Journal of Biological Sciences and Medicine
Journal home page: www.jbscim.com∗ Corresponding author
Email address: prabha0502@rediffmail.com (Dr. Chandra Prabha)
28
Review Article
Open Access
Photodynamic Therapy in Periodontics: A Review
Chandra Prabha1,* and Surendra Man Shrestha2
1
Sardar Patel Post Graduate Institute of Dental and Medical Sciences, Lucknow, India
2
Department of Periodontics and Oral Implantology, People’s Dental College and Hospital, Tribhuvan University, Kathmandu, Nepal
Received 20 December 2015; Accepted 28 December 2015; Available online 31 December 2015
Abstract
The use of photodynamic therapy has been encouraged as one of the substitutes to antimicrobial agents for suppressing subgingival species and for treatment of periodontitis. Bacteria loaded with dense biofilms such as those encountered in dental plaque or peri implant surfaces are becoming resistant due to the injudicious use of antibiotics, recently. The present review elucidates the evolution and use of photodynamic therapy with the emphasis on application of photosensitizing dyes and their excitation by visible lights that enables effective killing of periodontopathogens.
Keywords: Microbial resistance; Photodynamic therapy; Photosensitizers; Antibiotics
Introduction
The presence of bacterial species, particularly the periodontopathogenic species on the tooth or root surface is major cause of gingivitis or periodontitis (Kolenbrander 2000; Page et al. 1997; Chen 2001). It is well accepted that mechanical removal of dental biofilms is basis of any adjunctive periodontal therapy as microbial biofilms in the oral cavity involved in etiology of various oral conditions including caries, periodontal and endodontic disease, oral malodour, denture stomatitis, candidiasis and dental implant failures (Singh et al. 2014; Cortelli et al. 2014). In general, it is recognised that the growth of bacteria in biofilms imparts a substantial decrease in susceptibility to antimicrobial agents compared with culture growth in suspension (Soukos and Goodson 2011; Silva et al. 2012; Bjarnsholt et al. 2013; Singh et al. 2014). Therefore, dental plaque, a naturally occurring biofilm (Marsh 2005), display increased resistance to antimicrobial agents (Anderson and Toole 2008; Fux et al. 2005). Currently,
various treatment modalities with least possible side effects have been increasingly searched. Local and systemic administration of antibiotics may lead to resistance, gastrointestinal and other disorders besides patient compliance which is often an added problem (Pallasch 2003; Quirynen et al. 2003; Rodrigues et al. 2004).
29
History
The use of photodynamic therapy for inactivating microorganisms was first demonstrated more than 100 years ago, in 1900 when Oscar Rabb (Rabb 1900) reported the lethal effects of acridine hydrochloride and visible light on
Paramecia caudatum. PDT was introduced
in medical therapy in 1904, as the light induced inactivation of cells, microorganisms or molecules (Von Tappeiner and Jodlbauer 1904). In 1913, Friedrich Meyer Betz, the German physician performed the pioneering study which was at first called Photo-radiation Therapy with porphyrins. He tested the effect of hematoporphyrins on his own skin (Moan and Peng 2003). It was John Toth who acknowledged the photodynamic chemical effect of therapy with early clinical argon due lasers and wrote the first white paper renaming the therapy as photodynamic therapy. Thomas Dougherty formed international photodynamic association in 1986. PDT was first approved by drug and food administration in 1999 to treat precancerous skin lesions on face and scalp (Babilas et al. 2005). The photodynamic therapy in curing human infections is based on the conception that an agent which absorbs light known as a photosensitizer, is preferentially taken up by bacteria and subsequently photon of light; a molecule of the photosensitizer is activated by light of the appropriate wavelength in the presence of oxygen to generate oxygen and free radicals that are cytotoxic to microorganisms (De Melo et al. 2013). Because of the primitive molecular nature of singlet oxygen, it is unlikely that microorganisms would develop resistance to the cytotoxic action (Soukos and Goodson 2011). Photodynamic therapy has emerged as an alternative to antimicrobial regimes and mechanical means in eliminating dental plaque of species owing to the ground-breaking work of Professor Michael Wilson
and colleagues (Wilson 1993) at the Eastman Dental Institute, University College London, UK.
Principles
behind
the
photo
dynamic therapy
PDT is based on the principle that a photoactivable substance (photosensitiser) binds to the target cell can be activated by light of suitable wavelength. During this process, free radicals are formed (among them singlet oxygen) which then produce an effect that is toxic to the cells. For bactericidal effect on the cells, the respective photosensitiser needs to have selectivity for prokaryotic cells. Some authors have reported the possibility of lethal photosensitisation of bacteria in vivo
and in vitro (Martinetto et al. 1986; Wilson 1993; De Simone 1999; Bertoloni et al. 1992).
By irradiation with light in visible spectrum, the dye (photosensitizer) is excited to its triplet state, the energy of which is transferred to molecular oxygen. The product formed is highly reactive singlet oxygen capable of reacting with biological systems and destroying them, while only the first excited state with energy of 94 kj/mol (22kcal/mol) above the ground state is important, the second excited state does not react. The bactericidal effect of photo- dynamic therapy can be explained by two potential, but different, mechanisms- one is DNA damage and the other is the damage caused to the cytoplasmic membrane of the bacteria by cytotoxicity (Bertoloni et al. 1992).
30
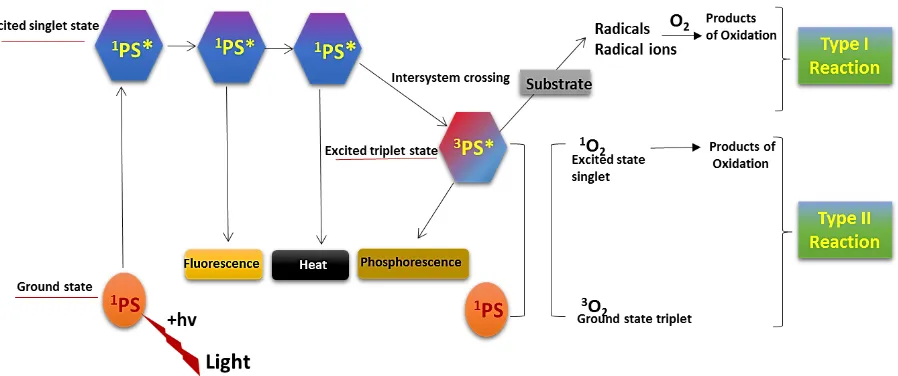
photosensitizer at ground state are activated to highly energized triplet state. The extended life of triplet state enables the interaction of excited photosensitiser that leads to generation of cytotoxic species produced during PDT. Two different pathways are followed by the triplet state photosensitizer involving Type I and Type II reactions-
Type I reactions
Type I reaction involves the hydrogen atom abstraction or electron transfer reaction between the excited state of photosensitizer and an organic substrate molecule of the cells which produces free radical and radical ions. These free radical species are generally highly reactive and interact with endogenous molecular oxygen to produce highly reactive oxygen species such as superoxide, hydroxyl radicals and hydrogen peroxide that harm cell membrane integrity causing irreparable biological damage (Rajesh et al. 2011; Khurana et al. 2014).
Type II reaction
In type II reaction, the triplet state photosensitizer reacts with oxygen to produce an electronically excited singlet oxygen which can interact with a large number of biological substrate as a result of its high chemical reactivity inducing oxidative damage and damaging the cell membrane and cell wall (Foote et al.1991; Sharman et al. 1999). Microorganisms that are killed by singlet oxygen includes viruses, bacteria, protozoa and fungi. Sites of initial cell damage from PDT are closely related to the localization of photosensitizer. Thus, the reaction takes place in limited space, leading to a localized response and making it ideal for application at localized sites without affecting distant molecules, cells or organs (Moan et al. 1991; Peng et al. 1996).
A schematic diagram of mechanisms involved during Type I and Type II reactions in photodynamic therapy is presented in Fig 1.
Photosensitizers
More than 400 compounds are known with photosensitizing properties including dyes, drugs, cosmetics, chemicals and many natural substances (Santamaria et al. 1972). Most of the sensitizers used for medical purposes belong to the following basic structures:
1. Trycyclic dyes with different meso atoms, acridine orange, proflavine, riboflavin, methylene blue, fluorescein, eosin, erythrosine, rose Bengal.
2. Tetrapyrroles, porphyrins and derivatives, chlorophyll, phylloerythrin, phthalocynins. 3. Furocoumarins, psoralen and its
methoxy derivatives- xanthotoxin, bergaptene.
Based on the advantages and characteristics of anti-microbial photodynamic therapy, it has been anticipated that periodontal and peri-implant diseases are potential targets of this novel antimicrobial photo-chemotherapy. Antimicrobial photodynamic therapy is expected to resolve the difficulties and problems of conventional antimicrobial therapy and can work as an adjunctive to conventional mechanical treatments.
31
Fig 1. The schematic representation of Type I and Type II reactions in photodynamic therapy (Adapted from Khurana et al. 2014).
After exposure to light, the activated photosensitizer during excited triplet state, tracks either of the two pathways. Type I pathway includes electron- transfer reactions from the photosensitizer triplet state by the participation of a substrate to produce radical ions which reacts with oxygen to produce cytotoxic species. Type II pathway includes energy transfer from the photosensitizer triplet state to the ground state molecular oxygen (triplet) to produce excited state singlet oxygen, which oxidizes biological molecules.
hv= photon energy; PS= photosensitizer.
technical simplicity of the method and the high effectiveness of bacterial killing, the use of antimicrobial photodynamic therapy in the treatment of periodontal and peri-implant diseases has extensively studied. The bactericidal effect of antimicrobial photo-dynamic therapy on periodontal pathogens has been demonstrated in several basic studies. In the early 1990s, Dobson & Wilson showed that low-level helium–neon laser irradiation with toluidine blue O or methylene blue was effective for killing P.
gingivalis, F. nucleatum, A.
actinomycetemcomitans and S. sanguinis. In comparison with other photosensitizers, toluidine blue O and methylene photosensitizers, toluidine blue O and methylene blue prove to be more effective for killing periodontal pathogens in antimicrobial photodynamic therapy (Wilson et al.1993). These authors also revealed that the most effective bactericidal effect was achieved with the combination of toluidine blue O and a helium–neon laser in a supragingival biofilm model study (Wilson M Dobson et al.1995). Bhatti et al. 2002 demonstrated that the optimal
concentration of toluidine blue O to kill P. gingivalis was 12.5 lg⁄ ml with helium–
neon laser irradiation. In addition, they revealed, by transmission electron microscopic examination, that the bactericidal effect of light-activated toluidine blue O against P. gingivalis was caused by disruption of the outer membrane proteins of the bacteria. Additionally, it has been indicated that in the presence of methylene blue, the wavelength of 632.8 nm (helium neon) laser and 665 and 830 nm (diode) have a high bactericidal effect on periodontal pathogens (Chan and Lai 2002).
32
against subgingival plaque biofilm that comprised both gram positive and gram negative bacteria. They demonstrated that the bacteria present in deep layers of biofilm were killed by extensive penetration of the photosensitizer into the biofilm following antimicrobial photodynamic therapy. In black pigmented bacteria, the endogenous porphyrins present on bacteria may also act as photosensitizer. It seems that antimicrobial photodynamic therapy not only kills the bacteria but may also lead to the detoxification of endotoxins because it has been demonstrated that LPS stimulate the production of pro-inflammatory cytokines by mono nuclear cells (Kennedy et al. 1990). Consequently, PDT may possibly inactivate endotoxins such as lipopolysaccharides by decreasing their biological activity.
Use of antimicrobial PDT in
treatment of peri-implant disease
It has been proven by the treatment of peri-implantitis that complete eradication of causative bacteria is responsible for development of periodontal disease and disinfection or detoxification of peri implant pockets are essential to achieve effective healing with regeneration of lost bone around the affected implants (Mombelli. 1992, 1987). In one of the examples of in vitro study carried out by Hass et al (1997), the efficacy of antimicrobial PDT was examined in killing bacteria associated with Peri-implantitis such as A. actinomycetemcomitans, P. gingivalis or Provetella intermedia which adhered to titanium plates with different surface characterstics. Plates were incubated with those bacteria and subjected to four different treatments - (i) PDT (toluidine blue O + diode laser) (ii) no treatment (iii) laser light alone (iv) toluidine blue O alone. None of the plates subjected to PDT showed bacterial growth of any of the micro-organisms. While in the other treatment groups, all the three species of
bacteria were detected after treatment. The scanning electron microscopic analysis revealed that antimicrobial PDT led to bacterial cell destruction without damage to the titanium surface.
Adverse effects
PDT has a potential of phototoxic or photoallergic unwanted side effects (Kubler et al. 2002). There can be impairment of benign oral flora which may lead to the overgrowth of single resistant species (Roberts et al. 2002). In order to avoid phototoxic reactions, it is more important to stain selectively the target leaving out the gingival mucosa or tongue. Burning sensation stinging or prickling is common complain experienced during PDT (Lui et al. 1993; Kalker et al 2002). It usually occurs in early part of light exposure. A clinically obvious scar is rarely observed. The histological evidence of scarring is evident (Fink et al 1998). Hyperpigmentation or hypopigmentation can occasionally be seen in treated areas and resolves within six months.
Conclusion
33
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank the Professors of the People’s Dental College, Tribhuvan University for providing necessary assistance and guidance.
References
Anderson GG, O Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Micbiol Immuno l322: 85-105
Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, Van Lier JE (1992) Photosensitizing Activity of water and lipid soluble
pthalocyanins on prokaryotic and
eukaryotic microbial cells .Microbios 71:33-46
Babilas P, Karrer S, Sidoroff A, Landthaler M,
Szeimies RM (2005) Photodynamic
therapy in dermatology--an update.
Photodermatol Photoimmunol Photomed 21(3):142-149
Bhatti M, Mac Robert A, Meghji S, Henderson B, Wilson M (1997) Effect of Dosimetric and physiological factors on the lethal
photosensitization of Porphyromonas
gingivalis in vitro. Photochem Photobiol
65: 1026-1031
Bhatti M, MacRobert A, Henderson B, Wilson
M (2002) Exposure of Porphyromonas
gingivalis to red light activated
antimicrobial agent toluidine blue
decreases membrane flexidity. Curr
Microbiol 45: 118-122
Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N (2013) Applying insights from biofilm biology to drug development — can a new approach be developed? Nature Reviews Drug Discovery 12: 791–808. doi: 10.1038/nrd4000
Chan Y, Lai CH (2003) Bactericidal effects of
different laser wavelengths on
periodontopathic germs in photodynamic therapy. Lasers Med Sci 18: 51-55
Chen C (2001) Periodontics as a biofilm infection. J Calif Dent Assoc 29: 362-369
Cortelli SC, Costa FO, Rode SM, Haas AN, Andrade AK, Pannuti CM, Escobar EC, Almeida ER, Cortelli JR, Pedrazzi V (2014) Mouth rinse recommendation for prosthodontic patients. Braz Oral Res 28: Spec No. pii: S1806-83242014000200201.
doi:
10.1590/1807-3107BOR-2014.vol28.0020.
De Melo WC, Avci P, de Oliveira MN, Gupta A, Vecchio D, Sadasivam M, Hamblin MR (2013) Photodynamic inactivation of biofilm: taking a lightly coloured approach to stubborn infection. Expert Rev
Anti-Infective Therapy 11(7): 669–693.
http://doi.org/10.1586/14787210.2013.811 861
De Simone NA, Christiensen C, Dore D (1999) Bactericidal effect of 0.95 mW helium
Neon and 5mW indium Gallium
Aluminium Phosphate laser irradiation at exposure times of 30, 60 and 120 seconds
on photosensitized Staphylococcus aureus
and Pseudomonas aeruginosa in vitro.
Phys Ther 79: 839-846
Fink –Punches R, Soyer HP, Hofer A, Kerl H, Wolf P (1998) Long term follow up and histological changes of superficial non-melanoma skin cancers treated with topical delta –aminolevulinic acid Photodynamic Therapy. Arch Dermatol 134: 821-826 Foote CS (1991) Definition of type I and type
II photosynthesized oxidation. Photochem Photobiol 54: 659
Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infection biofilms. Trends microbial 13: 34-40 Haas R, Dortbudok O, Mendsdorff-poully N,
Mailath G (1997) Elimination of bacteria on different implant surfaces through
photosensitization and soft laser. An in
vitro study. Clin Oral Implants Res 8:
249-254
Kalka K, Merk H, Mukhtar H (2000) Photodynamic therapy in dermatology. J Am Acad Dermatol 42: 389-413
Kennedy JC, Potteir RH, Poole S (1990) photodynamic Therapy with endogenous protoporphyrins IX: basic principles and present clinical experience. J Photochem Photobiol B 6: 143-148
34
Kolenbrander PE (2000) Oral microbial communities: biofilms, interaction and genetic systems. Ann Rev Microbial 54: 413-437
Kreisler M, Christoffers AB, Al Haj H, Willershausen B, d’Hoedtr B (2002) Low
level 809 nm diode laser induced in vitro
stimulation of proliferation of human gingival fibroblasts. Laser Surg Med 30: 365-369
Kubler A. Haase T, Rheinwald M, Barth T, Muhling J (2002) Treatment of oral leukoplakia by topical application of 5-aminolevulinic acid. Int J Oral Maxillofac implants 73:1292-1298
Lui H, Anderson RR (1993) Photodynamic
therapy in dermatology: Recent
Developments. Dermatol Clin 11:1-13 Marsh PD (2005) Dental Plaque biological
significance of biofilm and community lifestyle. J Clin Periodontal 32 (suppl 6): 7-15
Martinetto P, Gariglio M, Lombard GF, Fiscella B, Boggio F (1986) Bactericidal effects induced by laser irradiation and haematoporphyrin against gram-positive and gram-negative microorganisms. Drugs Exp Clin Res 12 (4): 335-342
Moan J, Peng Q (2003) An outline of the history of PDT in Thierry practice. Photodynamic
Therapy, comprehensive series in
photochemistry and photobiology 2.The Royal society of Chemistry pp 1-18 Moan J, Berg K (1991) The photodegradation
of porphyrins in cells can be used to estimate the lifetime of the single oxygen. Photochem Photobiol 53: 549-553
Mohr H, Lambrecht B, Schmitt H (1993)
Photoinactivation of Viurses in
therapeutical plasma. Dev Biol Stand 81:177-183
Mombelli A, Lang NP (1992) Antimicrobial treatment of peri-implant infection. Clin Oral Implants Res 3: 162-168
Mombelli A, van Oostern MA, Schurch EJ, Land NP (1987) The microbiota associated with successful or failing osseo integrated titanium implants. Oral Microbial Immunol 2: 145-151
Page RC, Offenbacher S, Schroeder HE,
Seymour GJ, Komman KS (1997)
Advances in the pathogenesis of
periodontitis: summary of Developments, clinical implications and future directions. Perio 2000 14: 216-248
Pallasch TJ (2003) Antibiotic resistance. Dent Clin North Am 47: 623-639
Peng Q Moan J, Nesland JM (1996) Correlation of subcellular and intra tumoral photosensitizer localization with ultrastructural features after Photodynamic Therapy. Ultrastruct Pathol 20: 109-129 Pfitzner A, Siguch BW, Albrecht V,
Glockmann E (2002) Killing of
periodontopathogenic bacteria by
Photodynamic Therapy. J Clin Periodontal 29: 437-445
Quirynen M, Teughels W, van Steenberghe D (2003) Microbial shifts after subgingival debridement and formation of bacterial resistance when combined with local or systemic antimicrobials. Oral Dis 9: 30-37 Rabb O (1900) The effect of fluorescent agents
on infusoria (in German). Z Biol 39: 524-526
Rajesh S, Koshi E, Philip K, Mohan A (2011) Antimicrobial photodynamic therapy: An overview. Journal of Indian Society of
Periodontology, 15(4), 323–327.
http://doi.org/10.4103/0972-124X.92563 Rodrigues RM, Gonclaves C, Souto R, Feres–
Filho EJ, Uzeda M, Colombo AP (2004) Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol 31: 420-427
Sharman WM Allen CM Van Lier JE (1999)
Photodynamics Therapeutics. Basic
principles and clinical applications. Drug Discov Today 4: 507-517
Santamaria L, Prino G (1972) List of
Photodynamic substances. Res Progr Org Biol Med Chem 3: 11-35
Sarkar S, Wilson M (1993) Lethal
photosensitization of bacteria in
subgingival plaque from patients with chronic periodontitis. J Periodontal Res 2 (28): 204-210
Silva MSP, Brandão DO, Chaves TP, Formiga Filho ALN, Costa EMM, de B, Santos VL,
Medeiros ACD (2012) Study Bio
prospecting of Medicinal Plant Extracts of the Semiarid Northeast: Contribution to the
Control of Oral Microorganisms.
Evidence-Based Complementary and
Alternative Medicine : eCAM, 2012, 681207.
http://doi.org/10.1155/2012/681207 Singh H, Khurana H, Singh H, Singh M (2014)
35
between a drug and a light. Muller J Med Sci Res 5: 48-55
Soukos NS, Goodson JM (2011) Photodynamic therapy in the control of oral biofilms. Periodontol 2000 55 (1): 143-166
Soukos NS, Mulholland SE, Socransky SS, Doukas AG (2003) Photo destruction of human dental plaque bacteria enhancement
of photodynamic effect by
photomechanical waves in an oral biofilm model. Laser Surg Med 33: 161-168 Von Tappeiner H, Jodlbauer A (1904) On the
effect of Photodynamic (fluorescent
Substance) on protozoa and enzymes. Deutch Arch Klin Medizin 39: 427-487 Wilson M (1993) Photolysis of oral bacteria
and its potential use in the treatment of caries and periodontal disease. J Appl Bacteriol 75: 299-306
Wilson M, Pratten J (1995) Lethal
photosensitisation of Staphylococcus
aureusin vitro: effect of growth phase,
serum and preirradiation time. Laser Surg Med 16: 272-276
Wilson M, Burns T, Pratters J, Pearson GJ (1993) Bacteria in supragingival Plaque samples can be killed by low power laser. Oral Microbiol Immunol 8: 182-187 Wilson M, Dobson J, Sarkar S (1995)
Sensitisation of Periodontopathogenic
bacteria to killing by light from a Low
Power laser light in presence of