volatilization, as was showed previously in the Figs. 2 and 5.
The volatilization of minor elements decreased with oxygen enrichment, as a result the generated dust decreased. Increasing the oxygen partial pressure of the system, enhancing the transfer to slag phase.
As was mentioned before, it is apparent that the operating temperature does not have significant effect on the dust generation.
In spite of the increase of the dust generation with increasing of dust recirculation, dust reduction could be achieved. For example, in case of 10% dust recirculation, if all the recirculated dust was transferred again toward the new dust, the final generated dust would reach 200 tons per day (recirculated dust) plus 20 tons per day (basic generated dust without recirculation, ¼22:40:9). As a result, the dust generation would be around 220 tons per day. However, the experimental result showed that the generated dust with 10% dust recirculation was around 100 tons per day. Therefore 10% dust recirculation contributes to the reduc-tion about 100 tons of dust.
Figure 7 shows the generated dust of the dust recirculated experiments. Dotted line represents the first type and broken line the second type of experiments. The solid line represents the amount of recirculated dust plus basic generated dust
0
20
40
60
80
100
0
1.0
2.0
3.0
4.0
0
0.02
0.04
0.06
0.08
0.10
Distr
ib
ution (%)
Distr
ib
ution (%)
X in Matte (%)
X in Matte (%)
% Cu in Matte
0
20
40
60
80
100
Gas, <%X>
Slag, (%X)
Matte, {%X}
%X in Matte
Sb
Bi
Pb
Zn
As
65
55
75
80
55
65
75
80
55
65
75
80
65
55
75
80
65
55
75
80
Fig. 5 Distribution of As, Sb, Bi, Pb and Zn among matte, slag and gas phases against matte grade. (5% dust recirculation, 1523 K,
21%O2)
Sb
( )Pb
( )As
( )Cu
( )%Cu in Matte
L
XS/M
10
110
010
-110
-210
-380
0
20
40
60
Fig. 6 Distribution ratios of As, Sb, Pb and Cu between slag and matte.
(Plots: Present work,PSO20:2atm, 1523 K, 5% of dust recirculation,
[image:5.595.84.515.74.381.2] [image:5.595.55.285.424.645.2]without recirculation. Actual dust generation in this study was very much lower than the solid line. This means that recirculation of dust could be effective to reduce the dust amount.
Dust generation behavior of two types of experiments was compared. The generated dust for both experiments showed the same tendency up to about 10% of recirculation, and when the dust recirculation ratio increases more than 10%, the dust generation of second type of experiments increased. On the other hand, generation of dust was almost unchanged on such conditions for the first type of experiments. These results suggest that bath-smelting processes such as the Teniente converter or the Noranda reactor are especially favorable for a process with a recirculation of high impurity content dust. A process with injection of charge from the top,
such as the Flash furnace considerably increases the generation of dust at higher dust recirculation ratios. In addition, if the heavy amount of dust is recirculated into the Flash furnace by injection, some parts are carried over to exhaust gas, which are not taken into the bath.
[image:6.595.49.549.83.369.2]The results suggested that the recirculation of dust was quite effective on the reduction of dust amount. However, the acceptable dust recirculation amount was limited by the produced matte quality. Comparing the concentration of impurities in matte obtained by the laboratory test with the one without the dust recirculation, the concentrations of As and Sb increased with dust recirculation as already shown in Fig. 2. However, the final concentration of them was still small, so it did not have a significant influence on the matte quality. Bi content in matte slightly increased. On the other hand, concentration of Pb and Zn in matte strongly increased, the final concentration in matte was much higher than the initial ones. Therefore, the behavior of Pb, Zn and Bi might limit the amount of dust recirculation in the process. Oxygen enrichment may be effective in suppressing the accumulation of these elements in matte.
4. Conclusions
The effect of dust recirculation in smelting process on dust generation and the behavior of selected impurities among the matte and slag phases were investigated on a laboratory scale under different process conditions. Copper can be effectively recovered by the dust recirculation. If the carry over of recirculated dust is effectively suppressed, the dust generation can be decreased by the recirculation into the smelting process. But the amount of dust
recircula-Temperature Dust Recirculation Matte Grade DG Generated Dust Dust Reduction Note
K % %Cu % td1 td1
1523 0 60 1.1 22.4 0
5 57 1.2 23.9 97
59 2.3 46.2 75
65 2.4 48.2 74 35%O2
67 2.1 41.6 81
67 2.2 43.2 79 35%O2
67 1.7 33.6 89 50%O2
79 4.1 81.3 40
10 60 4.8 95.3 125
60 3.5 69.0 151 35%O2
75 6.3 126.3 94
20 61 4.8 96.5 321
65 5.7 113.8 304
70 8.1 161.7 256
0 65 1.7 34.6 0
Second Type
5 67 2.5 49.6 85
(Injection of charge
10 64 4.6 91.9 143
from top)
20 64 8.3 165.7 269
1573 0 60 1.4 27.5 0 35%O2
5 60 2.5 50.0 76 35%O2
10 60 3.7 74.2 151 35%O2
Assuming 2000 tons charge per day
Gener
ated Dust,
W
D
/ t
Dust Recirculation (%)
Dust Reduction [Recirculated dust]
[Basic generated dust without recirculation]
First Type (Samples initially charged)
0 200 400 600
0
Second Type (Injection of charge from top of the furnace)
+
d
-1
20 15
10 5
Fig. 7 Effect of dust recirculation on the dust reduction. (1523 K, 21%O2,
[image:6.595.57.282.406.557.2]tion depends on the final quality of the matte. The distributions of Pb, Zn and Bi in matte increase remarkably with recirculation. Therefore, the behavior of those impuri-ties might limit the amount of dust recirculation in the process.
Acknowledgements
The authors wish to express their thanks to Mr. Toru Muto and Mr. Takeshi Sasada for their valuable contribution in this work. The authors are much indebted to Chuquicamata Smelter, Division Codelco-Norte, Codelco and Institute for Innovation in Mining and Metallurgy (IM2), Codelco for the sample supply.
REFERENCES
1) F. Alguacil, L. Magne, P. Navarro and J. Simpson: Rev. Metal Madrid
32(1996) 400–407.
2) M. A. Maldini, J. Osorio, M. Mella and M. Herrera: Proc. Hydroprocess
(Arturo Prat University, Iquique, Chile, 2006) pp. 402–414. 3) Standard of emission for the regulation of arsenic emission into the
atmosphere, National Commission of Environment, Official Homepage
http://www.conama.cl/.
4) A. Valenzuela, J. Palacios, D. Cordero and M. Sanchez: Yazawa international symposium, ed. by F. Kongoli, K. Itagaki, C. Yamauchi and H. Y. Sohn (TMS, San Diego, USA, 2003) pp. 239–252. 5) R. Alvarado, B. Lectora, F. Hernandez and C. Moya: Copper 95, Vol. 4,
Pyrometallurgy of Copper, ed. by W. J. Chen, C. Diaz, A. Luraschi and P. J. Mackey, (Canadian Institute of Mining, Metallurgy and Petroleum, Quebec, 1995) pp. 83–101.
6) A. Luraschi: Cade-Idepe Engineering Consultant, Personal discussion, Chile, 2004–2005.
7) A. Yazawa and T. Azakami: Can. Met. Quart.8(1970) 257–261.
8) K. Itagaki: Metallurgical Review of MMIJ3(1986) 87–100.
9) A. Yazawa, S. Nakazawa and Y. Takeda: Proc. of the Advances in Sulfide Smelting, ed. by H. Y. Sohn, D. B. George and A. D. Zunkel, AIME, (1983) pp. 99–117.
10) K. Itagaki and A. Yazawa: Proc. of the Advances in Sulfide Smelting, ed. by H. Y. Sohn, D. B. George and A. D. Zunkel, AIME, (1983) pp. 119–142.