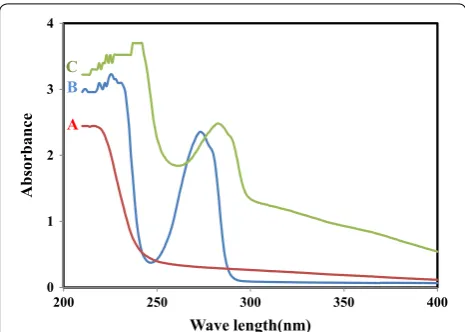
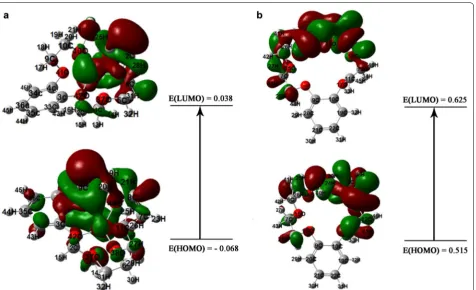
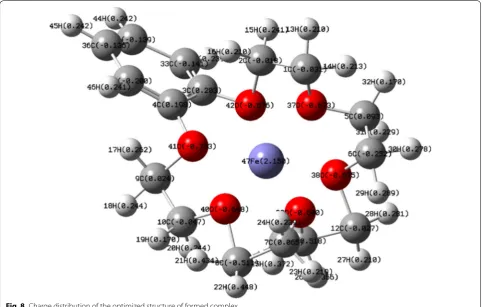
Potentiometric sensor for iron (III) quantitative determination: experimental and computational approaches
Full text
Figure




Related documents
1 and 2 A, the conditioned medium prepared with cells from subject 4, grown without added estrogen, had relatively higher mitogenic activity than conditioned media from
The usefulness of the method for transmission study was confirmed by detecting identical genotypes between the isolates and the plaque samples from which the isolates were cultured
Peyer's patch lymphoid follicle epithelial adherence of a rabbit enteropathogenic Escherichia coli (strain RDEC-1).. Role of plasmid-mediated pili in
Berdasarkan latar belakang dari uraian di atas, maka peneliti bermaksud mengadakan penelitian yang berjudul ”Hubungan Power Tungkai Dan Kelincahan Terhadap
As each project progressed, they built community capacity for research and fund- raising while also increasing the local involvement in the protection, management, and heritage
place common business practices, access to information, and services delivery. across the
Secondary outcomes (at 12 and 18 months unless indicated) were repetition of self-harm leading to hospital attendance in the 12 months after group assignment; cost per
The ionic conductivity increased with the increase of Lithium Triflate concentration as the number free ions increases [9].. However conductivity started to