organic papers
o2630
Akbalet al. C17H14N2O doi:10.1107/S1600536805022592 Acta Cryst.(2005). E61, o2630–o2631
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
4-(2,3,5-Trimethylphenoxy)phthalonitrile
Tufan Akbal,aNesuhi Akdemir,b Erbil Ag˘ar,bCihan Kantarcand Ahmet Erdo¨nmeza*
aDepartment of Physics, Faculty of Arts and
Sciences, Ondokuz Mayıs University, 55139 Kurupelit-Samsun, Turkey,bDepartment of
Chemistry, Art and Science Faculty, Ondokuz Mayıs University, 55139 Kurupelit-Samsun, Turkey, andcDepartment of Chemistry, Rize Arts and Sciences Faculty, Karadeniz Teknik University, Rize, Turkey
Correspondence e-mail: takbal@omu.edu.tr
Key indicators
Single-crystal X-ray study T= 293 K
Mean(Wae) = 0.000 A˚ Rfactor = 0.036 wRfactor = 0.100
Data-to-parameter ratio = 13.9
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
The crystal structure of the title compound, C17H14N2O, is
stabilized by weak van der Waals interactions.
Comment
Substituted phthalonitriles are generally used for preparing symmetrically and unsymmetrically peripherally substitu-ted phthalocyanine complexes and subphthalocyanines (McKeown, 1998; Leznoff & Lever, 1989–1996). Phthalo-cyanines, whose production for the use of dyes and pigments is around 80 000 tons per year (Worhle, 2001), are excellent pigments with good thermal and chemical stabilities. One of the most promising fields is the use of phthalocyanine deri-vatives as photosensitizers for photodynamic therapy (PDT), an emerging new bimodal strategy for treating a large variety of illnesses, such as psoriasis, cancer, dysplastic, infectious diseases and prevention of HIV-1 infection (Leznoff & Lever, 1989–1996; Vzorovet al., 2003).
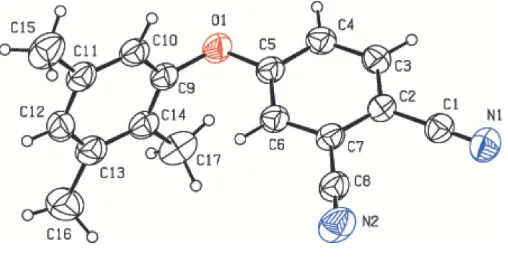
The triple-bond lengths are in agreement with reported values (Petek et al., 2004; Bu¨yu¨kgu¨ngo¨r et al., 2005). The dihedral angle between the C2–C7 and C9–C14 rings is 85.12 (5).
[image:1.610.207.461.586.717.2]Received 24 June 2005 Accepted 14 July 2005 Online 20 July 2005
Figure 1
Experimental
2,3,5-Trimethylphenol (1.17 g, 8.59 mmol) and 4-nitrophthalonitrile (1.0 g, 5.78 mmol) were dissolved in dry dimethylformamide (50 ml). After stirring for 1 h at room temperature, dry fine-powdered potassium carbonate (1.40 g, 10.0 mmol) was added portionwise over a period of 2 h with stirring. The reaction mixture was stirred for 48 h at room temperature and poured into ice–water (200 g). The product was filtered off and washed with (10%w/w) NaOH solution and water until the filtrate was neutral. Recrystallization from ethanol gave (I) (yield 1.11 g, 73.51%). Single crystals were obtained from absolute ethanol at room temperature by slow evaporation (m.p. 373 K); elemental analysis calculated for C17H14N2O: C 77.84, H 5.38, N 10.68%; found: C 75.70 H 5.46 N 10.60%.
Crystal data
C17H14N2O
Mr= 262.30 Orthorhombic,Pbca a= 7.8929 (8) A˚
b= 29.415 (4) A˚
c= 12.4679 (14) A˚
V= 2894.7 (6) A˚3
Z= 8
Dx= 1.204 Mg m 3
MoKradiation
Cell parameters from 10756 reflections
= 1.4–26.0
= 0.08 mm1
T= 293 (2) K Prism, colourless 0.380.200.20 mm
Data collection
Stoe IPDS-2 diffractometer
!scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002)
Tmin= 0.926,Tmax= 0.970 15698 measured reflections 2841 independent reflections
1343 reflections withI> 2(I)
Rint= 0.063
max= 26.0
h=9!9
k=36!36
l=15!14
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.036
wR(F2) = 0.100
S= 0.80 2841 reflections 205 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo2) + (0.056P)2] whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 0.09 e A˚
3
min=0.10 e A˚
3
Extinction correction:SHELXL97
[image:2.610.313.564.110.152.2]Extinction coefficient: 0.0030 (6)
Table 1
Selected geometric parameters (A˚ ,).
O1—C5 1.3621 (19)
O1—C9 1.4097 (19)
C6—C5 1.388 (2)
C1—N1 1.142 (2)
C8—N2 1.134 (2)
N1—C1—C2 179.3 (2) N2—C8—C7 179.1 (2)
The aromatic H atoms were found in a difference Fourier map and were refined isotropically [C—H = 0.877 (19)–0.991 (19) A˚ ]. The methyl H atoms were placed in geometrically idealized positions (C— H = 0.96 A˚ ) and constrained to ride on their parent atoms, with Uiso(H) = 1.5Ueq(methyl C).
Data collection:X-AREA(Stoe & Cie, 2002); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2002); program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:ORTEPIII (Burnett & Johnson, 1996); software used to prepare material for publication: WinGX (Farrugia, 1999) and PARST(Nardelli, 1995).
References
Burnett, M. N. & Johnson, C. K. (1996).ORTEPIII. Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
Bu¨yu¨kgu¨ngo¨r, O., Kos¸ar, B., Akdemir, N., Ag˘ar, E. & Gu¨mru¨kc¸u¨og˘lu, I´. (2005).Acta Cryst.E61, o335–o336.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Leznoff, C. C. & Lever, A. B. P. (1989–1996).Phthalocyanines: Properties and Applications, Vols. 1, 2, 3 and 4. Weinheim/New York: VHC Publishers Inc.
McKeown, N. B. (1998).Phthalocyanine Materials: Synthesis, Structure and Function. Cambridge University Press.
Nardelli, M. (1995).J. Appl. Cryst.28, 659.
Petek, H., Is¸ık, S¸., Akdemir, N., Kantar, C., Ag˘ar, E. & S¸enel, I˙. (2004).Acta Cryst.E60, o256–o257.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Stoe & Cie (2002).X-AREA(Version 1.18) andX-RED32(Version 1.04). Stoe & Cie, Darmstadt, Germany.
Vzorov, A. N., Marzilli, L. G., Compans, R. W. & Dixon, D. W. (2003).Antiviral Res.59, 99–109.
supporting information
sup-1
Acta Cryst. (2005). E61, o2630–o2631
supporting information
Acta Cryst. (2005). E61, o2630–o2631 [https://doi.org/10.1107/S1600536805022592]
4-(2,3,5-Trimethylphenoxy)phthalonitrile
Tufan Akbal, Nesuhi Akdemir, Erbil A
ğ
ar, Cihan Kantar and Ahmet Erd
ö
nmez
4-(2,3,5-Trimethylphenoxy)phthalonitrile
Crystal data C17H14N2O
Mr = 262.30
Orthorhombic, Pbca Hall symbol: -P 2ac 2ab a = 7.8929 (8) Å b = 29.415 (4) Å c = 12.4679 (14) Å V = 2894.7 (6) Å3
Z = 8
F(000) = 1104 Dx = 1.204 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 10756 reflections θ = 1.4–26.0°
µ = 0.08 mm−1
T = 293 K Prism, colourless 0.38 × 0.20 × 0.20 mm
Data collection Stoe IPDS-2
diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002) Tmin = 0.926, Tmax = 0.970
15698 measured reflections 2841 independent reflections 1343 reflections with I > 2σ(I) Rint = 0.063
θmax = 26.0°, θmin = 1.4°
h = −9→9 k = −36→36 l = −15→14
Refinement Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.036
wR(F2) = 0.100
S = 0.80 2841 reflections 205 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.056P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.09 e Å−3
Δρmin = −0.10 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.0030 (6)
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
H3 0.427 (2) 0.2576 (6) 0.9789 (13) 0.078 (5)* H6 0.089 (2) 0.3840 (6) 1.0526 (13) 0.083 (5)* H4 0.278 (2) 0.2934 (6) 0.8401 (15) 0.098 (6)* H10 0.172 (3) 0.4427 (6) 0.8267 (14) 0.085 (6)* H12 −0.270 (2) 0.4973 (7) 0.9005 (14) 0.090 (7)* O1 0.08617 (16) 0.35993 (4) 0.84833 (8) 0.0852 (4) C10 0.0513 (3) 0.44001 (6) 0.84694 (13) 0.0745 (5) C9 −0.0183 (2) 0.39806 (6) 0.86624 (12) 0.0701 (5) C11 −0.0455 (3) 0.47838 (6) 0.85891 (13) 0.0791 (5) C7 0.2486 (2) 0.33806 (6) 1.11797 (12) 0.0675 (5) C2 0.3498 (2) 0.30027 (6) 1.09748 (12) 0.0665 (5) C6 0.1570 (2) 0.35856 (6) 1.03733 (13) 0.0715 (5) C5 0.1695 (2) 0.34164 (6) 0.93371 (12) 0.0685 (5) C1 0.4413 (2) 0.27873 (6) 1.18280 (15) 0.0744 (5) C14 −0.1837 (3) 0.39120 (6) 0.89720 (12) 0.0746 (5) C13 −0.2811 (3) 0.42981 (8) 0.91191 (13) 0.0829 (6) C8 0.2392 (2) 0.35666 (6) 1.22459 (15) 0.0806 (5) C3 0.3594 (2) 0.28369 (7) 0.99302 (14) 0.0739 (5) C12 −0.2121 (3) 0.47217 (8) 0.89229 (14) 0.0853 (6) N1 0.5126 (2) 0.26150 (5) 1.25134 (14) 0.0933 (5) C4 0.2706 (2) 0.30446 (6) 0.91223 (14) 0.0751 (5) C15 0.0247 (3) 0.52510 (6) 0.83666 (16) 0.1115 (8)
H15A 0.0496 0.5400 0.9033 0.167*
H15B −0.0574 0.5426 0.7975 0.167*
H15C 0.1265 0.5225 0.7950 0.167*
N2 0.2328 (3) 0.37180 (6) 1.30813 (14) 0.1127 (6) C17 −0.2528 (3) 0.34413 (7) 0.91417 (15) 0.1031 (7)
H17A −0.1737 0.3222 0.8869 0.155*
H17B −0.3588 0.3411 0.8770 0.155*
H17C −0.2700 0.3390 0.9894 0.155*
C16 −0.4652 (3) 0.42626 (9) 0.94664 (19) 0.1253 (8)
H16A −0.5270 0.4084 0.8953 0.188*
H16B −0.5137 0.4561 0.9508 0.188*
H16C −0.4712 0.4119 1.0157 0.188*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-3
Acta Cryst. (2005). E61, o2630–o2631
C9 0.0869 (14) 0.0766 (11) 0.0468 (9) 0.0117 (11) −0.0022 (8) −0.0001 (8) C11 0.1035 (16) 0.0785 (12) 0.0552 (10) 0.0018 (12) −0.0042 (10) 0.0030 (8) C7 0.0808 (12) 0.0677 (10) 0.0540 (9) −0.0018 (10) 0.0020 (9) 0.0011 (8) C2 0.0703 (11) 0.0652 (10) 0.0642 (10) −0.0037 (9) 0.0012 (9) 0.0044 (8) C6 0.0880 (13) 0.0681 (11) 0.0585 (11) 0.0077 (10) 0.0048 (9) −0.0022 (8) C5 0.0841 (12) 0.0675 (11) 0.0538 (10) 0.0047 (9) 0.0032 (9) −0.0005 (8) C1 0.0762 (12) 0.0720 (11) 0.0751 (12) −0.0043 (10) 0.0011 (10) 0.0050 (9) C14 0.0847 (14) 0.0861 (13) 0.0530 (10) −0.0008 (11) −0.0043 (9) 0.0044 (8) C13 0.0806 (15) 0.1086 (16) 0.0597 (11) 0.0021 (12) −0.0057 (9) 0.0058 (10) C8 0.1007 (15) 0.0783 (11) 0.0628 (11) 0.0054 (11) −0.0023 (10) 0.0004 (9) C3 0.0808 (13) 0.0685 (11) 0.0724 (12) 0.0071 (10) 0.0076 (10) −0.0021 (9) C12 0.1003 (19) 0.0925 (16) 0.0630 (11) 0.0273 (14) −0.0044 (11) −0.0015 (10) N1 0.0936 (13) 0.0955 (11) 0.0909 (11) −0.0019 (10) −0.0123 (10) 0.0145 (9) C4 0.0892 (14) 0.0739 (12) 0.0624 (11) 0.0052 (10) 0.0082 (10) −0.0067 (9) C15 0.165 (2) 0.0799 (13) 0.0901 (14) −0.0095 (14) −0.0007 (15) 0.0051 (10) N2 0.1631 (19) 0.1066 (13) 0.0683 (11) 0.0037 (12) −0.0069 (11) −0.0118 (9) C17 0.1271 (19) 0.1032 (15) 0.0788 (13) −0.0274 (14) −0.0029 (12) 0.0075 (11) C16 0.0824 (16) 0.179 (2) 0.1143 (18) 0.0091 (16) 0.0115 (13) 0.0172 (16)
Geometric parameters (Å, º)
O1—C5 1.3621 (19) C14—C17 1.503 (3)
O1—C9 1.4097 (19) C13—C12 1.381 (3)
C10—C11 1.371 (2) C13—C16 1.520 (3)
C10—C9 1.372 (2) C8—N2 1.134 (2)
C10—H10 0.991 (19) C3—C4 1.371 (2)
C9—C14 1.376 (2) C3—H3 0.953 (17)
C11—C12 1.392 (3) C12—H12 0.877 (19)
C11—C15 1.507 (2) C4—H4 0.958 (19)
C7—C6 1.377 (2) C15—H15A 0.9600
C7—C2 1.393 (2) C15—H15B 0.9600
C7—C8 1.439 (2) C15—H15C 0.9600
C2—C3 1.393 (2) C17—H17A 0.9600
C2—C1 1.433 (3) C17—H17B 0.9600
C6—C5 1.388 (2) C17—H17C 0.9600
C6—H6 0.942 (17) C16—H16A 0.9600
C5—C4 1.380 (2) C16—H16B 0.9600
C1—N1 1.142 (2) C16—H16C 0.9600
C14—C13 1.384 (3)
C5—O1—C9 118.22 (12) N2—C8—C7 179.1 (2)
C11—C10—C9 119.9 (2) C4—C3—C2 120.23 (19)
C11—C10—H10 119.9 (10) C4—C3—H3 120.7 (10)
C9—C10—H10 120.1 (10) C2—C3—H3 119.0 (10)
C10—C9—C14 124.10 (17) C13—C12—C11 123.0 (2) C10—C9—O1 117.00 (18) C13—C12—H12 122.2 (13) C14—C9—O1 118.84 (16) C11—C12—H12 114.9 (13)
C10—C11—C15 121.8 (2) C3—C4—H4 120.3 (11) C12—C11—C15 121.45 (19) C5—C4—H4 119.2 (11)
C6—C7—C2 121.11 (15) C11—C15—H15A 109.5
C6—C7—C8 118.74 (16) C11—C15—H15B 109.5
C2—C7—C8 120.15 (15) H15A—C15—H15B 109.5
C3—C2—C7 118.82 (16) C11—C15—H15C 109.5
C3—C2—C1 120.81 (17) H15A—C15—H15C 109.5
C7—C2—C1 120.37 (15) H15B—C15—H15C 109.5
C7—C6—C5 119.02 (17) C14—C17—H17A 109.5
C7—C6—H6 120.1 (10) C14—C17—H17B 109.5
C5—C6—H6 120.9 (10) H17A—C17—H17B 109.5
O1—C5—C4 116.14 (14) C14—C17—H17C 109.5
O1—C5—C6 123.46 (16) H17A—C17—H17C 109.5
C4—C5—C6 120.39 (17) H17B—C17—H17C 109.5
N1—C1—C2 179.3 (2) C13—C16—H16A 109.5
C9—C14—C13 116.35 (18) C13—C16—H16B 109.5 C9—C14—C17 121.26 (18) H16A—C16—H16B 109.5
C13—C14—C17 122.4 (2) C13—C16—H16C 109.5
C12—C13—C14 119.8 (2) H16A—C16—H16C 109.5 C12—C13—C16 119.3 (2) H16B—C16—H16C 109.5 C14—C13—C16 120.8 (2)
C11—C10—C9—C14 0.4 (2) O1—C9—C14—C13 −178.96 (14) C11—C10—C9—O1 177.56 (14) C10—C9—C14—C17 178.36 (16) C5—O1—C9—C10 96.19 (18) O1—C9—C14—C17 1.2 (2) C5—O1—C9—C14 −86.49 (19) C9—C14—C13—C12 2.0 (2) C9—C10—C11—C12 0.9 (2) C17—C14—C13—C12 −178.24 (15) C9—C10—C11—C15 −178.88 (16) C9—C14—C13—C16 −179.67 (16) C6—C7—C2—C3 0.9 (3) C17—C14—C13—C16 0.1 (3) C8—C7—C2—C3 −178.54 (16) C7—C2—C3—C4 0.2 (3) C6—C7—C2—C1 −178.58 (16) C1—C2—C3—C4 179.67 (17) C8—C7—C2—C1 1.9 (2) C14—C13—C12—C11 −0.8 (3) C2—C7—C6—C5 −1.4 (3) C16—C13—C12—C11 −179.15 (17) C8—C7—C6—C5 178.06 (17) C10—C11—C12—C13 −0.7 (3) C9—O1—C5—C4 −179.81 (16) C15—C11—C12—C13 179.06 (17)
C9—O1—C5—C6 0.0 (3) C2—C3—C4—C5 −0.7 (3)
C7—C6—C5—O1 −178.96 (16) O1—C5—C4—C3 −179.94 (17)
C7—C6—C5—C4 0.8 (3) C6—C5—C4—C3 0.2 (3)