inorganic papers
Acta Cryst.(2005). E61, i129–i131 doi:10.1107/S160053680501723X Messouriet al. (NH
4)2[Mg(H2O)6]3(HPO3)4
i129
Acta Crystallographica Section EStructure Reports Online
ISSN 1600-5368
Diammonium tris[hexaaquamagnesium(II)]
tetrakis[hydrogenphosphate(III)],
(NH
4)
2[Mg(H
2O)
6]
3(HPO
3)
4Ibtissam Messouri,aBrahim El Bali,a* Francesco Capitelli,b Juan F. Piniella,cMohammed Lachkaraand Zineb Slimania
aLaboratoire d Analyses, Essais et Environnement
(LAEE), De´partment de Chimie, Faculte´ des Sciences Dhar Mehraz, BP 1796 Atlas, 30000 Fe´s, Morocco,bInstitute of Crystallography– CNR, via G. Amendola, 122/o, 70125 Bari, Italy, andcDepartment of Geology, Universitat Auto´noma de Barcelona, Campus Universitari UAB, 08193 Bellaterra, Spain
Correspondence e-mail: belbali@fsdmfes.ac.ma
Key indicators
Single-crystal X-ray study
T= 293 K
Mean(P–O) = 0.001 A˚
Rfactor = 0.022
wRfactor = 0.064
Data-to-parameter ratio = 11.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
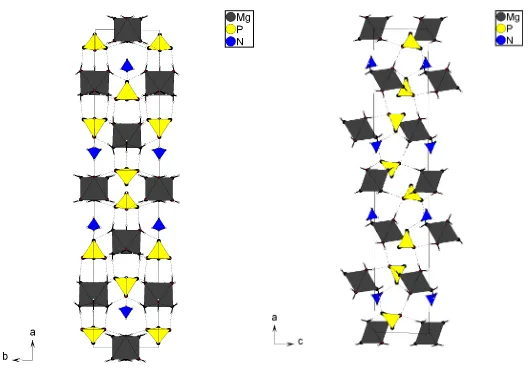
The framework of the title compound is made up of discrete
Mg(H2O)6 octahedra, and HPO3and NH4tetrahedra, which
are organized in planes parallel to (010). Strong hydrogen bonding between the building units stabilizes the structure. The hydrogenphosphate(III) tetrahedra, the ammonium tetrahedron and one of the two Mg atoms lie on positions
withmsymmetry, whereas the second Mg atom is located on a
position with 2/msymmetry.
Comment
The work reported in the present paper is a continuation of our investigations focusing on the synthesis of phosphates and phosphites using wet-chemical methods. In the course of this project we have synthesized and structurally characterized various compounds, such as diphosphates (Essehliet al., 2005, and references therein), phosphites (Ouarsalet al., 2003, 2004, and references therein) and monophosphates including (NH4)CoPO46H2O (El Baliet al., 2005).
The first structural investigation in the system Mg–PIII–O–H was carried out by Corbridge (1956), who determined the
crystal structure of MgHPO36H2O on the basis of
two-dimensional X-ray photographic data. This structure was
redetermined some years ago (Powell et al., 1994). In the
present work, we report the synthesis and crystal structure of the ammonium-containing phase (NH4)2Mg3(HPO3)418H2O.
The two independent Mg2+cations lie on positions with 2/m
(Mg2) and m(Mg1) symmetry. They are octahedrally
coor-dinated by six O atoms that all belong to water molecules
(Fig. 1). The average Mg—O distance of 2.072 (2) A˚ is
[image:1.610.207.459.533.707.2]Received 10 May 2005 Accepted 31 May 2005 Online 17 June 2005
Figure 1
comparable to that of 2.099 A˚ reported for MgHPO36H2O
(Corbridge, 1956) and to that of 2.086 A˚ for NaMg(H2PO3)3
-H2O (Ouarsal et al., 2004). The Mg(H2O)6 octahedra are
isolated in the structure, the shortest Mg Mg distance being 6.1666 (3) A˚ , which is considerably longer than the Mg Mg distance of 5.031 A˚ found in NaMg(H2PO3)3H2O.
The P atoms occupy two non-equivalent crystallographic
positions, both withmsymmetry. The surrounding tetrahedra
consist of three O atoms and one H atom. The average P—H and P—O distances are 1.25 (2) and 1.527 (2) A˚ , respectively. The distances are in good agreement with those found in (NH4)2HPO3H2O (1.36 and 1.523 A˚ ; Rafiqet al., 1982) or in
[Zn2(H2O)4](HPO3)2H2O (1.31 and 1.520 A˚ ; Ortiz-Avila et al., 1989).
The crystal structure of (NH4)2Mg3(HPO3)418H2O might
be described as a framework made up of isolated
[Mg(H2O)62(HPO3)]2 and [NH4]+ units that are stabilized
by an intricate network of hydrogen bonds (Table 2). Figs. 2 and 3 depict projections of the crystal structure. The three polyhedra Mg(H2O)6, HPO3and NH4lie on a plane parallel to
(010). The hydrogen-bond network ensures the interactions between two neighbouring planes.
Experimental
MgO (10 mg) was dissolved in H3PO3 (10 ml) to which aqueous
ammonia solution (around 5 ml, 0.4M) was added. The solution was heated for 2 h at 300 K and was then left to stand at room temperature. After a week, colourless crystals of (NH4)2Mg3(HPO3)4.18H2O deposited. They were filtered off and
washed with an ethanol–water (80:20) solution.
Crystal data
(NH4)2[Mg(H2O)6]3(HPO3)4
Mr= 753.21 Monoclinic,C2=m a= 34.330 (3) A˚
b= 7.0380 (3) A˚
c= 6.1666 (3) A˚
= 91.377 (6)
V= 1489.51 (16) A˚3
Z= 2
Dx= 1.679 Mg m3 MoKradiation Cell parameters from 72
reflections
= 5.1–27.5
= 0.43 mm1
T= 293 (2) K Prism, colourless 0.450.450.40 mm
Data collection
Nonius KappaCCD diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Siemens, 1996)
Tmin= 0.831,Tmax= 0.848
4735 measured reflections 1799 independent reflections
1687 reflections withI> 2(I)
Rint= 0.012
max= 27.5
h=44!41
k=9!9
l=7!8
inorganic papers
i130
Messouriet al. (NH [image:2.610.315.558.52.419.2] [image:2.610.42.573.54.428.2]4)2[Mg(H2O)6]3(HPO3)4 Acta Cryst.(2005). E61, i129–i131
Figure 2
[image:2.610.43.232.60.431.2]Projection of the crystal structure alongc. Dashed lines indicate hydrogen bonds.
Figure 3
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.022
wR(F2) = 0.064
S= 1.11 1799 reflections 158 parameters
All H-atom parameters refined
w= 1/[2
(Fo2) + (0.0385P)2
+ 0.6624P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001 max= 0.28 e A˚
3
[image:3.610.44.294.208.308.2]min=0.32 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
P1—O1 1.5235 (8)
P1—O2 1.5261 (11)
P1—H1 1.232 (18)
P2—O4 1.5240 (8)
P2—O3 1.5328 (11)
P2—H2 1.267 (19)
Mg1—O6 2.0545 (9)
Mg1—O7 2.0793 (13)
Mg1—O8 2.0929 (9)
Mg1—O5 2.0973 (12)
Mg2—O10 2.0504 (12)
Mg2—O9 2.0737 (9)
O1i
—P1—O1 112.41 (6)
O1—P1—O2 112.33 (4)
O1i—P1—H1 107.1 (4)
O2—P1—H1 105.0 (9)
O4ii
—P2—O4 112.92 (6)
O4—P2—O3 111.67 (4)
O4—P2—H2 106.6 (4)
O3—P2—H2 106.8 (9)
[image:3.610.45.296.379.494.2]Symmetry codes: (i)x;y;z; (ii)x;yþ1;z.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O8—H81 O3 0.75 (2) 1.98 (2) 2.7227 (12) 168 (2) O8—H82 O1 0.77 (2) 1.93 (2) 2.6883 (12) 173 (2) O10—H100 O1 0.85 (2) 1.83 (2) 2.6707 (10) 169 (2) N1—H110 O3 0.90 (3) 1.90 (3) 2.799 (2) 179 (2) O5—H5 O4i
0.87 (2) 1.81 (2) 2.6758 (10) 169 (2) O6—H62 O4v 0.80 (2) 1.94 (2) 2.7193 (12) 166 (2) O6—H61 O4vi
0.75 (2) 2.00 (2) 2.7436 (12) 170 (2) O7—H71 O5vii
0.71 (3) 2.23 (3) 2.9363 (19) 172 (3) O7—H72 O2vii
0.87 (3) 1.80 (3) 2.6657 (18) 172 (2) O9—H91 O2iv
0.85 (2) 1.87 (2) 2.7207 (12) 173 (2) N1—H112 O8viii
0.87 (2) 2.20 (2) 3.0242 (17) 157 (2)
Symmetry codes: (i)x;y;z; (iv)x;y;z; (v)x;y;zþ1; (vi)xþ1 2;y
1 2;zþ1;
(vii)x;y;zþ1; (viii)x;yþ1;z1.
H atoms were found in difference Fourier maps and refined isotropically without any restraints.
Data collection: COLLECT (Nonius, 1998); cell refinement:
EVALCCD (Duisenberg, 2003); data reduction: EVALCCD; program(s) used to solve structure:SIR97 (Altomare et al., 1999); program(s) used to refine structure:SHELXL97(Sheldrick, 1997); molecular graphics:DIAMOND(Brandenburg, 1999); software used to prepare material for publication:SHELXL97.
BEB thanks Dr M. Bouchaib [Centre for the Study of Matter at Extreme Conditions (CeSMEC), Florida Interna-tional University, VH-140, University Park, Miami, FL 33199, USA] for his unquestioning collaboration. JFP is thankful for the financial support of the Spanish Ministerio de Educacion y Ciencia (Programa nacional de ayudas para la movilidad de profesores espano˜lesyextranjeros; Ref. PR-2004-0403).
References
Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999).J. Appl. Cryst.32, 115–119.
Brandenburg, K. (1999).DIAMOND. Version. 2.1c. Crystal Impact GbR, Bonn, Germany.
Corbridge, D. E. C. (1956).Acta Cryst.9, 991–994.
Duisenberg, A. J. M., Kroon-Batenburg, L. M. J. & Schreurs, A. M. M. (2003).
J. Appl. Cryst.36, 220–229.
El Bali, B., Essehli, R., Capitelli, F. & Lachkar, M. (2005).Acta Cryst.E61, i52– i54.
Essehli, R., Lachkar, M., Svoboda, I., Fuess, H. & El Bali, B. (2005).Acta Cryst.E61, i64–i66.
Nonius. (1998).COLLECT. Nonius BV, Delft, The Netherlands.
Ortiz-Avila, C. Y., Squattrito, P. J., Shieh, M. & Clearfield, A. (1989).Inorg. Chem.28, 2608–2615.
Ouarsal, R., Alaoui, T. A., Lachkar, M., Dusek, M., Fejfarova, K. & El Bali, B. (2003).Acta Cryst.E59, i33–i35.
Ouarsal, R., Essehli, R., Lachkar, M., Zenkouar, M., Dusek, M., Fejfarova, K. & El Bali, B. (2004).Acta Cryst.E60, i66–i68.
Powell, D. R., Smith, S. K. & Farrar, T. C. (1994).Acta Cryst.C50, 342–346. Rafiq, M., Durand, J. & le Cot, L. (1982).Z. Anorg. Allg. Chem.484, 187–194. Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Siemens (1996). SADABS. Siemens Analytical X-ray Instruments Inc.,
Madison, Wisconsin, USA.
inorganic papers
Acta Cryst.(2005). E61, i129–i131 Messouriet al. (NH
supporting information
sup-1 Acta Cryst. (2005). E61, i129–i131
supporting information
Acta Cryst. (2005). E61, i129–i131 [https://doi.org/10.1107/S160053680501723X]
Diammonium tris[hexaaquamagnesium(II)] tetrakis[hydrogenphosphate(III)],
(NH
4)
2[Mg(H
2O)
6]
3(HPO
3)
4Ibtissam Messouri, Brahim El Bali, Francesco Capitelli, Juan F. Piniella, Mohammed Lachkar and
Zineb Slimani
Diammonium trimagnesium tetrakis[hydrogenphosphate(III)] octadecahydrate
Crystal data
(NH4)2[Mg(H2O)6]3(HPO3)4 Mr = 753.21
Monoclinic, C2/m Hall symbol: -C 2y a = 34.330 (3) Å b = 7.0380 (3) Å c = 6.1666 (3) Å β = 91.377 (6)° V = 1489.51 (16) Å3 Z = 2
F(000) = 796 Dx = 1.679 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5254 reflections θ = 5.1–27.5°
µ = 0.43 mm−1 T = 293 K Prism, colourless 0.45 × 0.45 × 0.40 mm
Data collection
Nonius KappaCCD diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Siemens, 1996) Tmin = 0.831, Tmax = 0.848
4735 measured reflections 1799 independent reflections 1687 reflections with I > 2σ(I) Rint = 0.012
θmax = 27.5°, θmin = 5.1° h = −44→41
k = −9→9 l = −7→8
Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.022 wR(F2) = 0.064 S = 1.11 1799 reflections 158 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
All H-atom parameters refined w = 1/[σ2(F
o2) + (0.0385P)2 + 0.6624P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.28 e Å−3
supporting information
sup-2 Acta Cryst. (2005). E61, i129–i131
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full
covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
P1 0.047588 (10) 0.0000 0.34406 (6) 0.01405 (11) P2 0.204833 (11) 0.5000 0.37833 (6) 0.01539 (11) Mg1 0.166919 (14) 0.0000 0.75159 (8) 0.01693 (13) Mg2 0.0000 0.5000 0.0000 0.01638 (16) O1 0.05798 (2) 0.17990 (11) 0.47012 (12) 0.02087 (18) O2 0.06427 (3) 0.0000 0.11689 (17) 0.0219 (2) O3 0.16056 (3) 0.5000 0.40510 (19) 0.0238 (3) O4 0.21869 (2) 0.31952 (12) 0.26747 (13) 0.02258 (19) O5 0.19134 (3) 0.0000 0.44310 (19) 0.0224 (2) O6 0.20496 (2) −0.21016 (14) 0.84966 (15) 0.0255 (2) O7 0.14077 (4) 0.0000 1.0523 (2) 0.0379 (4) O8 0.12952 (3) 0.21956 (13) 0.65128 (14) 0.02337 (19) O9 −0.03569 (3) 0.29247 (13) 0.12762 (15) 0.0267 (2) O10 0.03689 (4) 0.5000 0.2665 (2) 0.0309 (3) N1 0.11722 (5) 0.5000 0.0148 (3) 0.0328 (4) H1 0.0120 (5) 0.0000 0.315 (3) 0.019 (5)* H2 0.2201 (6) 0.5000 0.567 (3) 0.022 (5)* H5 0.2033 (5) −0.098 (2) 0.388 (3) 0.041 (5)* H61 0.2253 (6) −0.211 (3) 0.807 (3) 0.042 (5)* H62 0.2060 (5) −0.255 (2) 0.968 (3) 0.038 (5)* H71 0.1513 (8) 0.0000 1.154 (4) 0.038 (7)* H72 0.1162 (7) 0.0000 1.087 (4) 0.035 (6)* H81 0.1400 (5) 0.286 (3) 0.577 (3) 0.036 (5)* H82 0.1092 (5) 0.198 (2) 0.603 (2) 0.031 (4)* H91 −0.0430 (5) 0.195 (3) 0.055 (3) 0.039 (4)* H92 −0.0394 (5) 0.737 (3) 0.243 (3) 0.045 (5)* H100 0.0418 (5) 0.403 (3) 0.344 (3) 0.040 (4)* H110 0.1314 (7) 0.5000 0.140 (4) 0.037 (6)* H112 0.1230 (5) 0.602 (3) −0.060 (3) 0.055 (5)* H113 0.0913 (10) 0.5000 0.046 (5) 0.069 (9)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-3 Acta Cryst. (2005). E61, i129–i131
Mg1 0.0149 (2) 0.0201 (3) 0.0158 (2) 0.000 0.00105 (18) 0.000 Mg2 0.0193 (3) 0.0158 (3) 0.0141 (3) 0.000 0.0003 (3) 0.000 O1 0.0267 (4) 0.0177 (4) 0.0181 (4) 0.0004 (3) −0.0017 (3) −0.0024 (3) O2 0.0242 (5) 0.0272 (6) 0.0145 (5) 0.000 0.0047 (4) 0.000 O3 0.0169 (5) 0.0257 (6) 0.0290 (6) 0.000 0.0044 (4) 0.000 O4 0.0250 (4) 0.0183 (4) 0.0247 (4) 0.0004 (3) 0.0065 (3) −0.0039 (3) O5 0.0277 (6) 0.0176 (5) 0.0222 (6) 0.000 0.0076 (5) 0.000 O6 0.0192 (4) 0.0337 (5) 0.0237 (4) 0.0047 (3) 0.0019 (3) 0.0074 (4) O7 0.0193 (6) 0.0785 (11) 0.0160 (6) 0.000 0.0026 (5) 0.000 O8 0.0165 (4) 0.0246 (4) 0.0288 (4) −0.0003 (3) −0.0014 (3) 0.0034 (4) O9 0.0391 (5) 0.0237 (4) 0.0174 (4) −0.0098 (4) 0.0063 (3) 0.0001 (3) O10 0.0514 (8) 0.0160 (6) 0.0245 (6) 0.000 −0.0173 (6) 0.000 N1 0.0309 (8) 0.0355 (9) 0.0316 (9) 0.000 −0.0034 (7) 0.000
Geometric parameters (Å, º)
P1—O1i 1.5235 (8) Mg2—O9iii 2.0737 (9)
P1—O1 1.5235 (8) Mg2—O9iv 2.0737 (9)
P1—O2 1.5261 (11) Mg2—O9 2.0737 (9) P1—H1 1.232 (18) Mg2—O9ii 2.0737 (9)
P2—O4ii 1.5240 (8) O5—H5 0.874 (17)
P2—O4 1.5240 (8) O6—H61 0.753 (19) P2—O3 1.5328 (11) O6—H62 0.798 (19) P2—H2 1.267 (19) O7—H71 0.71 (3) Mg1—O6i 2.0545 (9) O7—H72 0.87 (3)
Mg1—O6 2.0545 (9) O8—H81 0.751 (19) Mg1—O7 2.0793 (13) O8—H82 0.766 (17) Mg1—O8i 2.0929 (9) O9—H91 0.85 (2)
Mg1—O8 2.0929 (9) O10—H100 0.848 (17) Mg1—O5 2.0973 (12) N1—H110 0.90 (3) Mg2—O10 2.0504 (12) N1—H112 0.87 (2) Mg2—O10iii 2.0504 (12) N1—H113 0.91 (3)
O1i—P1—O1 112.41 (6) O10iii—Mg2—O9iii 93.16 (4)
O1i—P1—O2 112.33 (4) O10—Mg2—O9iv 86.84 (4)
O1—P1—O2 112.33 (4) O10iii—Mg2—O9iv 93.16 (4)
O1i—P1—H1 107.1 (4) O9iii—Mg2—O9iv 89.55 (5)
O1—P1—H1 107.1 (4) O10—Mg2—O9 93.16 (4) O2—P1—H1 105.0 (9) O10iii—Mg2—O9 86.84 (4)
O4ii—P2—O4 112.92 (6) O9iii—Mg2—O9 180.00 (4)
O4ii—P2—O3 111.67 (4) O9iv—Mg2—O9 90.45 (5)
O4—P2—O3 111.67 (4) O10—Mg2—O9ii 93.16 (4)
O4ii—P2—H2 106.6 (4) O10iii—Mg2—O9ii 86.84 (4)
O4—P2—H2 106.6 (4) O9iii—Mg2—O9ii 90.45 (5)
O3—P2—H2 106.8 (9) O9iv—Mg2—O9ii 180.00 (4)
O6i—Mg1—O6 92.10 (6) O9—Mg2—O9ii 89.55 (5)
O6i—Mg1—O7 91.29 (4) Mg1—O5—H5 123.7 (10)
supporting information
sup-4 Acta Cryst. (2005). E61, i129–i131
O6i—Mg1—O8i 178.34 (4) Mg1—O6—H62 124.8 (12)
O6—Mg1—O8i 86.36 (4) H61—O6—H62 106.9 (18)
O7—Mg1—O8i 89.35 (4) Mg1—O7—H71 124 (2)
O6i—Mg1—O8 86.36 (4) Mg1—O7—H72 131.0 (15)
O6—Mg1—O8 178.34 (4) H71—O7—H72 105 (2) O7—Mg1—O8 89.35 (4) Mg1—O8—H81 109.7 (13) O8i—Mg1—O8 95.18 (6) Mg1—O8—H82 121.2 (13)
O6i—Mg1—O5 90.11 (4) H81—O8—H82 109.1 (17)
O6—Mg1—O5 90.11 (4) Mg2—O9—H91 122.4 (11) O7—Mg1—O5 177.98 (6) Mg2—O10—H100 124.3 (11) O8i—Mg1—O5 89.29 (4) H110—N1—H112 109.0 (14)
O8—Mg1—O5 89.29 (4) H110—N1—H113 109 (2) O10—Mg2—O10iii 180.00 (7) H112—N1—H113 110.0 (15)
O10—Mg2—O9iii 86.84 (4)
Symmetry codes: (i) x, −y, z; (ii) x, −y+1, z; (iii) −x, −y+1, −z; (iv) −x, y, −z.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O8—H81···O3 0.751 (19) 1.983 (19) 2.7227 (12) 168.2 (18) O8—H82···O1 0.766 (17) 1.927 (18) 2.6883 (12) 172.5 (18) O10—H100···O1 0.848 (17) 1.833 (18) 2.6707 (10) 169.2 (16) N1—H110···O3 0.90 (3) 1.90 (3) 2.799 (2) 179 (2) O5—H5···O4i 0.874 (17) 1.812 (17) 2.6758 (10) 169.0 (16)
O6—H62···O4v 0.798 (19) 1.938 (19) 2.7193 (12) 166.0 (17)
O6—H61···O4vi 0.753 (19) 1.999 (19) 2.7436 (12) 170.3 (19)
O7—H71···O5vii 0.71 (3) 2.23 (3) 2.9363 (19) 172 (3)
O7—H72···O2vii 0.87 (3) 1.80 (3) 2.6657 (18) 172 (2)
O9—H91···O2iv 0.85 (2) 1.87 (2) 2.7207 (12) 173.0 (17)
N1—H112···O8viii 0.87 (2) 2.20 (2) 3.0242 (17) 157.3 (17)