R E V I E W
Open Access
H. pylori
infection and extra-gastroduodenal
diseases
Feng-Woei Tsay
1,2and Ping-I Hsu
1*Abstract
Helicobacter pylori
infection is the principal cause of peptic ulcer disease, gastric adenocarcinoma and gastric
mucosa-associated lymphoid tissue lymphoma. Recent studies have shown that it may interfere with many
biological processes and determine or influence the occurrence of many diseases outside the stomach. Currently,
the role of
H. pylori
in idiopathic thrombocytopenic purpura and iron deficiency anemia is well documented.
Emerging evidence suggests that it may also contribute to vitamin B12 deficiency, insulin resistance, metabolic
syndrome, diabetes mellitus and non-alcoholic liver disease. Additionally, it may increase the risk of acute coronary
syndrome, cerebrovascular disease, neurodegenerative disease and other miscellaneous disorders. Different
pathogenic mechanisms have been hypothesized, including the occurrence of molecular mimicry and the
induction of a low-grade inflammation. This review summarizes the results of the most relevant studies on the
extra-gastroduodenal manifestations of
H. pylori
infection.
Keywords:
Helicobacter pylori
, Iron deficiency anemia, Idiopathic thrombocytopenic purpura and vitamin B12
deficiency
Background
Helicobacter pylori
infection is the principal cause of
chronic gastritis, gastric ulcer, duodenal ulcer, gastric
adenocarcinoma and gastric mucosa-associated lymphoid
tissue lymphoma [
1
,
2
]. In recent decades, many articles
have published on the fascinating topic of
extragastroduo-denal manifestations of
H. pylori
infection, including
hematological, metabolic, cardiovascular,
neurodegenera-tive and allergic disorders [
3–13
]. Different pathogenic
mechanisms have been hypothesized, including the
occur-rence of molecular mimicry and the induction of a
low-grade inflammation. Indeed,
H. pylori
infection is a
very good model for studying host-bacterial interactions
and very attractive for those interested in the role of gut
microbiota in health and diseases. Here, we summarize
the results of the most relevant studies on the
extragastro-duodenal manifestations of
H. pylori
infection.
Iron deficiency anemia
The link between Iron deficiency anemia (IDA) and
H.
pylori
infection was reported firstly in 1991 by Blecker et
al., who cured IDA of a 15 year-old female presenting
with anemia-related syncope and
H. pylori
-induced
chronic active hemorrhagic gastritis by eradication
therapy without iron supplements [
14
]. The association
of
H. pylori
infection with unexplained IDA has been
proven in adult and pediatric populations [
15
,
16
] though
a few investigations didn
’
t show this link [
17
,
18
].
Recently, Qu et al. conducted a meta-analysis of 15
case-control studies to investigate the relation between
H.
pylori
infection and IDA [
19
].
H. pylori
infection was
diagnosed by endoscopy and histological examination in
five studies, in which patients with peptic ulcer disease
and gastric cancer were not included. The other 10 studies
confirmed
H. pylori
infection by serology or urea breath
test. The data showed an increased risk of IDA in patients
with
H. pylori
infection with an odds ratio (OR) of 2.2
(95% confidence interval [CI]:1.5
–
3.2) [
19
]. Several works
also demonstrated recovery from IDA by successful
eradication of
H. pylori
without iron supplements [
20
].
Yuan et al. performed a meta-analysis of 16 randomized
controlled trials involving 956 patients to assess the
* Correspondence:williamhsup@yahoo.com.tw
1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kaohsiung Veterans General Hospital and National Yang-Ming University, 386 Ta Chung 1st Road, Kaohsiung 813, Taiwan, Republic of China Full list of author information is available at the end of the article
impact of
H. pylori
eradication therapy on IDA [
21
]. In
this work, the diagnosis of
H. pylori
infection was based
on rapid urease test or histology in eight studies, in which
patients with peptic ulcer disease were excluded. The
other eight studies confirmed
H. pylori
infection by urea
breath test. The follow-up time in these studies ranged
from 1 to 3 months. The difference from baseline to
end-point of hemoglobin, serum iron, and serum ferritin in the
meta-analysis was statistically significantly different
be-tween anti-
H. pylori
treatment plus oral iron and oral iron
alone (differences: Hb, 1.48 g/dL; serum iron: 1.15 mol/L;
serum ferritin, 1.84 ng/mL) [
21
].
H. pylori
causes IDA by several mechanisms. First,
in-creased iron loss can be due to hemorrhagic gastritis,
peptic ulcer disease and gastric adenocarcinoma [
22
].
Second, CagA protein of
H. pylori
has been shown to
participate in iron acquisition from interstitial
holotrans-ferrin [
23
]. Iron uptake by
H. pylori
is enhanced during
the growth of the bacteria [
24
]. Third,
H. pylori
-related
corporal gastritis may decrease acid secretion due to
gland atrophy and results in the reduction of iron
absorption from diet [
25
].
In summary, the association of
H. pylori
and IDA has
been conclusively proven in numerous studies. Current
international and national guidelines recommend
eradi-cation of
H. pylori
infection in patients with unexplained
IDA [
26
,
27
].
Immune thrombocytopenic purpura
Gasbarrini et al. reported the first case of
H. pylori
infec-tion associated with immune thrombocytopenic purpura
(ITP) in 1998 [
28
]. An observation study from Japan also
found a good platelet response in ITP patients treated
by
H. pylori
eradication [
29
]. A randomized controlled
trial by Brito et al. revealed that
H. pylori
eradication
resulted in a significant platelet response in children and
adolescents affected by ITP [
30
]. The role of
H. pylori
infection in ITP has also been confirmed by several
other studies [
31
,
32
]. Nonetheless, some studies from
countries with low prevalence of infection, like France
and the United States, did not find the link between
H.
pylori
infection and ITP [
33
,
34
]. Recently, Stasi et al.
conducted a meta-analysis of 25 studies to investigate
the impact of anti-
H. pylori
therapy on ITP [
34
]. The
assessing time for platelet response ranged from one to
six months. The data showed that the rates of complete
response (platelet count
≧
100 × 10
9/L) and overall
re-sponse (platelet count
≧
30 × 10
9/L and at least doubling
of the basal count) after successful eradication of
H.
pylori
were 42.7 and 50.3%, respectively [
35
]. The
predictors of a good response to eradication therapy
were countries with higher prevalence of
H. pylori
infec-tion (such as Japan and Italy) and patients with milder
degree of thrombocytopenia [
35
]. In the majority of ITP
patients responding to anti-
H. pylori
therapy, the
dur-ability of platelet response is more than 7 years,
indicat-ing the disease is cured [
36
]. Another meta-analysis by
Arnold et al. performed a meta-analysis to determine
the effect of
H. pylori
eradication therapy in patients
with ITP by comparing the platelet response in ITP
patients with and without
H. pylori
infection [
37
]. The
odds of achieving a platelet count response following
eradication therapy were 14.5 higher (95% CI: 4.2 to
83.0) in patients with
H. pylori
infection than in those
without infection (response rate: 51.2% vs. 8.8%). These
findings strengthen the causal association between
H.
pylori
infection and ITP. Several mechanisms regarding
H. pylori
-associated ITP have been proposed [
38
]. One
intriguing hypothesis concerning molecular mimicry is
that cross-reactive antibodies are produced that react
both
H. pylori
components and platelet surface antigens.
Takahashi et al. showed that platelet elutes from
H.
pylori
-infected ITP patients recognized CagA protein in
immunoblots, but those from
H. pylori
-infected non-ITP
patients did not [
39
]. Bai et al. also reported that
mono-clonal antibodies generated against
H. pylori
urease B
react with GP IIb/IIIa expressed on the platelet surface
[
40
]. While these findings suggest molecular mimicry
be-tween
H. pylori
components and platelet surface antigens,
the exact pathogenic roles of these cross-reactive
anti-bodies remain obscure. In another potential mechanism,
H. pylori
infection may alter Fcγ
receptor balance of
mon-cytes/macrophages and induce autoantibody formation. A
recent study showed that the FcγR II B expression on
cir-culating monocytes was down-regulated in
H. pylori
-in-fected ITP patients [
41
]. Therefore,
H. pylori
may alter
Fcγ
receptor balance of moncytes/macrophages through
downregulation of the inhibitory receptor FcγR II B.
In conclusion, many studies support the association
between
H. pylori
infection and ITP. Current
inter-national and inter-national guidelines recommend that
H.
pylori
infection should be sought and treated in patients
with ITP [
27
].
Vitamin B12 deficiency
The link between vitamin B12 deficiency and
H. pylori
infection was reported firstly in 1984 by O
’
Connor et al.
who showed Campylobacter-like organisms in patients
with type A gastritis and pernicious anemia [
42
]. Studies
have demonstrated a link between chronic
H. pylori
in-fection and malabsorption of vitamin B12 [
43
]. Sarari et
al. showed that vitamin B12 deficiency was present in
67.4% (29/43) of the patients with
H. pylori
infection
[
44
]. Shuval-Sudai et al. found a higher prevalence of
H.
status and measuring serum levels of vitamin B12. No
adequate interventional studies proving the effect of
anti-
H. pylori
therapy on vitamin B12 deficiency exist.
Metabolic syndrome and diabetes mellitus (DM)
Many epidemiological studies have supported a link
between insulin resistance, metabolic syndrome and
H.
pylori
infection [
46
,
47
]. Chen et al. demonstrated that
H. pylori
-infected subjects had a higher prevalence of
metabolic syndrome than those without
H. pylori
infec-tion [
48
]. Additionally, Yang et al. showed a significant
association between
H. pylori
infection and DM [
49
].
Similar results were also observed by other investigators
[
50
]. Furthermore, Horikawa et al. revealed that
H. pylori
infection worsened glycemia control in diabetic patients
[
51
]. Polyzos et al. conducted a systemic review
includ-ing nine studies and showed a trend toward a positive
association between
H. pylori
infection and insulin
resistance [
47
]. In contrast, several studies did not find
the link between
H. pylori
infection and insulin resistance
or metabolic syndrome [
52
]. Naja et al. showed no
associ-ation between
H. pylori
infection and metabolic syndrome
in a Lebanese population [
53
]. A meta-analysis of 18
stud-ies found no strong correlation between
H. pylori
infection
and serum concentrations of total cholesterol and
trigly-ceride [
54
]. Wada et al. also found that successful
eradica-tion of
H. pylori
could not improve glucose control of DM
in Japanese patients [
55
]. Furthermore, a recent
random-ized controlled trial involving 49
H. pylori
-infected
subjects in a prediabetes stage showed that
H. pylori
eradi-cation resulted in an increased Homeostatic model
assess-ment of insulin resistance (HOMA-IR) [
56
].
Several studies reported a reverse link between
H.
pylori
infection and obesity [
57–60
]. A case-control
study from Taiwan demonstrated an inverse relationship
between morbid obesity and
H. pylori
seropositivity [
57
].
An ecological study also showed an inverse correlation
between
H. pylori
prevalence and rate of overweight/
obesity in countries of the developed world [
58
].
However, a large case-control study including 8820
participants from China showed body mass index was
significantly and positively associated with
H. pylori
in-fection [
59
]. An intervention trial demonstrated serum
ghrelin concentrations were inversely related to the
severity of
H. pylori
-associated gastritis in prepubertal
children [
60
]. Eradication of
H. pylori
infection resulted
in a significant increase in body mass index along with a
significant decrease in circulating ghrelin levels and an
increase in leptin levels [
60
].
In summary, the issue of the association between
H.
pylori
infection and metabolic syndrome or DM remains
contradictory.
Nonalcoholic fatty liver disease (NAFLD)
A cohort study by Kim et al. demonstrated that the
sub-jects with
H. pylori
infection had a higher incidence of
NAFLD than those without infection (hazard ratio: 1.21
[95% CI: 1.1
–
1.3]) [
61
]. Polyzos et al. also revealed that
patients with NAFLD had higher anti-
H. pylori
IgG
titers, together with lower circulating adiponectin and
higher tumor necrosis factor-α
levels, compared to
non-NAFLD subjects [
62
]. However, opposite results
from Korea and Japan showed no association between
H. pylori
infection and NAFLD [
63
,
64
]. Recently, a
meta-analysis demonstrated a significantly increased risk
of NAFLD in patients with
H. pylori
infection [
65
].
Nonetheless, the mechanism underlying the association
between
H. pylori
infection and NAFLD remains unclear,
and interventional studies proving the effect of anti-
H.
pylori
therapy on NAFLD are fairly limited.
In summary, the association between
H. pylori
infec-tion and NAFLD remains contradictory.
Coronary artery disease (CAD)
Mendall et al. first showed a link between
H. pylori
and
CAD in 1994 [
66
]. Several studies reported that
CagA-postive strains of
H. pylori
were associated with
atherosclerosis [
67
–
69
]. Al-Ghamdi et al found that
H.
pylori
plays an important role in the development of
CAD by altering the lipid profile and enhancement of
chronic inflammation [
70
]. Figura et al. also revealed
that CagA-postive strains of
H. pylori
were associated
with high serum levels of interleukin-6 and B-type
natri-uretic peptide in patients with CAD [
71
]. A nationwide
retrospective cohort study demonstrated that
H. pylori
infection increased the risk of acute coronary syndrome
[
72
]. In addition, a meta-analysis of 26 studies involving
more than 20,000 patients also showed a significant
as-sociation between
H. pylori
infection and the risk of
myocardial infarction (OR: 2.10; 95% CI: 1.8
–
2.5) [
73
].
However some studies from Indian and German did not
find the association between
H. pylori
and CAD [
74
,
75
].
Additionally, there are still no interventional studies
proving the beneficial effect of
H. pylori
eradication in
decreasing the incidence of CAD.
There are several proposed mechanisms underlying
the association between
H. pylori
infection and CAD.
H.
In conclusion, there is controversial evidence linking
H. pylori
infection and CAD. No adequate interventional
trials demonstrating a lower incidence of CAD as a
result of anti-
H. pylori
therapy exit.
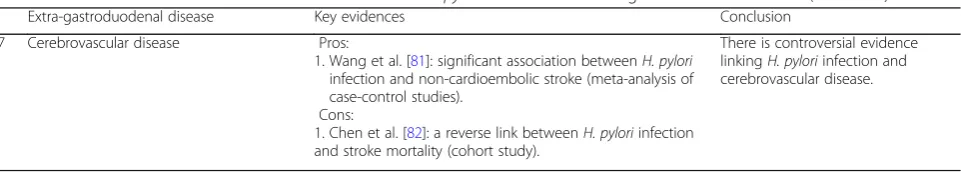
Cerebrovascular disease
Wincup et al. first reported a link between
H. pylori
infection and stroke in 1996 (OR = 1.57, 95% CI 0.95 to
2.60) [
79
]. A Mexican study found that levels of
antibodies to
H. pylori
predict incident stroke in fully
adjusted models (OR: 1.58; 95% CI: 1.1 to 2.3) [
80
].
Recently, Wang et al. performed a meta-analysis of 4041
Chinese patients, and found an association between
H.
pylori
infection and non-cardioembolic stroke [
81
].
However, a cohort study of 9895 cases from the United
States found a reverse link between
H. pylori
infection
and stroke mortality, and this reverse association was
stronger for
H. pylori
cagA positivity [
82
]. In summary,
there is controversial evidence linking
H. pylori
infection
and cerebrovascular disease.
Other miscellaneous disorders
Some studies also disclosed the relationship of
H. pylori
with dementia and Alzheimer
’
s disease (AD) [
83
,
84
]. A
study in Greece by Kountouras et al. found higher
prevalence of
H. pylori
infection in patients with AD
than in the control group [
85
]. Hung et al. designed a
study for the relationship between
H. pylori
infection
and non-Alzheimer
’
s dementia (non-AD) using a
nation-wide population-based dataset in Taiwan, and found that
patients with
H. pylori
infection were 1.6-fold more
likely to develop non-AD than those without infection
[
83
]. A retrospective cohort study using nationwide
database in Taiwan showed that eradication of
H. pylori
was associated with a decreased progression of dementia
as compared to no eradication of
H. pylori
in AD
pa-tients with peptic ulcers [
86
]. However, further
prospect-ive randomized control trials are needed to clarify these
findings.
The inverse relationship between
H. pylori
infection
and allergic asthma has been reported. A meta-analysis
by Zhou et al.
..
in 2013 found lower prevalence rate of
H. pylori
infection in patients with allergic asthma [
87
].
Higher prevalence rate of
H. pylori
infection has been
found in cirrhotic patients with hepatoencephalopathy
than in those without hepatoencephalopathy [
88
]. Jaing
et al also showed the association of
H. pylori
infection
with elevated blood ammonia levels in cirrhotic patients
Table 1
The relevant studies on the associations between
H. pylori
infection and extra-gastroduodenal diseases
Extra-gastroduodenal disease Key evidences Conclusion
1 Iron deficiency anemia (IDA) Pros:
1. Qu et al. [19]: an increased risk of IDA in patients with H. pyloriinfection (meta-analysis of case-control studies). 2. Yuan et al. [21]: Eradication ofH. pyloricould improve
the levels of hemoglobin and serum ferritin in patients with IDA (meta-analysis of intervention trials). Cons:
1. Sandstrom et al. [18]: no association betweenH. pylori infection and IDA in female adolescents (case-control study).
Eradication ofH. pyloriinfection is recommended for patients with unexplained IDA.
2 Immune thrombocytopenic purpura (ITP) Pros:
1. Stasi et al. [35]: The overall response rate of increased platelet count was 50.3% after successful eradication of H. pyloriin ITP patients (meta-analysis of intervention trials).
2. Arnold et al. [37]: The odds of achieving a platelet count response following eradication therapy were 14.5 higher in ITP patients withH. pyloriinfection than in those without infection (response rate: 51.2% vs. 8.8%) (meta-analysis of intervention trials).
Cons:
1. Michel et al. [34]: Seroprevalence ofH. pyloriin patients with ITP was not significantly different from that in control subjects (case-control study).
H. pyloriinfection should be sought and treated in patients with ITP.
3 Vitamin B12 deficiency Pros:
1. Sarari et al. [44]: There was significant association between the presence ofH. pyloriinfection and vitamin B12 deficiency (case-control study).
2. Shuval-Sudai et al. [45]: Prevalence ofH. pylori seropositivity was significantly higher among subjects with borderline (> 145–180 pg/mL) or low normal (> 180–250 pg/mL) vitamin B12 levels than among those with vitamin B12 > 250 pg/mL (case-control study).
H. pyloriinfection is associated with vitamin B12 deficiency.
4 Metabolic syndrome and diabetes mellitus (DM)
Pros:
1. Chen et al. [48]:H. pylori-infected subjects had a higher prevalence of metabolic syndrome than those without H. pyloriinfection (case-control study).
2. Yang et al. [49]:H. pyloriinfection was associated with risk of DM (case-control study).
Cons:
1. Naja et al. [53]: no association betweenH. pyloriinfection and metabolic syndrome (case-control study).
2. Wada et al. [55]: The eradication of Helicobacter pylori does not affect glycemic control in Japanese subjects with type 2 diabetes (intervention trial).
The association betweenH. pylori infection and metabolic syndrome or DM is contradictory.
5 Nonalcoholic fatty liver disease (NAFLD) Pros:
1. Kim et al. [61]: The subjects withH. pyloriinfection had a higher incidence of NAFLD than those without infection (cohort study).
2. Wijarnpreecha et al. [65]: a significantly increased risk of NAFLD in patients withH. pyloriinfection (meta-analysis of case-control studies).
Cons:
1. Okushin et al. [63]: no association betweenH. pylori infection and NAFLD (case-control study).
The association betweenH. pylori infection and NAFLD remains contradictory.
6 Coronary artery disease (CAD) Pros:
1. Yu et al. [73]: significant association betweenH. pylori infection and the risk of myocardial infarction (meta-analysis of case-control studies). Cons:
1. Schottker et al. [75]: no association betweenH. pylori infection and the risk of CAD (cohort study).
[
89
]. Several studies have also reported that
H. pylori
infection increases the risk of colon adenocarcinoma
and adenoma [
90–92
]. Recently, an association between
H. pylori
infection and chronic spontaneous urticaria
has been reported but remains controversial. Fukuda et
al. demonstrated a significant improvement of chronic
spontaneous urticaria by anti-
H. pylori
therapy in
Japa-nese patients [
93
]. This work was consistent with a
sys-temic review of 10 studies by Federman et al. [
94
].
However, Moreira et al. did not find the association
between
H. pylori
infection and chronic spontaneous
urticaria [
95
].
In summary, there are still controversial evidences
linking
H. pylori
infection and aforementioned
miscel-laneous disorders. Adequate interventional trials are
needed to clarify these associations.
Conclusions
Recent studies have shown that
H. pylori
may interfere
with many biological processes and determine or
influ-ence the occurrinflu-ence of many diseases outside the
stom-ach (Table
1
and Fig.
1
). Currently, its role in ITP and
IDA is well documented. Emerging evidence suggests
that it may also contribute to vitamin B12 deficiency,
in-sulin resistance, metabolic syndrome, diabetes mellitus
and non-alcoholic liver disease. Additionally, it may also
increase the risk of acute coronary syndrome,
cerebro-vascular disease, and neurodegenerative disease,
H.
pyl-ori
infection is a perfect model for the study of interplay
between human beings and bacteria. Further studies are
mandatory to clarify the pathogenesis of
extragastroduo-denal diseases induced by
H. pylori
infection.
Abbreviations
AD:Alzheimer’s disease; CI: Confidence interval; DM: Diabetes mellitus; IDA: Iron deficiency anemia; ITP: Immune thrombocytopenic purpura; NAFLD: Nonalcoholic fatty liver disease; OR: Odds ratio
Authors’contributions
Drs. PIH and FWT reviewed the articles and wrote the manuscript. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher
’
s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author details
1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kaohsiung Veterans General Hospital and National Yang-Ming University, 386 Ta Chung 1st Road, Kaohsiung 813, Taiwan, Republic of China. 2
Cheng Shiu University, Kaohsiung, Taiwan, Republic of China.
Received: 4 May 2018 Accepted: 24 August 2018
References
1. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–86.
2. Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148(4):719–31.
3. Realdi G, Dore MP, Fastame L. Extradigestive manifestations of helicobacter pylori infection: fact and fiction. Dig Dis Sci. 1999;44(2):229–36.
4. Suzuki H, Franceschi F, Nishizawa T, et al. Extragastric manifestations of helicobacter pylori infection. Helicobacter. 2011;16(Suppl 1):65–9. 5. Banic M, Franceschi F, Babic Z, et al. Extragastric manifestations of
helicobacter pylori infection. Helicobacter. 2012;17(Suppl 1):49–55. 6. Deng B, Li Y, Zhang Y, et al. Helicobacter pylori infection and lung cancer: a
review of an emerging hypothesis. Carcinogenesis. 2013;34(6):1189–95. 7. Papagiannakis P, Michalopoulos C, Papalexi F, et al. The role of helicobacter
pylori infection in hematological disorders. Eur J Intern Med. 2013;24(8):685–90.
8. Buzas GM. Metabolic consequences of helicobacter pylori infection and eradication. World J Gastroenterol. 2014;20(18):5226–34.
9. Campuzano-Maya G. Hematologic manifestations of helicobacter pylori infection. World J Gastroenterol. 2014;20(36):12818–38.
10. Franceschi F, Tortora A, Gasbarrini G, et al. Helicobacter pylori and extragastric diseases. Helicobacter. 2014;19(Suppl 1):52–8.
11. Franceschi F, Zuccala G, Roccarina D, et al. Clinical effects of helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol.
2014;11(4):234–42.
12. Wong F, Rayner-Hartley E, Byrne MF. Extraintestinal manifestations of helicobacter pylori: a concise review. World J Gastroenterol. 2014;20(34):11950–61.
13. Chmiela M, Gajewski A, Rudnicka K. Helicobacter pylori vs coronary heart disease - searching for connections. World J Cardiol. 2015;7(4):187–203. 14. Blecker U, Renders F, Lanciers S, et al. Syncopes leading to the diagnosis of
a helicobacter pylori positive chronic active haemorrhagic gastritis. Eur J Pediatr. 1991;150(8):560–1.
15. Ortiz M, Rosado-Carrion B, Bredy R. Role of helicobacter pylori infection in Hispanic patients with anemia. Bol Asso Med P R. 2014;106:13–8. 16. Sato Y, Yoneyama O, Azumaya M, et al. The relationship between iron
deficiency in patients with helicobacter pylori-infected nodular gastritis and the serum prohepcidin level. Helicobacter. 2015;20:11–8.
17. Bazmamoun H, Razavi Z, Esfahani H, et al. Evaluation of iron deficiency anemia and BMI in children suffering from helicobacter pylori infection. Iran J Ped Hematol Oncol. 2014;4:167–71.
18. Sandstrom G, Rodjer S, Kaijser B, et al. Helicobacter pylori antibodies and iron deficiency in female adolescents. PLoS One. 2014;9:e113059.
Table 1
The relevant studies on the associations between
H. pylori
infection and extra-gastroduodenal diseases
(Continued)
Extra-gastroduodenal disease Key evidences Conclusion
7 Cerebrovascular disease Pros:
1. Wang et al. [81]: significant association betweenH. pylori infection and non-cardioembolic stroke (meta-analysis of case-control studies).
Cons:
1. Chen et al. [82]: a reverse link betweenH. pyloriinfection and stroke mortality (cohort study).
19. Qu XH, Huang XL, Xiong P, et al. Does helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis World J Gastroenterol. 2010;16(7):886–96.
20. Annibale B, Marginani M, Monarca B, et al. Reversal of iron deficiency anemia after helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131:668–72.
21. Yuan W, Li Y, Yang K, et al. Iron deficiency anemia in helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45(6):665–76.
22. Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012;13:342–9. 23. Boyanova L. Role of helicobacter pylori virulence factors for iron acquisition
from gastric epithelial cells of the host and impact on bacterial colonization. Future Microbiol. 2011;6(8):843–6.
24. Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systemic review and meta-analysis. Helicobacter. 2008;13:323–40. 25. Capurso G, Lahner E, Marcheggiano A, et al. Involvement of the corporal
mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:1753–61.
26. Goddard AF, James MW, Mclntyre AS, et al. Guidelines for the management of iron deficiency anemia. Gut. 2011;60:1309–16.
27. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut. 2012;61(5):646–64.
28. Gasbarrini A, Franceschi F, Tartaglione R, et al. Regression of autoimmune thrombocytopenia after eradication of helicobacter pylori. Lancet. 1998;352(9131):878.
29. Kikuchi T, Kobayashi T, Yamashita T, et al. Eight-year follow-up of patients with immune thrombocytopenic purpura related toH. Pyloriinfection. Platelets. 2011;22(1):61–4.
30. Brito HS, Braga JA, Loggetto SR, et al. Helicobacter pylori infection and immune thrombocytopenia purpura in children and adolescents: a randomized controlled trial. Platelet. 2014;15:1–6.
31. Kim H, Lee WS, Lee KH, et al. Efficacy of helicobacter pylori eradication for the 1st-line treatment of immune thrombocytopenia patients with moderate thrombocytopenia. AnnHematol. 2015;94:939–46.
32. Noonavath RN, Lakshmi CP, Dutta TK, et al. Helicobacter pylori eradication in patients with chronic immune thrombocytopenic purpura. World J Gastroenterol. 2014;20:6918–23.
33. Michel M, Cooper N, Jean C, et al. Does helicobacter pylori initiate or perpetuate immune thrombocytopenic purpura? Blood. 2004;103(3):890–6. 34. Michel M, Khellaf M, Desforges L, et al. Autoimmune thrombocytopenic
purpura and helicobacter pylori infection. Arch Intern Med. 2002;162(9):1033–6.
35. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113(6):1231–40.
36. Tsumoto C, Tominaga K, Okazaki H, et al. Long-term efficacy of helicobacter pylori eradication in patients with idiopathic thrombocytopenia purpura: 7-year follow-up prospective study. Ann Hematol. 2009;88:789–93. 37. Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H. Pylori
treatment in patients with immune thrombocytopenic purpura with and without H. Pylori infection: a systematic review. Haematologica. 2009;94(6):850–6.
38. Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714–23.
39. Takahashi T, Yujiri T, Shinohara K, et al. Molecular mimicry by helicobacter pylori CagA protein may be involved in the pathogenesis of H. Pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91–6.
40. Bai Y, Wang Z, Bai X, et al. Cross reaction of antibody against helicobacter pylori urease B with platelet glycoprotein IIIa and its significance in the pathogenesis of immune thrombocytopenic purpura. Int J Hematol. 2009;89:142–9.
41. Wu Z, Zhou J, Prsoon P, et al. Low expression of FCCRIIB in macrophages of immune thrombocytopenia-affected individuals. Int J Hematol.
2012;96:588–93.
42. O'Connor HJ, Axon AT, Dixon MF. Campylobacter-like organisms unusual in type a (pernicious anaemia) gastritis. Lancet. 1984;2(8411):1091.
43. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368:2041–2. 44. Sarari AS, Farraj MA, Hamoudi W, et al. Helicobacter pylori, a causative agent
of vitamin B12 deficiency. J Infect Dev Ctries. 2008;2(5):346–9. 45. Shuval-Sudai O, Granot E. An association between helicobacter pylori
infection and serum vitamin B12 levels in healthy adults. J Clin Gastroenterol. 2003;36(2):130–3.
46. Eshraghian A, Hashemi SA, Hamidian Jahromi A, et al. Helicobacter pylori infection as a risk factor for insulin resistance. Dig Dis Sci. 2009;54(9):1966–70. 47. Polyzos SA, Kountouras J, Zavos C, et al. The association between
helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16(2):79–88.
48. Chen TP, Hung HF, Chen MK, et al. Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: a cross-sectional study. Helicobacter. 2015;20:184–91.
49. Yang GH, Wu JS, Yang YC, et al. Gastric helicobacter pylori infection associated with risk of diabetes mellitus, but not prediabetes. J Gastroenterol Hepatol. 2014;29:1794–9.
50. Bajai S, Rekwal L, Misra SP, et al. Association of Helicobacter pylori infection in patients with type 2 diabetes. Indian J Endocrinol Metab. 2014;18:694–9. 51. Horikawa C, Kodama S, Fujihara K, et al. High risk of failing eradication of
helicobacter pylori in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2014;106:81–7.
52. Gillum RF. Infection with helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the third National Health and nutrition examination survey. J Natl Med Assoc. 2004;96:1470–6. 53. Naja F, Nasreddine L, Hwalla N, et al. Association of H. Pylori infection with
insulin resistance and metabolic syndrome among Lebanese adults. Helicobacter. 2012;17(6):444–51.
54. Danesh J, Peto R. Risk factors for coronary heart disease and infection with helicobacter pylori: meta-analysis of 18 studies. BMJ. 1998;316:1130–2. 55. Wada Y, Hamamoto Y, Kawasaki Y, et al. The eradication of helicobacter
pylori does not affect glycemic control in Japanese subjects with type 2 diabetes. Jpn Clin Med. 2013;4:41–3.
56. Kachuei A, Amini M, Sebghatollahi V, et al. Effect of helicobacter pylori eradication on insulin resistance among prediabetic patients: a pilot study and single-blind randomized controlled clinical trial. J Res Med Sci. 2016;21:8.
57. Wu MS, Lee WJ, Wang HH, et al. A case-control study of association of helicobacter pylori infection with morbid obesity in Taiwan. Arch Intern Med. 2005;165:1552–5.
58. Lender N, Talley NJ, Enck P, et al. Associations between helicobacter pylori and obesity--an ecological study. Aliment Pharmacol Ther. 2014;40:24–31. 59. Xu C, Yan M, Sun Y, et al. Prevalence of helicobacter pylori infection and its
relation with body mass index in a Chinese population. Helicobacter. 2014;19:437–42.
60. Pacifico L, Anania C, Osborn JF, et al. Long-term effects of helicobacter pylori eradication on circulating ghrelin and leptin concentrations and body composition in prepubertal children. Eur J Endocrinol. 2008;158:323–32. 61. Kim TJ, Sinn DH, Min YW, et al. A cohort study on helicobacter pylori
infection associated with non-alcoholic fatty liver disease. J Gastroenterol. 2017;52(11):1201–10.
62. Polyzos SA, Kountouras J, Papatheodorou A, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. 2013;62:121–6.
63. Okushin K, Takahashi Y, Yamamichi N, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. 2015;15:25. 64. Tang DM, Kumar S. The association between helicobacter pylori infection
and nonalcoholic liver disease. Curr Gastroenterol Rep. 2017;19:5. 65. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, et al. Helicobacter
pylori and risk of nonalcoholic fatty liver disease: a systemic review and meta-analysis. J Gastroenerol. 2017; Jan 17;https://doi.org/10.1097/MCG. 0000000000000784.
66. Mendall MA, Goggin PM, Molineaux N, et al. Relation of helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71(5):437–9. 67. Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu QB. Increased risk of
atherosclerosis is confined to CagA-positive Helicobacter pylori strains prospective results from the Bruneck study. Stroke. 2003;34:610–5. 68. Park MJ, Choi SH, Kim D, et al. Association between helicobacter pylori
69. Huang B, Chen Y, Xie Q, et al. CagA-positive helicobacter pylori strains enhanced coronary atherosclerosis by increasing serum OxLDL and HsCRP in patients with coronary heart disease. Dig Dis Sci. 2011;56(1):109–14. 70. Al-Ghamdi A, Jiman-Fatani AA, El-Banna H. Role of chlamydia pneumoniae,
helicobacter pylori and cytomegalovirus in coronary artery disease. Pak J Pharm Sci. 2011;24(2):95–101.
71. Figura N, Palazzuoli A, Vaira D, et al. Cross-sectional study: CagA-positive helicobacter pylori infection, acute coronary artery disease and systemic levels of B-type natriuretic peptide. J Clin Pathol. 2014;67(3):251–7. 72. Lai CY, Yang TY, Lin CL, et al. Helicobacter pylori infection and the risk of
acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2015;34:69–74.
73. Yu XJ, Yang X, Feng L, et al. Association between helicobacter pylori infection and angiographically demonstrated coronary artery disease: a meta-analysis. Exp Ther Med. 2017;13:787–93.
74. Padmavati S, Gupta U, Agarwal HK. Chronic infections & coronary artery disease with special reference to Chalmydia pneumoniae. Indian J Med Res. 2012;135(2):228–32.
75. Schottker B, Adamu MA, Weck MN, et al. Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population-based cohort study. Atherosclerosis. 2012;220(2):569–74.
76. Ameriso SF, Fridman EA, Leiguarda RC, et al. Detection of helicobacter pylori in human carotid atherosclerotic plaques. Stroke. 2001;32:385–91. 77. Oshima T, Ozono R, Yano Y, et al. Association of Helicobacter pylori
infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45:1219–22.
78. Kucukazman M, Yavuz B, Sacikara M, et al. The relationship between updated Sydney system score and LDL cholesterol levels in patients infected with helicobacter pylori. Dig Dis Sci. 2009;54:604–7. 79. Whincup PH, Mendall MA, Perry IJ, et al. Prospective relations between
helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart. 1996;75(6):568–72.
80. Sealy-Jefferson S, Gillespie BW, Aiello AE, et al. Antibody levels to persistent pathogens and incident stroke in Mexican Americans. PLoS One. 2013;8(6):e65959.
81. Wang ZW, Li Y, Huang LY, et al. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol. 2012;259(12):2527–37.
82. Chen Y, Segers S, Blaser MJ. Association between helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62(9):1262–9.
83. Huang WS, Yang TY, Shen WC, et al. Association between helicobacter pylori infection and dementia. J Clin Neurosci. 2014;21(8):1355–8. 84. Honjo K, van Reekum R, Verhoeff NP. Alzheimer's disease and infection: do
infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement. 2009;5(4):348–60.
85. Kountouras J, Tsolaki M, Gavalas E, et al. Relationship between helicobacter pylori infection and Alzheimer disease. Neurology. 2006;66(6):938–40. 86. Chang YP, Chiu GF, Kuo FC, et al. Eradication of helicobacter pylori is
associated with the progression of dementia: a population-based study. Gastroenterol Res Pract. 2013;2013:175729.
87. Zhou X, Wu J, Zhang G. Association between helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25(4):460–8. 88. Hu BL, Wang HY, Yang GY. Association of Helicobacter pylori infection with
hepatic encephalopathy risk: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37(6):619–25.
89. Jiang HX, Qin SY, Min ZG, et al. Association of Helicobacter pylori with elevated blood ammonia levels in cirrhotic patients: a meta-analysis. Yonsei Med J. 2013;54(4):832–8.
90. Zhang Y, Hoffmeister M, Weck MN, et al. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol. 2012;175(5):441–50.
91. Wu Q, Yang ZP, Xu P, et al. Association between helicobacter pylori infection and the risk of colorectal neoplasia: a systematic review and meta-analysis. Color Dis. 2013;15(7):e352–64.
92. Chen YS, Xu SX, Ding YB, et al. Helicobacter pylori infection and the risk of colorectal adenoma and adenocarcinoma: an updated meta-analysis of different testing methods. Asian Pac J Cancer Prev. 2013;14(12):7613–9. 93. Fukuda S, Shimoyama T, Umegaki T, et al. Effect of helicobacter pylori
eradication in the treatment of Japanese patients with chronic idiopathic urticaria. J Gastroenterol. 2004;39(9):827–30.
94. Federman DG, Kirsner RS, Moriarty JP, et al. The effect of antibiotic therapy for patients infected with helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49(5):861–4.