Patient reported outcomes from a phase III study of baricitinib in patients with conventional synthetic DMARD refractory rheumatoid arthritis
Full text
Figure
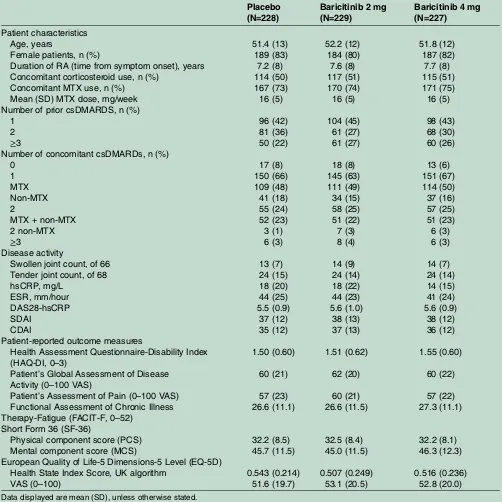

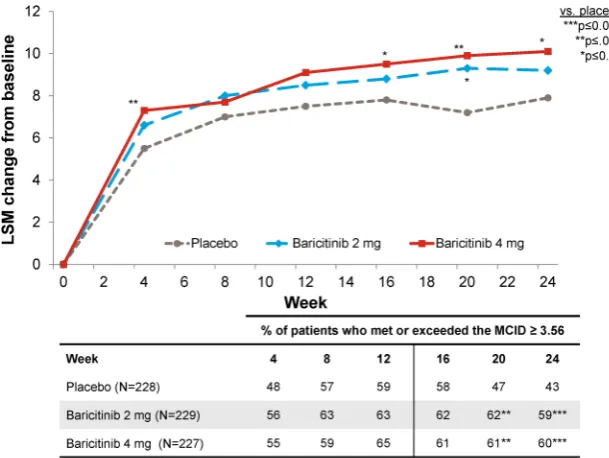
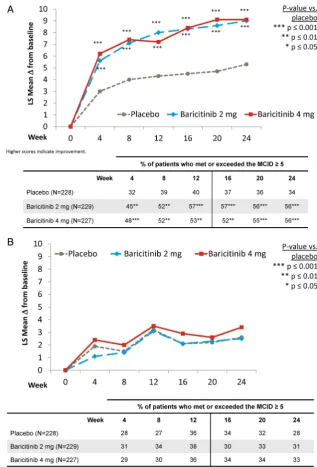
Related documents
Assess- ment of QOL was based on the Spanish version of the Health Assessment Questionnaire Disability Index (Span- ish HAQ-DI) [26] administered the same day as the foot
(ETN): Etanercept; (MTX): Methotrexate; (DMARDs): Disease modifying antirheumatic drugs; (PRO): Patient reported outcome; (RA): Rheumatoid arthritis; (HAQ): Health
HRQoL: Health related quality of life; MCS: Mental Component Summary; MDHAQ: Multi-Dimensional Health Assessment Questionnaire; PCS: Physical Component Summary; PN: Pain;
To measure lim- itations in physical functioning, the multi-item Health Assessment Questionnaire (HAQ), expressed as a disability index (DI) from 0 (no disability) to 3
Measures of rheumatoid arthritis disease activ- ity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and
Assessments were conducted before and after intervention using the Pain Disability Index (PDI) to measure pain and functioning, the Health Assessment Questionnaire (HAQ) for
Four of the patient-reported outcome measures considered (the Ankle Osteoarthritis Scale, the Manchester Foot Pain and Disability Index, the Foot and Ankle Ability Measure and
Nevertheless, this study indicated that PROs reflecting physical aspects (pain, functional disability, PGA, physical quality of life) were more modulated by this biologic