www.wjpr.net Vol 4, Issue 06, 2015.
1777
SYNTHESIS, MOLECULAR PROPERTIES PREDICTION AND
BIOLOGICAL ACTIVITY OF BENZIMIDAZOLE
TRITHIOCARBAMATE DERIVATIVES
A.N.V.Sunitha, S. Sharada, M.S.N.Sandhya, T.Saritha Jyostna*
Medicinal Chemistry Division, Sarojini Naidu Vanita Pharmacy Maha Vidyalaya, Hyderabad-500017, India.
ABSTRACT
In the present investigation, a series of novel Benzimidazole trithiocarbamates have been synthesized. It deals with the design and calculation of Molecular Properties, Druglikeness, Lipophilicity and solubility parameters using Molinspiration, Molsoft softwares. Toxicity parameters were calculated using Osiris software. All the compounds are non-toxic, fulfill the solubility requirements and passing oral bioavailibility criteria. The compounds were synthesized and characterized by IR, 1H NMR and Mass Spectral analysis followed by antimicrobial screening. Most of the synthesized compounds (4a-4f) were found to be on conformity with Lipinski’s “Rule of Five” and other parameters, for their onward screening for antimicrobial activity as oral active drugs.
KEYWORDS: Benzimidazole, Trithiocarbamate, Molinspiration, Molsoft, Osiris, Antimicrobial activities.
INTRODUCTION
Benzimidazole is a heterocyclic aromatic organic compound. The most prominent benzimidazole compound in nature is N-ribosyl-dimethyl-benzimidazole, which serves as an axial ligand for cobalt in vitamin B12.[1] Benzimidazole class of compounds are found in
several therapeutic agents such as antiulcer[2], antihypertensive[3], antifungal[4], antiprotozoal[5], antihistamine[6], antiviral[7] antimalarial[8] and most importantly in antimicrobials[9]. Extensive biochemical and pharmacological studies have confirmed that its derivatives are effective against various strains of microorganisms.
Volume 4, Issue 6, 1777-1784. Research Article ISSN 2277– 7105
*Correspondence for Author
Dr. T.Saritha Jyostna
Medicinal Chemistry
Division, Sarojini Naidu
Vanita Pharmacy Maha
Vidyalaya,
Hyderabad-500017, India. Article Received on 04 April 2015,
www.wjpr.net Vol 4, Issue 06, 2015.
1778 Beside this Trithiocarbamates[10] have industrial, synthetic and medicinal properties. They have been used extensively as pharmaceuticals, organic chemicals and as intermediates in organic synthesis for the protection of thiol functional group. There is no literature data available on benzimidazole possessing trithiocarbamate as a side chain. Moreover dithiocarbamates have various biological activities which are capable of forming mono and bidentate co-ordination complexes to transition metal centers. Transition metal complexes of dithiocarbamates present a wide range of biological activities.[11,16] Drugs which possessing dithiocarbamates are Oxaramate, Sulfaramate and natural products like brassinin and isobrassin found to have chemotherapeutic activity. And there is structural similarity between dithiocarbamates and trithiocarbamates. Hence, we incorporated trithiocarbamate as a side chain in the place of dithiocarbamate and to find out its biological activity.
MATERIALS AND METHODS
All reagents were purchased from commercial suppliers like Sigma Aldrich, Merck India Ltd., Sd fine chemicals. All reagents were of AR grade and were used without purification. Melting points of the synthesized compounds were determined using MELTER FP-51 apparatus and were found uncorrected. The IR spectra of the synthesized compounds were recorded using KBr pellets on BRUKER (Perkin Elmer Model 283B and Nicolet-740 FTIR spectrophotometer and frequencies were recorded in wave numbers (cm-1). The 1HNMR spectra were recorded on Varian Gemini-400 spectrometer, chemical shifts were expressed as δ-values in ppm, downfield from internal standard TMS. Mass spectrum was recorded by electrospray ionization mass spectrometer as a value of m/z. Purity of the compounds were checked by TLC on silica gel 60F-254 (Merck) in an appropriate solvent.
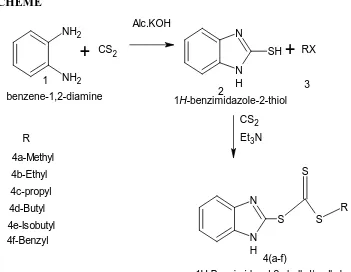
Synthesis of 2-mercapto benzimidazole
A mixture of O-phenylene diamine (0.01 mol) was dissolved in Alc.KOH (25 ml) and to the mixture carbon disulphide (0.015 mol) was added and refluxed for 3 hrs at 45-50 ⁰C and then cooled and poured into ice cold water and neutralized with 0.1N HCl. The resulting solid was filtered, dried and purified by the recrystallization from methanol to yield 2- mercaptobenzimdazole.[17]
General method of Synthesis of Benzimidazole trithiocarbamate
www.wjpr.net Vol 4, Issue 06, 2015.
1779 monitored by TLC. The mixture was extracted with ethyl acetate and dried over sodium sulfate and later was subjected to purification by passing through silica gel using mixture of n-hexane and ethyl acetate as eluent.
Spectral data of synthesized compounds[4] 1H-benzimidazol-2-yl methyl carbonotrithioate
IR (KBr) ν cm-1 2838 aliphatic (C-H), 1535 Aromatic (C=C), Amine 1237 (C-N). 1H NMR [CDCl3, 400MHz] ppm 2.81 (s, 3H, CH3), δ 7.25-7.30 (t, 1H, H), δ 7.38-7.45 (t, 1H,
Ar-H),δ7.76-7.78 (d, 2H, Ar-H) 7.87-7.88 (d, 2H, Ar-H). ESI-MS m/z [M+1] 241.
1H-benzimidazol -2-yl ethyl carbonotrithioate IR (KBr) ν cm-1
2927 aliphatic (C-H), 1423 Aromatic (C=C), Amine 1308 (C-N). 1H NMR [CDCl3, 400MHz] ppm 1.45-1.50 (t, 3H, CH3 ), δ 3.31-3.38 (q, 2H, CH2), δ 7.25-7.31 (t, 1H,
Ar-H), δ 7.38- 7.42 (t, 1H, Ar-H), δ 7.73-7.76 (d, 2H, Ar-H), δ7.85-7.89 (d, 2H, Ar-H). ESI-MS m/z [M+1] 255.
1H-benzimidazol -2-yl propan-2-yl carbonotrithioate IR (KBr) ν cm-1
2962 aliphatic (C-H), 1424 Aromatic (C=C) 1237 Amine (C-N). 1H NMR [CDCl3, 400MHz] ppm 1.49-1.50 (d, 6H, 2CH3), δ 4.04-4.11(m, 1H, CH) δ 7.25-7.31 (t, 1H,
Ar-H), δ 7.38- 7.43 (t, 1H, Ar-H), δ 7.74-7.76 (d, 2H, Ar-H), δ 7.87-7.89 (d, 2H, Ar-H). ESI-MS m/z [M+1] 269.
1H-benzimidazol -2-yl butyl carbonotrithioate IR (KBr) ν cm-1
2962 aliphatic (C-H), 1424 aromatic (C=ssssC), 1237 Amine (C-N). 1H NMR [CDCl3, 400MHz] ppm 1.04-1.08 (t, 3H, CH3) δ 1.78-1.88 (m, 4H, CH2CH2CH3) δ
3.28-3.31 (t, 2H, CH2), δ 7.22-7.27 (t, 1H, Ar-H), δ 7.35-7.40 (t, 1H, Ar-H), δ 7.69-7.72 (d,
1H, Ar-H), δ 7.84-7.87 (d, 1H, Ar-H). ESI-MS m/z [M+1] 283.
1H-benzimidazol -2-yl - 2-methylpropyl carbonotrithioate IR (KBr) ν cm-1
2961 aliphatic (C-H), 1424 Aromatic (C=C), 1237 Amine (C-N). 1H NMR [CDCl3, 400MHz] ppm 1.36-1.39 (d, 6H, 2CH3) δ 1.69-1.80 (t, 1H, CH) δ 3.89-3.94 (d, 2H,
CH2), δ 7.23-7.28 (t, 1H, Ar-H), δ 7.31-7.36 (t, 1H, Ar-H), δ 7.67-7.70 (d, 2H, Ar-H), δ
www.wjpr.net Vol 4, Issue 06, 2015.
1780 1H-benzimidazol -2-yl benzyl carbonotrithioate
IR (KBr) ν cm-1
3056 Aromatic (C-H), aliphatic 2926(C-H), 1424 Aromatic (C=C), 1309 Amine (C-N). 1H NMR [CDCl3, 400MHz] ppm 4.04-4.11 δ (m, 2H, SCH2),δ 7.25-7.43 (t,
4H, Ar-H), δ 7.74-7.89 (d, 5H, Ar-H). ESI-MS m/z [M+1] 317.
MOLECULAR PROPERTIES PREDICTION
A molecular property is a complex balance of various structural features which determine whether a particular molecule is similar to the known drugs. It generally means “molecules which contain functional groups and/or have physical properties consistent with most of the known drugs”. These properties mainly, hydrophobicity, molecular size, flexibility and presence of various pharmacophoric features, influence the behaviour of molecules in a living organism, including bioavailability. The bioavailability related properties such as solubility, lipophilicity are important before actual synthesis to reduce the chemical expenses and valuable time. Computational chemists have a wide array of tools and approaches available for the assessment of molecular diversity. Diversity analysis has been shown to be an important ingredient in designing drugs. So, computational sensitivity analysis and structural analysis have been used to study the drug likeness of the candidate molecule.[18,19] The molecular properties were predicted and presented in Table-2.
Biological activity
Antibacterial activity: The synthesized novel compounds 4a-f were screened for their antibacterial activity against Escherichia Coli, a gram negative bacteria and BacillusSubtilis, a gram positive bacteria by agar cup plate method.[20] The test compounds were dissolved in methanol to prepare stock solutions. The concentrations of test compounds were 100 and 200µg / ml in comparison to standard drug streptomycin. For agar cup plate method, nutrient media was prepared and sterilized by autoclaving at 121oC for 15min to which loop full bacteria from 24 hours culture was added, and gently shaken for uniform distribution of organism. The media was poured in petri plates, allowed to solidify after which, bore wells of 10 mm diameter at equal distance were made and a drop of test and standard compound was placed in the plates evenly distributed with bacteria and incubated at 37oC for 24-48 hours, for the inhibition of bacteria. Zone of inhibition of compounds were compared with zone of inhibition of standard drug streptomycin.
www.wjpr.net Vol 4, Issue 06, 2015.
1781 of 100 and 200 µg /ml. Sabouraud-dextrose agar media was prepared and sterilized and incubated with fungi for 3 days under aseptic conditions to get new mycelium for activity. A drop of test and standard compounds was placed in the bore wells made in the plates, and allowed to incubate for 72 hours, for the inhibition of fungi, the zone of inhibition of test compounds was compared with standard drug fluconazole.
RESULT AND DISCUSSION
All the synthesized compounds (4a-4f) were evaluated for antibacterial and antifungal activites and results were present in Table-1. In the series homologation of side chain form methyl, ethyl, propyl to isobutyl there is increase in antibacterial activity and by the introduction of aromatic substituent (benzyl), there is further increase in activity observed which is nearly equipotent with the standard Streptomycin against E.coli. All the compounds exhibited mild activity against gram positive bacteria B.subtilis, when compared to the standard. In a series, none of the compounds were found to be active against fungus,
Pencillium Chrysogenum, it indicating that the compounds were not selective for the
organism. The molecular properties were predicted and presented in Table-2. All the compounds followed oral bioavailability criteria. The pharmacokinetics parameters and molecular bioactivity scores were good, all the compounds showed good drug likeness score. Toxicity prediction data showed that all the compounds are nontoxic.
Table1: The Antimicrobial data of title compounds
Compounds
Zone of Inhibition
Antibacterial Antifungal
E.Coli Bacillus Subtilis Pencillium chrysogenum
100µg 200µg 100µg 200µg 100µg 200µg
4a 8 12 5 7 - -
4b 11 13 6 8 - -
4c 12 15 8 10 - -
4d 15 17 9 10 - -
4e 16 19 10 12 - -
4f 20 22 11 13 8 12
STREPTOMYCIN 23 25 - - - -
www.wjpr.net Vol 4, Issue 06, 2015.
[image:6.595.82.433.253.525.2]1782 Table 2: Toxicity studies of 1H-benzimidazole trithiocarbamate.
Compound MUT TUM IRR RE ClogP Solubility Mol.wt DLS DS
4a + + + + 2.46 -3.3 241 0.12 0.67
4b + + + + 2.96 -3.53 255 -0.06 0.62
4c + + + + 3.41 -3.8 269 -0.07 0.58
4d + + + + 3.36 -3.72 269 0.67 0.66
4e + + + + 3.63 -3.96 283 -0.04 0.56
4f + + + + 3.94 -4.88 317 -3.21 0.33
MUT: mutagenic, TUM: tumorigenic, IRR: irritant, RE: reproductive effect, DLS: drug likeness score, DS: drug score.
SCHEME
NH2
NH2
+
CS2Alc.KOH
N
N H
SH
2
+
RX3
N
N H
S S
S
R CS2
Et3N R
4a-Methyl 4b-Ethyl 4c-propyl
1H-benzimidazole-2-thiol benzene-1,2-diamine
1
4(a-f) 4d-Butyl
4e-Isobutyl 4f-Benzyl
1H-Benzimidazol-2-yl-alkyl/aralkyl carbonotrithioate
CONCLUSION
In conclusion a series of Benzimidazole Trithiocarbamate derivatives have been synthesized and screened for their antimicrobial activities. Most of the compounds have shown moderate to promising activity against gram negative bacteria when compared to gram positive bacteria. More importantly, compound 4f exhibited potential anti bacterial activity agisainst
E.coli, indicating that benzyl side chain with trithiocarbamate on 2nd position of
www.wjpr.net Vol 4, Issue 06, 2015.
1783 ACKNOWLEDGEMENT
The authors are thankful to the principal and management of Sarojini Naidu Vanitha Pharmacy Maha Vidhyalaya and Central Facilities for Research and Development, Osmania University, Hyderabad, India for providing spectral data.
REFERENCES
1. Barker HA, Smyth RD, Weissbach H, Toohey JI, Ladd JN and Volcani BE. Isolation and properties of crystalline cobamide coenzymes containing Benzimidazole. J of Biol Chem., 1960; 235(2): 480- 488.
2. Patil A, Ganguly S and Surana S. A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J Chem, 2008; 1(3): 447-460.
3. Jat RK, Jat JL and Pathak DP. Synthesis of benzimidazole derivatives as anti-hypertensive activity. Eur J of Chem, 2006; 3: 278-285.
4. Goker H, kus C, Boykin DW, Yildiz S, Altanlar. Synthesis of some new 2-substituted-phenyl-1-H-benzimidazol-5-carbonitriles and their potent activity against Candida albicans species. Biorg Med Chem, 2002; 10: 2589-96.
5. Hernandez-Luis F, Hernandez-Campos A, Castillo R, NavarreteVazquez G, Soria-Arteche O, Hernandez Hernandez M and YepezMulia L. Synthesis and biological activity of 2-(trifluoromethyl)-1Hbenzimidazole derivatives against some protozoa and Trichinella spiralis. Eur J Med Chem, 2010; 45(7): 3135- 3141.
6. Lemura R, Manabe H, Todayaki S. Bioisosteric transformation of H1-antihistaminic benzimidazole derivatives. Chem. Pharm. Bull. 1989; 37: 2723–2726.
7. Tewari AK and Mishra A. Synthesis and antiviral activities of N-substituted -2-substituted - benzimidazole derivatives. Indian J Chem, 2006; 45(B): 489-493.
8. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf., 2004; 27: 25-61. 9. Ayhan-Kilcigil G and Altanlar N. Synthesis and antimicrobial activities of some new
benzimidazole derivatives. Farmaco, 2003; 58: 1345-1350.
10. Barahman Movassagh, Mohammad Soleiman-Beig. Triehylamine catalysed one-pot Synthesis of trithiocarbamates from carbondisulfide, thiols, alkyl halides in water. Monatsh Chem, 2008; 139: 927-930.
www.wjpr.net Vol 4, Issue 06, 2015.
1784
12. Erian, AW, Sherif, SM. The chemistry of thiocyanic esters. Tetrahedron, 1999; 55: 7957-8024.
13. Wood TF, Gardner, JH, Synthesis of some dialkylaminoalkyl arythiourethanes and thioureas. J Am. Chem Soc, 1941; 63: 2741-2742.
14. Bowden K, Chana RS. Synthesis and biological activity of benzimidazole trithiocarbamates derivatives. J Chem Soc, Perkin Trans, 1990; 2: 2163.
15. Beji, M. Sbihi, H.; Baklouti, A.; Cambon, A. Synthesis of F-alkyl N-sulfonyl carbamates and thiocarbamates. J Fluor. Chem, 1999; 99: 17-24.
16. Goel A, Mazur SJ, Fattah RJ, Hartman TL, Turpin JA, Huang M, Rice WG, Appella E, Inman JK. Bioorg Med Chem Lett. 2002; 12: 767–770.
17. Umarani N, Illango K, Arun Kumar Mishra, Mohamed Rabik Raja R. Synthesis of Newer
bioactive 2- Mercapto benzimidazoles. Indian J of Heterocyclic Chem, 2011; 20: 347-350.
18. Mohammed AB, Shahar Yar M , Sami Gaber Abdel-Hamid, Saleh I, Qasoumi S.Al, Abdul, Eur J Med Chem, 2010; 45: 5862-5869.
19. Mohamed JA, Jeyabalan GS, Habibullah I, MdShivliNomani PS, Ramakant G, Abhimanyu S Molecular properties prediction and synthesis of novel 1,3,4- oxadiazole analogues as potent antimicrobial and antitubercular agents. Bio Org Med Chem. Letters, 2011; 21(24): 7246-7250.
20. Goker H, Ozden S, Yildiz S, Boykin D. Synthesis and potent antibacterial activity against MRSA of some novel 1,2-substituted-1-H-benzimidazole-N-alkylated-5-carbox-amidines. Eur J Med Chem, 2005; 40: 1062-1069.