PREPARATION AND CHARACTERIZATION OF CURCUMIN GOLD
NANO SUSPENSION FOR BREAST CANCER
Pankti P. Dalwadi*1,2, Dr. Pragnesh Patani1 and Dr. Indrajeet Singhavi2
1,2
Department of Pharmacology, A-One Pharmacy College Naroda, Ahmedabad, Gujarat,
India.
2
Dean Faculty of Pharmacy, Department of Pharmaceutical Chemistry, Pacific Academy of
Higher Education and Research, Udaipur, Rajasthan, India.
ABSTRACT
Breast cancer is world third leading cause of death in the world among
women. Breast cancer is spreads from milk duct. Nano suspension
improves bioavailability of poorly soluble drugs improves therapeutic
effectiveness of drug to target at the site of effectiveness. Curcumin
gold nano suspension (CGNS) is prepared by Bottom –Up technology.
Curcumin is obtained from rhizomes of curcuma longa. Curcumin
modulate anti cancer activity by activation of NF- κB pathway.Gold
has surface plasmon resonance property which reduces the dose of the
chemotherapy and radiation therapy. Curcumin gold nano suspension
solid particles were obtained by lyophilisation. Particle size of
optimized batch of CGNS is 322.6±4.98nm and poly dispersity index of 0.18±0.020. Zeta
potential of CGNS is -27.42 ±0.32. Differential scanning calorimetry shows that nano particle
was in amorphous in nature. Improves dissolution rate of CGNS because of reduces particle
size. Transmission Micrograph of the CGNS was show particle size of 322.6nm. The results
suggested that precipitation was an effective way to prepare CGNS improves the dissolution
rate for targeting cancer cells.
KEYWORDS: Curcumin, Gold nano particle, Precipitation method, Nano suspension,
Dissolution.
INTRODUCTION
Breast cancer is major health problem in developed and developing countries. As per
epidemiological survey various parameters such as mortality and survival rates varying
Article Received on 15 Nov. 2018,
Revised on 04 Dec. 2018, Accepted on 25 Dec. 2018
DOI: 10.20959/wjpr20191-13958
*Corresponding Author
Pankti P. Dalwadi
Department of
Pharmacology, A-One
Pharmacy College Naroda,
Ahmedabad, Gujarat, India.
between countries.[1] Breast cancer is accredited to various factors such as life style changes dietary changes, obesity, hormonal imbalance as well as exposure to radiation, alteration
menstrual changes. A Women with predispose to genetic mutation such as BRCA 1 and
BRCA 2. Current therapy of breast cancer is based on complementary and alternative
medicine .treatment approach for breast cancer.[2] It compiles both and involves different therapies such as surgery, Radiation and oncological treatment such as chemotherapy,
hormonal therapy and immunological therapy or systemically destroy or control cancer cell
throughout the body. Gold is newer approach for the treatment of cancer. Gold particles are
smaller in size and penetrate throughout the body preferentially accumulates at the tumor site
owing to EPR effect and importantly they can bind to many proteins and actively targeted to
cancer cells and by over expressing cell surface receptor.[3] Gold has characteristics of surface plasmon resonance and as chemically inert for the proteins, antibodies and genes and also has
higher biologically compatible and provide high surface area so large amount of drug can be
loaded.[4] Natural phytoconstitute from various medicinal plant has now widely interested as having promising effect in development of new class of anticancer drugs since they have
multiple targets in cancer cells with minimum toxicity of the constitute. curcumin is obtained
from rhizomes of turmeric curcuma longa which is widely used as Indian spice traditional
medicine for various disorders.[5] curcumin has anti antioxidant effect. Curcumin modulates biological pathways involved in mutagenesis, cell cycle regulation, apoptosis, angiogenesis,
tumour genesis and metastasis.[6] curcumin with paclitexal in combination produce increases antitumor efficacy. Curcumin reduces tumor growth and cell proliferation as well as
suppression of angiogenesis by intra peritoneal administration of curcumin in xenograft
model of breast cancer.[8] Reduces human epidermal receptors HER2 expression as well as phosphorylation of Akt MAPK in different cell line. curcumin induce apoptosis by inhibiting
fatty acid synthase.[9]
A nano suspension is a submicron colloidal dispersion of drug particle. It is a very finely
colloid, biphasic, dispersed and solid drug particle in aqueous vehicle, size below 1 µm,
stabilized by surfactants and polymers.[10] The particle size distribution of the solid particle in nano suspension is usually less than one micron with an average particle size ranging
between 200 to 600nm.[11] Nano suspension approach improves bioavailability of drug. Nano suspension approach is most suitable for the drug with high log P value and high melting
Nano suspension is prepared by various approaches like Top-down technology, bottom-up
technology, micro emulsion and emulsion as template.[13] In the present research work nano suspension was prepared by bottom -up technology in which drug is dissolved in organic
solvent which is then added to non organic solvent that cause precipitation of the nano
particle and the system is stabilized by stabilizer or surfactant to prevent further
agglomeration of particle.[14] The anti solvent approach precipitation approach is cost effective and rapid method.[15]
MATERIAL AND METHOD
Material
Gold chloride was procured from Astron Chemicals, Ahmedabad. Curcumin was procured
Yucca Enterprise, Mumbai, India. Poloxamer-188 was procured from Astron Research
Center. Ahmedabad, Gujarat. PVP K-90 was procured from SD chemicals, Mumbai.
Hydroxy propyl methyl cellulose was procured as gift sample from Cadila pharma, Gujarat
Indi.
Method
Preparation of Gold nano particle (GNP):
All glassware’s were cleaned with an aqua regia solution (1:3 nitric acid /hydrochloric acid).
1mM solution of 250 ml of hydrogen tetrachloroaurate trihydrate was brought to a boil for
vigorous stirring. To this solution 25ml of 33.8 mM of sodium citrate was added. Sodium
citrate is used as reducing agent. Gold solution was yellow at starting of reaction. Solution is
allow to boil. After adding sodium citrate act as reducing agent which sodium citrate turned
to citric acid.[16] Conversion of Au+3 to Au0 yellow coloured solution become transparent and colorless and It changed to black than slowly to wine red. The reaction can be summarized as.
2HAuCl4 + 3C6H8O7 (citric acid) 2Au + 3C5H6O5 + 8HCl+3CO2
Nano suspension was prepared by “Bottom –Up” technology. In the first step of Curcumin
was dissolved in the acetone to prepare organic phase and solution was then filter to clear
impurities at room temperature.[17] Different concentration of surfactant were dissolve in water as anti solvent and to this equal quantities of gold nano particle was added in different
batches. In second step drug was dispersed in gold nano particle using overhead stirrer at
2000 RPM for 24 hr leading to precipitation of nano suspension of phytoconstituents. The
Formulation
Ingredients F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 F14 F15 F16
Drug
(mg/ml) 30 30 30 30 30 30 30 30 30 30 30 30 30 30 30 30
Poloxamer
-407(g/ml) 0.25 0.5 1 5 - - - -
Poloxamer-188(g/ml) - - - - 0.25 0.5 1 5 - - - -
PVP-K-90
(g/ml) - - - 0.25 0.5 1 5 - - - -
HPMC(g/ml) - - - 0.25 0.5 1 5
Acetone (ml) 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
GNP (ml) 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10
Water (ml) 05 20 25 50 05 20 25 50 05 20 25 50 05 20 25 50
CHARACTERIZATION OF PARTICLE SIZE
Particle Size and Polydispersity Index
Mean particle size of nano suspension and poly dispensability index of formulations were
determined by Malvern Zetasizer using water as dispersion medium. The sample of different
batches were scanned for determination of particle size. The samples of batches were
measured after appropriate dilution with distilled water. Mean particle size and distribution of
particle size in nanodispersion measured by dynamic light scattering. The zeta sizer measure
particle in the range of 0.6 nm- 6µm. The reading of samples were taken at 900 angle of incident beam. The zeta potential was measured by laser doppler anemometer coupled with
the same instrument. For accuracy the analysis of the sample was carried out in triplicate.[18]
Zeta potential
Zeta potential of the suspension is measured by Malvern zeta sizer. The zeta sizer is mainly
consist of the laser which is used to provide light source to illuminate particle within the
sample. For measurement of zeta potential light splits into an incident and reference beam.
The incident beam passes through the center of the sample cell. Zeta sizer produces a
frequency spectrum from which the electrophoretic mobility hence zeta potential is
calculated. The mobility and potential of nano dispersion particles with in range of 3nm-10
µm.[19]
Total Drug Content
An aliquot (0.5 ml) was evaporated to dryness. The residue was dissolved in acetone and
filtered with 0.45µm filter paper. The sample was analyzed using UV spectrophotometer at
Total Drug Content= (Total volume of nano suspension * Amount of drug in aliquot
)/Volume of aliquot.[20]
Entrapment Efficiency
High concentration of the free drug present in the supernant after centriguation can
determined by entrapment efficiency.10 ml of the freshly prepared nano suspension was
centrifuged at 10,000 rpm for 10 minutes using micro centrifuge. The supernant was
collected and unincorporated drug measured by taking absorbance of supernatant at 411 nm
by using UV spectro photo metrically.
Fourier Transform Infrared Spectroscopy
The Fourier transform infrared spectroscopy (FT-IR) spectra were scanned in the range of
3600-400cm-1 at an ambient temperature. FT-IR was scanned in the sequence of Curcumin, Polymer, Gold chloride and optimized batch of formulation using KBr technique. The pellet
were prepared using KBr hydraulic press under hydraulic pressure of 150 kg/m2.
Differential Scanning Calorimetry
DSC of pure Curcumin, Polymer, Gold chloride and Curcumin gold nano suspension were
studied using DSC (Shimadzu 60 with TDA trend line software, Shimadzu Co. Kyoto,
Japan). In DSC analysis, the samples were weighed (5mg) hermetically sealed in flat bottom
aluminum pans were heated over a temperature range of 50 ℃ to 300 ℃ at a constant
increasing rate of temperature of 10℃/min. Nitrogen gas 50 mL/min. Calibration of
instrument with respect to temperature and enthalpy was achieved.[21]
Transmission Electron Microscopy
Transmission electron microscopy (TEM) studies were performed using transmission
electron microscope(Philips Technai-20). The liquid nano suspension formulation was
dropped on copper-gold carbon grid and allowed to dry. This grid was then mounted in the
instrument and photographs were taken at various magnifications.[22]
In Vitro Drug Release Study
Dissolution studies were carried out in dissolution apparatus (TDT-08L, Electro lab) by using
the USP apparatus 2 paddle method. The bath temperature and paddle speed were set at 37℃
and 50 rpm respectively. The dissolution media include 900 ml of distilled water and
during dissolution process. An accurately weight bulk drug and nano suspension were
dispersed to the vessels. 5 ml samples were withdrawn at 5,10,20,30 40, 50, 60 min from
dissolution medium and replace with same buffer solution. The accumulation of dissolution
amount of Curcumin was determined by Spectro photo metrically.[24, 25]
RESULT AND DISCUSSION
Curcumin gold nano suspension was prepared by Bottom –Up approach. Polymers, solvent
and anti solvent ratio were selected based on preliminary formulation study of CGNS
batches. Nano suspension can be visualized by bluish white transparent ring appearance at the
[image:6.595.105.493.306.581.2]top. Characterization of particle size of different batches are as seen in table 2:
Table 2: Particle Size, Poly dispersity index and Zeta potential of different batches.
BATCH NO PARTICLE SIZE(nm) (MEAN±S.D) PDI (MEAN±S.D) ZETA POTENTIAL(mV) (MEAN±S.D)
F1 608.5±5.88 0.46±0.01 -9.42±0.17
F2 570.9±12.71 0.35±0.02 -10.59±0.27
F3 322.6±4.98 0.18±0.02 -27.42 ±0.32
F4 472.4±5.19 0.25±0.03 -20.42±0.21
F5 695.3±10.27 0.53±0.04 -4.38±1.92
F6 600.1±11.72 0.43±0.03 -12.27±0.17
F7 547.8±3.68 0.35±0.03 -20.53±0.22
F8 215.7±6.9 0.36±0.05 -22.54±0.14
F9 425.9 ±3.29 0.27±0.05 -4.79±1.52
F10 393.7±6.54 0.71±0.03 -15.64±0.16
F11 372.3±10.78 0.57±0.06 -22.49±0.22
F12 188.6±3.26 0.45±0.02 -20.37±0.15
F13 1107.4±4.18 0.68±0.03 -12.65±0.39
F14 906.9±3.68 0.48±0.04 -12.81±0.20
F15 847.3±5.71 0.37±0.04 -19.52±0.19
F16 650.1±4.98 0.53±0.04 -23.81±0.15
Determination of Particle Size
Particle size of different batches were taken are as below. Batch size from F1 to F16,
maximum particle size was observed of batch F13 because of low concentration of polymers
induce aggregation of particles. The optimized shows particle size 322.6 nm as shown below
Polydispersity Index
Poly dispersity index of nano suspension batches were found to be in the range of o.71 to
0.18. F3 batch show the particle size distribution 0.18 which is most suitable for even
distribution of particle size.
Zeta Potential
Zeta potential means the distribution of charges over dispersed particle of nano suspension. It
indicates stability of nano suspension. Zeta potential of formulation batch of F1 to F16 were
found to be -9.42 to -27.42. F3 batch of formulation show zeta potential of -27.42 was found
[image:7.595.96.498.285.512.2]to be stable curcumin gold nano suspension.
Figure 1: Particle size graph of optimized batch of curcumin gold nano suspension.
Total Drug Content
Drug content of all CGNS formulation was found to be greater than 80% except some of
batches indicates suitability of these method for particle size reduction. The optimized batch
Graph 1: Percentage total drug content of different CGNS batches.
Entrapment Efficiency
Entrapment efficiency all the batches were found to be greater than 60% except some of
batches. The optimized batch F3 shows entrapment efficiency 89.15% as shown in graph2.
Graph 2: Percentage entrapment efficiency of different CGNS batches.
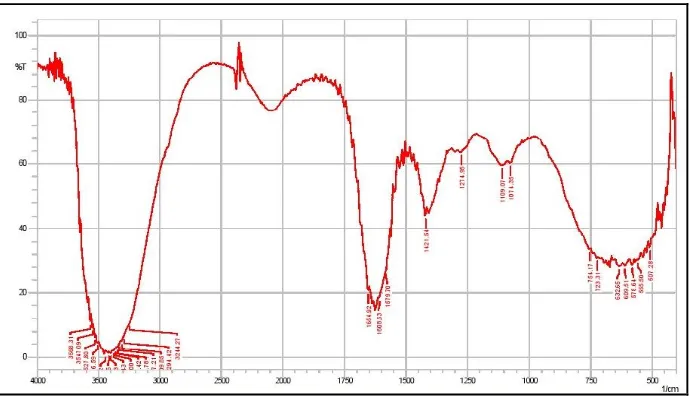
Fourier Transform Infrared Spectroscopy
FTIR Spectrum of curcumin, polymer and nano suspension were taken to determine
physicochemical property and intermolecular bonding of drug and polymer. The FTIR
spectra of pure curcumin and curcumin gold nano suspension curves were well matched.
FTIR spectra shows O-H stretching was shift from 3299 cm-1 to 3205 cm-1 indicates intermolecular bonding. Broadening of peak was due to loading of curcumin with gold and
Figure 2: FT-IR spectra of pure Curcumin.
Figure 3: FT-IR spectra of Poloxamer 188.
[image:9.595.126.471.517.718.2]Figure 5: FT-IR spectra of Curcumin gold nano suspension.
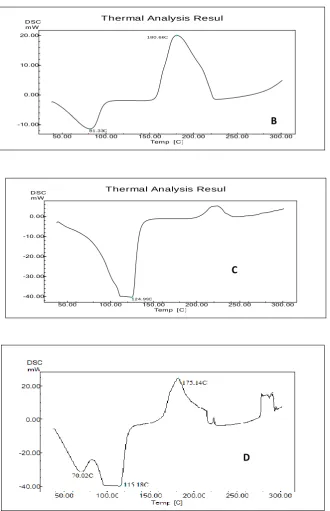
Differential Scanning Calorimetry
DSC performed to determine molecular state of the drug and to elucidate the physical nature
of drug in formulation. The DSC curve of Curcumin, Poloxamer 188, Gold nano particle and
Curcumin gold nano suspension (CGNS) and gold nano particle were carried out. Curcumin
exhibit endothermic peak at its melting point at 180.66 ℃ indicates the crystalline nature of
the drug. curcumin gold nano suspension showed melting peak at 175.14 ℃ indicates the
absence of the crystalline nature of the drug. Reduction in height of the peak this changes
indicates as result of reduction of particle size. curcumin loaded gold nanoparticle
endothermic peak at 115.18 ℃. This indicates changes in nature of drug giving a more
amorphous type as the product this may increase rate of dissolution of CGNS.(Fig:6).
50.00 100.00 150.00 200.00 250.00 300.00 Temp [C ]
-10.00 0.00 10.00 20.00 mW DSC
81.33x100C
180.66x100C
Thermal Analysis Result
50.00 100.00 150.00 200.00 250.00 300.00
Temp [C ] -40.00
-30.00 -20.00 -10.00 0.00 mW DSC
124.99x100C
Thermal Analysis Result
FIGURE 6: DSC Thermogram of A) Poloxamer -188 B) Curcumin C) Gold Nano
Particle (D).
Transmission electron microscopy
TEM image of curcumin gold nano suspension indicate that curcumin particle exhibit
spherical shape with a diameter of about 322.6 nm. It shows uniform dispersion of particles.
The results suggested that curcumin nano particles were well stabilized by precipitation
method.(Fig:7).
B
[image:11.595.132.463.69.581.2]Figure 7: TEM micrograph of optimized formulation batch.
In Vitro Drug Release
Most important feature of nano particle is the increase in dissolution of formulation with
decreasing in particle size. As decrease in particle size increase surface area for absorption
because of increase saturation solubility. In vitro drug release from the nano suspension were
carried out for 60 min. It was graphically represented as percentage drug release versus time
in minutes. The percentage of drug release was carried out of pure drug, physical mixture
formulation batch of CGNS. From study reveals that F3 batch shows faster release of drug as
compare to pure drug and physical mixture. Optimized formulation batch was enhanced
dissolution by 85% in 60min as compared to pure Curcumin showed 65%. By reduction of
particle size of formulation faster the action at target site of cancer in 60 min. As shown in
Graph 3.
CONCLUSION
Curcumin gold nano suspension was precipitated by solvent –anti solvent precipitation
method. Gold loaded curcumin target the cancer cells. The mean particle size of amporphous
CGNS was about 322.6nm with a uniform dispersion of particles.Smaller particle size and
large surface area of nano particle enhances dissolution rate of CGNS. DSC therm of the
CGNS indicates amorphous nature of the nanoparticle. This study demostrated the usefulness
of the precipitation technique to enhance dissolution of drugs.
REFERENCES
1. Rossi RE, Pericleous M, Mandair D, Whyand T, Caplin ME, The role of dietary factor in
prevention and progression in breast cancer, Anticancer Res, 2014; 34(12): 6861-6875.
2. Raikhlin A, Curpen, B, Warner, E, Betel C, Wright B, Jong, R., Breast MRI as an adjunct
mammography for breast cancer screening in high – Risk Patients: Retrospective review,
AJR, 2014: 204: 889-897.
3. Lopes CM, Dourado A, Oliveira R, Phytotherapy and nutritional supplements on breast
cancer, Biomed Res Int, 2017; 1-43.
4. Saqui J et al, Classification of CAM use and its correlates in patients with early stage
breast cancer, Integr cancer Ther, 2011; 10(2): 138-147.
5. Day ES, Morton JG, West JL, Nanoparticles for thermal cancer therapy. J Biomech Eng,
2009; 31(7): 074001-05.
6. O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL, Photo-thermal tumor ablation in
mice using near infrared-absorbing nanoparticles. Cancer Letter, 2004; 209: 171–176.
7. Krup A, Prakash LH, Harini A, Pharmacological activities of turmeric (curcuma longa
Linn): a review, J home ayur med, 2013; 2: 133-136.
8. Liu D, Chen Z, The effect of curcumin on breast cancer cells, J breast cancer, 2013;
16(2): 133-137.
9. Kunnumakkara AB, Anand P, Aggarwal BB, Curcumin inhibits proliferation, invasion,
angiogenesis and metastasis of different cancers through interaction with multiple cell
signalling proteins, Cancer letters, 2008; 269(2): 199-325.
10.Zhan Y, Chen RL, Zhang Y, Potentiation of paclitaxel activity by curcumin in human
breast cancer cell by modulating apoptosis and inhibiting EGFR signalling, Archi Pharma
11.Ferreira, LC., Arbab, AS., Jardim-Perassi, BV., Effect of curcumin on pro-angiogenic
factors in the xenograft model of breast cancer, Anti-cancer agents Med Chem, 2015;
15(10): 1285-1296.
12.Chiiu TL, Su CC, Curcumin inhibits proliferation and migration by increasing the Bax to
Bcl2 ration and decrease NF-kappa Bp65 expression in breast cancer MDA-MB-231
cells, International Journal of Molecule Medicine, 2009; 23(4): 469-475.
13.Fan H, Liang Y, Jiang, Curcumin inhibits intracellular fatty acid synthase and induces
apoptosis in human breast cancer MDA-MB-231 cells, Oncology reports, 2016; 35(5):
2651-2656.
14.Prabhakar C, Krishna KB, A review on nanosuspensions in drug delivery, Int J Pharma
Biosci, 2011; 2(1): 549-558.
15.Rabinow BE, Nano suspension in drug delivery, Nature Review Drug discover, 2004;
9(3): 785-796.
16.Patravale VB, Date A, Kulkarni RM, Nanosuspensions: a promising drug delivery
strategy, J Pharma Pharmaco, 2004; 4(2): 342-349.
17.Mohanty, S., Role of nanoparticle in drug delivery system.Int J Res Pharma Biomed Sci,
2010; 1(2): 41-66.
18.Ch, P, A review on nanosuspension in drug delivery, Int J Pharma Biosci, 2011; 2:
549-558.
19.Michal EM., Margaret AH, Keith PJ, Robert OW, Drug nano particle by anti solvent
precipitation mixing enery.Langmuir, 2006; 22: 8951-8959.
20.Mirza AZ, Shamshad H, Preparation and characterization of doxorubicin functionalized
gold nanoparticle, Eur J Med .Chem, 2011; 46: 1857-1860.
21.Liu D, Fabrication of carvediol nano suspension through the solvent precipitation-
ultrasonication method for the improvement of dissolution rat rate and oral
bioavailability, AAPS Pharma Sci Tech, 2012; 13(1): 295-304.
22.Shaker MA, Shaaban MI, Formulation of carbapenam loaded gold nanoparticle to combat
multi antibiotic bacterial resistance: In vitro antibacterial study, Int J Pharma, 2017:
71-84.
23.Detroja C, Chavhan S, Sawant K, Enhanced antihypertensive activity of candesartan
cilexetil nanosuspension: Formulation, characterization and pharmacodynamic study, Sci
24.Paun JS, Tank HM, Screening of formulating and processing parameters of candesartan
cilexetil nano suspension prepared by nano precipitation-Ultrasonication technique, Int J
PharmaRes, 2016; 8(4): 8-13.
25.Papadiwal A, Sagar K, Pande V, Formulation and characterization of nateglinide
nanosuspension by precipitation method, Int J Pharma Sci Nanotech, 2014; 7(4):