Effect of Surface Treatment on Glossiness of Al
Mg
Zn Alloy Casting
+Makoto Hino
1, Koji Murakami
1, Norihito Nagata
2, Chie Ibata
2, Hideki Kanetsuki
3and Sadao Kawai
31Industrial Technology Research Institute of Okayama Prefecture, Okayama 701-1296, Japan 2SURTECH NAGATA CO., LTD., Akashi 673-0028, Japan
3HIKARI LIGHT METALS CO., LTD., Okayama 709-0854, Japan
In this study, the effects of various surface treatments on the glossiness of AC4CH-T6 casting and newly developed bright aluminum alloy casting were examined.
With the AC4CH-T6 casting, it was difficult to obtain a bright surface by buffing, because of the surface irregularity between the hard eutectic silicon and the aluminum matrix. On the other hand, with the developed silicon-free alloy casting, it was possible to produce a bright surface by buffing, and this glossiness was superior to that of the AC4CH-T6 casting covered with the decorative chrome electroplating. The glossiness of the developed casting decreased sharply after chemical polishing, depending on the generation of local dissolution close to the intermetallic compound and pin-hole. However, electropolishing under an optimum conditions helped realize a smooth and bright surface.
As with the anodization from sulphuric acid solution, glossiness of the developed alloy casting decreased because of the prior dissolution of the intermetallic compound. However, anodization from the developed solution suppressed this dissolution, forming a uniform oxidefilm. As a result, bright surface could be produced for the developed alloy casting by anodization. [doi:10.2320/matertrans.F-M2013830]
(Received February 14, 2013; Accepted September 4, 2013; Published October 25, 2013)
Keywords: aluminum alloy, casting, glossiness, surface treatment
1. Introduction
The lightening of the car body is the most important issue regarding the reduction of the carbon dioxide emissions for realization of a low carbon society in the automobile industry. Aluminum, which can be expected to help lightening, has been applied in the place of the iron and steel, and the needs for these aluminum materials in the automobile industry has increased year by year.1)Especially, the substitution from iron
and steel to aluminum alloys is pronounced for the wheels of automobiles.2)
The aluminum wheel, which has an excellent workability, is extremely effective for the exterior use in automobiles. In particular, the aluminum wheel with a decorative plating, which has a bright surface like a mirror, is in popular demand. However, since aluminum is such an active element and is passivated by oxidation, the loss of adhesion by the platedfilm on the aluminum alloy substrate due to the oxide
film is one of the problems of this process. For the purpose of improving the adhesion, the double zincate treatment, which repeats the conversion treatment twice, is commonly adopted for the plating onto the aluminum alloy substrate.3)Although
the double zincate treatment was made on the aluminum alloy substrate, bad plating, such as blister, nib, tarnish and poor adhesion, often occur. These bad platings become a significant problem during production. Therefore, the devel-opment of a new technology with a bright surface, which is similar to plated products without the plating process, is desired.
In general, JIS AC4CH-T6 (AlSiMg alloy),4)which has an excellent strength and castability, is mainly used for the raw materials of aluminum wheels for casting. However, it is extremely difficult for this casting to obtain a bright surface without a surface treatment such as a plating and drying process.
In this study, new aluminum alloy for the casting, which is similar in mechanical properties to the AC4CH-T6 casting and capable of having a glossy surface by another process such as the plating and dry coating, was developed.5,6) The developed alloy casting was adjusted on the basis of the JIS AC7A (AlMg alloy) casting, and became a heat-treatable aluminum alloy by adding zinc in order to improve its mechanical property. The effects of various surface treat-ments on the glossiness of the AC4CH-T6 casting and newly developed bright aluminum alloy casting were examined.
2. Experimental
Experiments were conducted with the AC4CH-T6 casting used for making aluminum wheels, developed bright aluminum alloy castings, and the A5052 sheet. The chemical compositions are shown in Table 1. The molten metal of the AC4CH casting and developed alloy casting were cast in metal mold (molten metal temperature: 1003 K, shape of metal mold: boat-shaped specimen,7) pouring weight: about
500 g, mold temperature: 523 K), and the heat treatment of the solution and aging (AC4CH casting: T6 treatment) was then carried out for these samples. The heat treatment conditions are shown in Table 2.
Buffing, chemical polishing, and electropolishing were respectively undertaken for each specimen. The buffing equipment was used in this study, since the surface glossiness significantly changes by the buffing. The conditions of the buffing and the electropolishing are shown in Tables 3 and 4, respectively. Furthermore, anodizing was conducted for the electropolished specimen in order to improve the corrosion resistance and paint adhesion. The anodizing conditions are shown in Table 5.
The visual observations, surface shape measurement using the noncontactive three-dimensional structure analysis microscope (Zygo New View 5000), and SEM observations and element mapping using field emission electron probe microanalysis were carried out on the surface of the various
+This Paper was Originally Published in Japanese in J. JFS84(2012) 438
444.
treated specimens. The effects of the various surface treatments on the glossiness were examined by measurement of the glossiness by a glossmeter (Murakami color research laboratory, Digital glossmeter (GM-3D)).
3. Results and Discussion
[image:2.595.48.557.84.138.2]3.1 Glossiness by buffing
Figure 1 shows the appearances of the AC4CH-T6 casting and the developed casting after the buffing under the same conditions. The lattice of the background against the AC4CH-T6 casting is not clear, but that against the developed one is clear. The glossiness of the AC4CH-T6 casting and the developed one are 470 and 785%, respectively. In this way, the glossiness of the developed casting is superior to the glossiness of the AC4CH-T6 one with buffing. On the other hand, the glossiness of the specimen (current aluminum wheel with decorative plating) with the covered decorative plating (double zincate treat-ment/copper cyanide plating/copper sulfate plating/nickel plating/decorative chrome plating) onto AC4CH-T6 casting
is 750%. The glossiness of the developed casting after the buffing makes it possible to show a significantly better glossiness than that of the decorative plating.
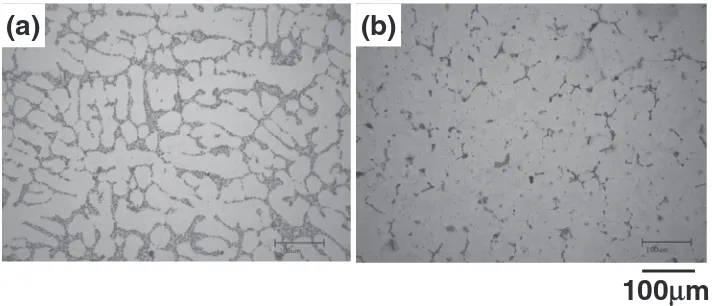
[image:2.595.48.557.187.237.2]Figure 2 shows the microstructures of the AC4CH-T6 casting and the developed one. The dendritic structure, which was developed during casting process, is observed for the AC4CH-T6 casting, however, it is not obviously observed for the developed one. In spite of both materials made by the
Table 2 Heat treatment conditions for JIS AC4CH casting and developed alloy casting.
Alloy Solution heat treatment temperature (K)
Solution heat treatment time (ks)
Aging temperature (K)
Aging time (ks)
JIS AC4CH 808 10.8 433 14.4
Developed alloy 703 21.6 433 28.8
Table 3 Buffing conditions.
Compound type U lime white (Alumina) Compound addition quantity 3 g
Buff type Cotton bias Rotational frequency 1800 rpm
[image:2.595.296.549.282.359.2]Buffing time (s) 16
Table 4 Electropolishing conditions.
Composition of electrolytic solution
Mixed acid
(Sulfuric acid+Phosphoric acid): 8 mol/L
pH 0
Voltage 20
[image:2.595.43.548.405.494.2]Temperature (K) 338 Treatment time (s) 60
Table 5 Anodizing conditions.
Anodizing type Sulphuric acid solution Developed solution
Composition of electrolytic solution Sulfuric acid: 1.8 mol/L
Dissolution aluminum: 5 g/L Phosphate+additive
pH 1 12
Voltage 8 8
Temperature (K) 295 295
Treatment time (s) 180 180
(a) (b)
10mm
Fig. 1 Appearances of the various specimens after the buffing: (a) JIS AC4CH-T6 casting, (b) Developed alloy casting.
Table 1 Chemical composition of various specimens (mass%).
Alloy Si Mg Zn Fe Cu Ni Ti Pb Sb Mn Cr B Be Al
[image:2.595.306.546.535.645.2]same gravity casting method, these microstructures are remarkably different each other as shown in Fig. 2. This difference is attributable to the chemical composition of each casting. That is to say, the AC4CH casting consisting of the AlSiMg alloy, which hardly dissolves silicon into the Al-rich solid phase, is a typical eutectic alloy. On the other hand, the amounts of magnesium and zinc in the developed casting consisting of the AlMgZn alloy are both under the solid solubility limit in the aluminum under the eutectic temperature. Therefore, these microstructures are remarkably different each other.
Figure 3 shows secondary electron images of the AC4CH-T6 casting and the developed casting after the buffing. On both castings, the polishing seams due to the buffing were observed, so there was no difference regarding this point. However, on the AC4CH casting, the polishing seams were missed in the part of the precipitates along the grain boundary and these were then discontinuous as shown in Fig. 2. Based on this result, these crystallizations seem to be harder than the aluminum matrix.
Figure 4 shows secondary electron images and X-ray maps for the aluminum, silicon, magnesium, zinc and oxygen obtained by the FE-EPMA analysis for the surface of the AC4CH-T6 casting and the developed one after the buffing. On the AC4CH-T6 casting, aluminum is the main compo-nent, silicon and magnesium as alloying elements were
nonuniformly distributed, and it was found that precipitates along the grain boundary observed in Fig. 2 were eutectic silicon. Magnesium was only slightly segregated banded, and the oxygen was also segregated in this area. The aluminum alloys, such as the 5000 series alloy and AC7A casting (Al Mg alloy) containing the magnesium, have been used as anticorrosive alloys. This anticorrosive performance as well as the enrichment of the oxygen in proportion to that of magnesium from the results of the EPMA element mapping suggest that the magnesium in the aluminum alloy accelerates the passivation.
On the other hand, magnesium in the developed casting has slightly segregated along the grain boundary, and the oxygen was also concentrated in this area. This result is in good agreement with the result of the AC4CH-T6 casting. Zinc was uniformly distributed, and no remarkable segrega-tion, such as the eutectic composition on the AC4CH-T6 casting, could be observed.
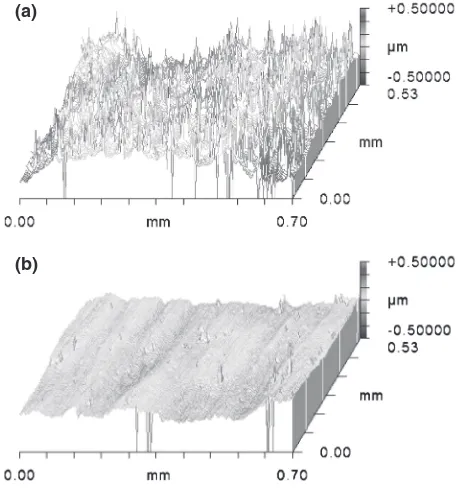
Figure 5 shows the surface morphology of the AC4CH-T6 casting and the developed one obtained by a three-dimen-sional structure analysis microscope after the buffing. On the AC4CH-T6 casting, the eutectic silicon, which crystallized along the grain boundary increases. This convex surface is caused by polishing the soft matrix in preference to the eutectic silicon, since the eutectic silicon is harder than the aluminum solid solution of the matrix.
100
μμ
m
(a)
(b)
Fig. 2 Optical microstructures of the various specimens: (a) JIS AC4CH-T6 casting, (b) Developed alloy casting.
10
μ
m
(a)
(b)
[image:3.595.120.476.69.222.2] [image:3.595.122.477.267.434.2]On the other hand, since there was no eutectic silicon on the developed casting, it was possible to obtain a smooth surface (surface roughness Ra: 0.48 µm) with an excellent glossiness. The microstructure of the developed alloy casting based on TEM observations after the solution heat treatment and aging treatment is shown in Fig. 6. The fine T-phase (Al2Mg3Zn3) precipitated in the matrix by this heat treatment.
Thus, the developed alloy casting as the age hardenable alloy is aimed at improving the mechanical property by the T-phase precipitation.
3.2 Effect of chemical polishing and electropolishing on the glossiness
In section 3.1, it was demonstrated that the glossiness of the developed alloy casting is better than that of the AC4CH-T6 casting by buffing. However, the buffing may not be applicable for the developed alloy casting, because this
material is used for castings with complicated shapes. Moreover, buffing is well known to increase the manufactur-ing cost due to its manual operation, and thus the effects of chemical polishing and electropolishing on the glossiness of the developed alloy casting were examined. In addition, the A5052 sheet was also evaluated as a comparison for this casting.
Figure 7 shows the appearances of the developed alloy casting and the A5052 sheet after the chemical polishing. Regard of the conditions (electrolyte composition, temper-ature, treatment time) for the chemical polishing, which were changed for the developed alloy casting, it was not possible to obtain the glossy surface. However, it was capable of
(a) (b)
SEI Al-Kαα Si-Kα
Mg-Kα O-Kα
SEI Al-Kα Mg-Kα
O-Kα
Zn-Kα 100 μm
Fig. 4 Secondary electron images and X-ray maps for aluminum, silicon, magnesium, zinc and oxygen by FE-EPMA analysis for the various specimens after the buffing: (a) JIS AC4CH-T6 casting, (b) Developed alloy casting.
(a)
(b)
Fig. 5 Surface morphology of the various specimens after the buffing: (a) JIS AC4CH-T6 casting, (b) Developed alloy casting.
Fig. 6 TEM image of the developed alloy casting after the solution heat treatment and aging treatment.
(a)
(b)
10mm 10mm
[image:4.595.58.540.70.246.2] [image:4.595.314.533.292.458.2] [image:4.595.54.283.294.538.2] [image:4.595.306.547.507.584.2]producing a glossy surface for the A5052 sheet without difficulty. The difference in the glossiness between the developed casting and the A5052 sheet seems to depend on the manufacturing process. That is to say, in the case of the chemical polishing depending on a chemical reaction, the heterogeneity of the microstructure affects the dissolution. Therefore, compared to the castings, the rolled sheet with a uniform microstructure has made it possible to have a uniform dissolution, and then obtain a glossy surface. At the present time, the chemical polishing as a glossy surface treatment has been applied to forged aluminum wheels made from the A5052 alloy. On the other hand, castings such as those developed in this study cannot uniformly dissolve because of the heterogeneity of the microstructure, inclusions and casting defects such as pinholes and shrinkage cavities.
Figure 8 shows the appearance of the developed alloy casting after the electropolishing from sulfuric acid and
phosphoric acid solutions. The lattice of the background against the developed alloy casting is clear. The electro-polishing, which is anodic electrolysis using an external power source has made it possible to control the oxidation and dissolution of the specimen surface by optimization of the solution composition and electrolysis conditions (voltage, bath temperature). Therefore, the glossiness (754%) after the electropolishing is similar to that of the buffing.
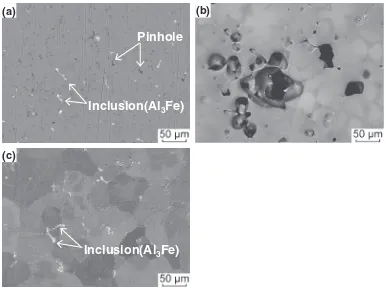
Figure 9 shows backscattered electron images of the developed alloy casting surfaces after various surface treat-ments such as buffing, chemical polishing and electro-polishing. In the surface after the polishing (Fig. 9(a)), a number of inclusions consisting of an intermetallic com-pound (Al3Fe) based on the iron as well as a number of
pinholes were observed.
During the chemical polishing (Fig. 9(b)), a local dis-solution was generated, and thus the glossiness was reduced. These local dissolutions formed in terms of the anodic dissolution of the aluminum close to the intermetallic compound (Al3Fe) and the local dissolution at the pinholes.
Therefore, the intermetallic compound observed on the buffing surface was thoroughly missed.
On the other hand, the intermetallic compound, which was missed by the chemical polishing, was observed after the electropolishing, and it was then found that the local dissolution was suppressed. In this way, for the electro-polishing, it is possible to suppress the nonuniform dissolution, which originates from the metal structure, by optimization of the electrolysis conditions, and thus this electropolishing makes it possible to obtain a smooth and bright surface.
10mm
Fig. 8 Appearance of the developed alloy casting after the electro-polishing.
(a)
(b)
(c)
Inclusion(Al
3Fe)
Pinhole
Inclusion(Al
3Fe)
[image:5.595.105.491.473.761.2]3.3 Effect of anodization on the glossiness
Although the 7000 series aluminum alloy possesses excellent mechanical property, its corrosion resistance is inferior to other aluminum alloys.8) Since the developed
casting consisting of the AlMgZn alloy is similar to the 7000 series aluminum alloy, the surface treatment when considering the anticorrosion is important for the developed casting. The anodization for the developed casting was examined, because the anodization has been widely applied as a surface treatment for aluminum alloys.
Figure 10 shows the appearances of the developed alloy casting with the electropolishing shown in Fig. 8 after anodizing from the sulfuric acid solution by the standard procedure9)and the newly developed solution.10)The lattice
of the background against the developed casting treated by the anodization from the sulfuric acid solution is not clear. This glossiness was 382%, and the sulfuric acid anodization remarkably lowered the glossiness.
On the other hand, the lattice of the background against the developed casting treated by the anodization from the developed solution was clear, and this glossiness was 701%. Although this glossiness is slightly inferior to that of a commercial aluminum wheel covered with the decorative chrome electroplating, this result indicates that the developed anodization is extremely effective for reducing the deterio-ration of the glossiness as well as the resistance to corrosion by the developed casting.
Based on the above results, it was proven that the glossiness after the anodizing between the sulfuric acid solution and the developed solution remarkably differed. The anodizing from the sulfuric acid solution and the developed solution were conducted using the A5052 sheet,
and then cross-sectional observations of the obtained oxide coating were carried out in order to clarify the factor.
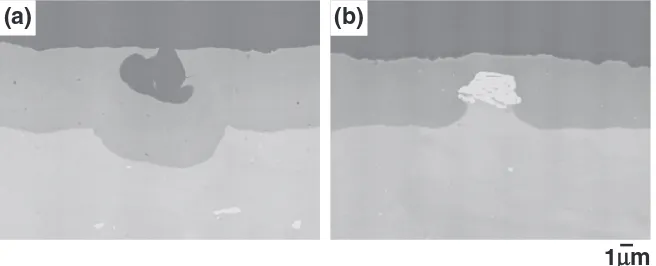
Figure 11 shows the backscattered electron images of the cross-sectional oxide coating from the various solutions. A large number of voids were observed in the oxidefilm from the sulfuric acid solution. An irregular reflection of light occurred on this nonuniform oxide film, it will cause the lower glossiness as in Fig. 10(a). Upon anodic electrolysis in the sulfuric solution with a strong acidity, the formation of an oxide film at the intermetallic compound (Al3Fe) is further
suppressed than that at the aluminum matrix. Therefore, the intermetallic compound preferentially dissolves, and voids were formed as shown in Fig. 11(a).
On the other hand, the intermetallic compound (Al3Fe) was
observed in the oxide film from the developed anodizing. Since the pH of the anodizing solution is adjusted for the suppression of dissolution of the intermetallic compound, the intermetallic compound in the oxide film possibly remains. Thus, the developed anodizing is capable of forming an oxide
film with an excellent glossiness.
4. Conclusions
The effects of various surface treatments on the glossiness of an AC4CH-T6 casting and newly developed AlMgZn aluminum alloy casting, which has the strength similar to the AC4CH-T6, were examined. The following results were obtained.
(1) For the AC4CH-T6 casting, it was difficult to obtain a bright surface by buffing due to surface irregularities between the hard eutectic silicon and the aluminum matrix. However, the developed casting without the silicon makes it possible to obtain a bright surface by buffing. The glossiness of the developed casting is superior to that of the AC4CH-T6 casting covered with a decorative chrome electroplating.
(2) The glossiness of the developed casting treated by the chemical polishing was remarkably reduced, depending on the generation of the local dissolution close to the intermetallic compound (Al3Fe) and pinholes. However,
based on the electropolishing, it was possible to suppress the nonuniform dissolution, which originated from the metal structure by optimizing the electrolysis condition, and thus this electropolishing made it possible to obtain a bright surface.
(a)
(b)
[image:6.595.48.291.480.569.2]1
μ
m
Fig. 11 Backscattered electron images of the cross-sectional oxide coating from the various solutions: (a) Sulphuric acid solution, (b) Developed solution.
(a) (b)
[image:6.595.135.463.624.757.2]10mm 10mm
(3) A nonuniform oxide film was formed by the anodiza-tion from a sulfuric acid soluanodiza-tion in terms of the preferential dissolution of the intermetallic compound (Al3Fe). Therefore,
the glossiness was reduced. However, a uniform oxide film was formed by the developed anodization because of the uniform dissolution of the intermetallic compound (Al3Fe).
As a result, it was possible to establish a new surface treatment technology which can produce a glossy surface.
Acknowledgment
Authors would like to express sincere thanks to the Small and Medium Enterprise Agency, the Ministry of Economy, Trade and Industry, Japan for providing the Research Grant-in-Aid for the promotion of this research.
REFERENCES
1) H. Horikawa: J. Jpn. Inst. Light Met.58(2008) 259273 (in Japanese). 2) Aluminum Wheel Statistical Data, Japan Aluminium Association,
No. 24 (2010) p. 20 (in Japanese).
3) M. Hino, K. Murakami and T. Kanadani: Science and Industry 85
(2011) 1218 (in Japanese).
4) Y. Kitaoka: J. Jpn. Inst. Light Met.61(2011) 485503 (in Japanese). 5) S. Kawai, H. Kanetsugi, M. Hino and K. Murakami: Japan Patent
journal 2010-56677.
6) S. Kawai and H. Kanetsugi: ALUTOPIA41(2011) 816 (in Japanese). 7) Chuzo Gijutsu Sirizu6, Kei Kinzoku Imono Daikasuto no Seisan
Gijutsu, Revised edition, 2000, p. 27 (in Japanese).
8) Aruminiumu no Soshiki to Seisitsu, (Jpn. Inst. Light Met., 1991) p. 311 (in Japanese).
9) Hyomen Gijutsu Binran, (The Surf. Finising Soc. of Japan, 1998) p. 519 (in Japanese).