Breast and Ovarian Cancer
Hereditary Breast and Ovarian Cancer
A Guide for Clinicians
Hereditary Breast and Ovarian Cancer Panel
Advances in molecular genetics have led to the identification of numerous genes associated with inherited susceptibility to breast and/or ovarian cancer. Approximately 5-10% of all cases of breast cancer1 and ~25% of all cases
of ovarian cancer are associated with a mutation in a cancer predisposition gene.2 Mutations in the BRCA1 and BRCA2 genes account for 20-60% of
hereditary breast cancer and 75% of hereditary ovarian cancer. 2,3,4 However, in
patients with a negative BRCA1 or BRCA2 result, other cancer predisposition genes may be considered and additional testing may be appropriate
depending on an individual’s personal and family history. Identifying those at increased risk of developing breast and ovarian cancer due to a hereditary cancer syndrome can allow for early detection of cancer or risk reduction. The Breast/Ovarian Cancer Panel offered at GeneDx is a comprehensive panel that utilizes next generation sequencing and exon level microarray to test for mutations in 21 genes associated with increased risk for developing breast and/or ovarian cancer and includes the BRCA1 and BRCA2 genes.
Genes Included in the Breast/Ovarian Cancer Panel
The 21 genes included in the Breast/Ovarian Cancer Panel can be categorized into three main groups: High-Risk, Moderate-Risk and Newer-Risk Genes as described in Table 1.
Breast Cancer High Risk Panel
The Breast Cancer High Risk Panel is a six-gene test focusing analysis on the highest risk breast cancer susceptibility genes found on the Breast/
High-Risk Genes Moderate-Risk Genes Newer-Risk Genes
•BRCA1, BRCA2, CDH1, EPCAM, MLH1, MSH2, MSH6, PMS2, PTEN, STK11, TP53
• Well studied
• Significantly increased risk of developing breast and/ or ovarian cancer (often at least 4-fold)
• Can increase risk for other cancers
• Guidelines for screening and prevention established
• BARD1, BRIP1, FANCC, NBN, RAD51C, RAD51D, XRCC2
• Not as well studied, data based on a small number of patients or patients within a specific ethnicity • Precise lifetime risks not
yet determined
• May increase risk for other cancers
• Guidelines for screening and prevention not yet established
• ATM, CHEK2, PALB2 • Well studied • Moderately increased
risk of developing breast and/or ovarian cancer (generally 2-3 fold) • Can increase risk for other
cancers
• Limited guidelines for screening and prevention
Lifetime Cancer Risks Associated with High
Risk Genes
BRCA1/BRCA2-Hereditary Breast and Ovarian Cancer
Mutations in BRCA1 and BRCA2 increase the lifetime risk for breast and ovarian cancer significantly over the general population risk. In general, the risk of breast and ovarian cancer is slightly higher for BRCA1 than BRCA2, and usually the cancers occur at a younger age in BRCA1 than BRCA2. The risk to develop breast cancer begins increasing when a woman is in her mid 20’s. The risk of ovarian cancer begins to increase in the mid 30’s, but becomes most significant in the 50’s and beyond. Other associated gynecologic cancers in women with BRCA mutations include fallopian tube carcinoma, primary peritoneal carcinoma, and uterine serous carcinoma.8,9
In addition to breast and ovarian cancer, BRCA1 and BRCA2 mutation carriers have increased lifetime
risks for male breast, pancreatic and prostate cancers, as presented in Figure 1. Women with BRCA1 mutations have a likelihood of 57-87% to develop breast cancer and 24-54% to develop ovarian cancer. Women with BRCA2 mutations have a likelihood of 41-84% to develop breast cancer and 11-27% to develop ovarian cancer. BRCA2 mutation Figure 1
Ovarian Cancer Panel. These genes include BRCA1, BRCA2, CDH1, PTEN, STK11, and TP53. This unique test offers clinicians the ability to target well-described genes that confer a significantly increased risk for breast cancer and for which explicit management guidelines have been published.5,6
In addition, there is the option to order the Breast Cancer High Risk Panel and PALB2 (7 gene panel) given recent data that shows that PALB2 mutation carriers with a positive family history of breast cancer may face up to a 58% lifetime risk of breast cancer.5,6 Of note, unlike the other six genes on the
Breast Cancer High Risk Panel, NCCN management guidelines for PALB2 mutation carriers are limited.
41-84% 57-87%
4-7% 24-54%
11-27%
BRCA1/2-Associated Lifetime Risks by Cancer Type
5-7% 20-34% 0 100 80 60 40 20 Pr obability (%) Cancer Type
*Individuals with BRCA1 mutations have increased risk for male breast, prostate, and pancreatic cancers, however the exact risks are not well-defined.
Female
Breast Breast*Male Ovarian Pancreatic* Prostate* BRCA2 BRCA1*
carriers also have a 20-34% risk of prostate cancer, and up to a 7% risk for both male breast cancer and pancreatic cancer. While individuals with BRCA1 mutations also have increased chances to develop these cancers, the exact risks are not well-defined.
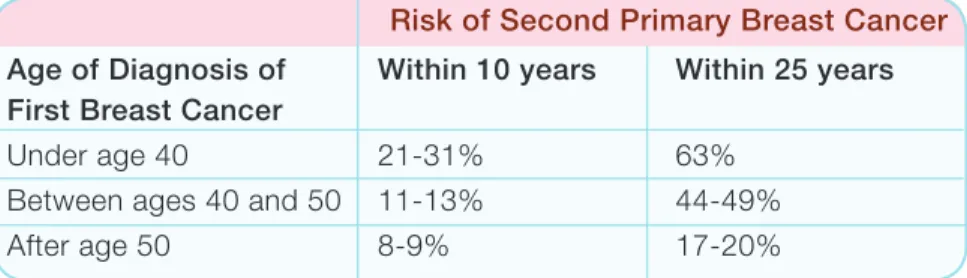
Women with BRCA1/2 mutations who have had breast cancer also have an increased risk for a second primary breast cancer. This risk may depend on the age at which the first breast cancer was diagnosed. Table 2 describes the risk of a second primary breast cancer based on age of diagnosis of the first breast cancer.
Additional High Risk Genes
In addition to BRCA1 and BRCA2, the Breast/Ovarian Cancer Panel includes other high penetrance genes that are associated with well-defined cancer syndromes associated with an increased risk for breast and/or ovarian cancer and other cancers. These include CDH1 (hereditary diffuse gastric cancer syndrome), PTEN (Cowden syndrome), STK11 (Peutz-Jeghers syndrome), TP53 (Li-Fraumeni syndrome), and MLH1, MSH2, MSH6, PMS2, and EPCAM (Lynch syndrome). Table 3 provides lifetime cancer risks (when available).
Moderate Risk and Newer-Risk Genes
In addition to the high risk genes, this panel includes genes that impart a moderate risk for breast and/or ovarian cancer and for which there are limited guidelines for screening and prevention available. These genes include ATM, CHEK2 and PALB2 (Table 3). Finally, 7 genes included in the panel have been identified in families with apparent hereditary breast and/or ovarian cancer, but precise lifetime cancer risks for these genes have not been determined
Age of Diagnosis of First Breast Cancer
Within 10 years Within 25 years
Under age 40 21-31% 63% Between ages 40 and 50 11-13% 44-49% After age 50 8-9% 17-20%
Risk of Second Primary Breast Cancer
Table 2: Risk of second primary breast cancer based on age of diagnosis of first breast
Gene Associated Cancers and Risks
BRCA1 Female Breast (57-87%), Ovarian (24-54%), Prostate, Male Breast, Pancreatic, Fallopian tube, Primary peritoneal, Endometrial (serous)8, 9,10,11,12,13,14,15,16,17,18,19
BRCA2 Female Breast (41-84%), Ovarian (11-27%), Prostate (20-34%), Pancreatic (5-7%), Male Breast (4-7%), Melanoma, Fallopian tube, Primary peritoneal, Endometrial (serous)3,9,10,13,17,19,20,21,22,23
CDH1 Female Breast (39-52%), Diffuse gastric cancer (40-83%), Colon24, 25,26
EPCAM* MLH1 MSH2 MSH6* PMS2*
Ovarian (1-24%), Colorectal (15-80%), Endometrial (12-61%), Gastric, Pancreatic, Biliary tract, Urothelium, Small bowel, Brain, Sebaceous neoplasms5,27,28,29,30,31,32,33,34
PTEN Female Breast (25-50%), Thyroid (10%), Endometrial (5-10%), Colon, Renal, Melanoma35,36,37
STK11 Female Breast (32-54%), Ovarian tumors (21%), Colorectal (39%), Pancreatic (11-36%), Gastric (29%), Lung (15%), Small intestine (13%), Cervical (10%), Endometrial (9%), Testicular tumors (9%)6,38,39
TP53 Female Breast, Soft tissue sarcoma, Osteosarcoma, Brain, Hematologic malignancies, Adrenocortical carcinoma Overall risk for cancer: nearly 100% in females, 73% in males4,40,41,42,43,44
ATM Female Breast, Colon, Pancreatic45,46,47
CHEK2 Female Breast, Male Breast, Colon, Prostate, Thyroid, Endometrial (serous), Ovarian2,8,48,49
PALB2 Female Breast (25-58%), Male Breast, Pancreatic, Ovarian2,50,51,52,53,54
BARD1 Female Breast, Ovarian2,55
BRIP1 Female Breast, Ovarian56,57
FANCC Female Breast58
NBN Female Breast, Melanoma, Non-Hodgkin lymphoma59,60
RAD51C Female Breast, Ovarian61
RAD51D Female Breast, Ovarian62
XRCC2 Female Breast, Pancreatic63,64
High-Risk Genes
Moderate- Risk Genes
Newer
-Risk Genes
Table 3: Cancers and lifetime risks associated with genes in the breast/ovarian cancer panel * Tumor spectrum is representative of Lynch syndrome; data are limited with regard to the
association of certain cancers with mutations in MSH6, PMS2, and EPCAM.
and/or need confirmation. They include: BARD1, BRIP1, FANCC, NBN, RAD51C, RAD51D, and XRCC2 (Table 3). Identifying mutations in these genes may be helpful for families because, as more data becomes available, targeted cancer screening and prevention may be offered.
Inheritance of the Genes in the Breast/Ovarian
Cancer Panel
The genes in the Breast/Ovarian Cancer Panel are inherited in an autosomal dominant manner. Therefore, an individual carrying a disease-causing mutation has a 50% chance of transmitting the mutation to a child, either male or female. Almost all individuals with a mutation in one of the genes on the panel inherited it from a parent and therefore the siblings of an individual with a mutation have a 50% chance to have the same disease-causing mutation. The risk for other family members to carry the familial mutation depends on which side of the family transmits the mutation, and how closely related the family member is to your patient.
While each of the genes on the panel is associated with an increased risk for breast and/or ovarian cancer when a single mutation in the gene is present, some of the genes on the panel are also associated with rare autosomal recessive syndromes if an individual has a mutation in both copies of the gene (biallelic mutations). The genes known to be associated with recessively inherited syndromes are listed in Table 4. If both a mother and father are carriers for a mutation within the same gene*, each of their children has a 25% chance to inherit both mutations, resulting in the recessive syndrome associated with that particular gene. If your patient tests positive for one of these mutations and wishes to have children in the future, then careful review of his or her partner’s family history and possibly genetic testing of the partner are needed to assess the reproductive risks associated with mutations these genes.
ATM Ataxia-Telangiectasia
EPCAM, MLH1, MSH2, MSH6, PMS2 Constitutional mismatch repair deficiency syndrome *there is a case report of a child with this condition that has one mutation in EPCAM and one mutation in MSH2
BRCA2, BRIP1, FANCC, PALB2,
RAD51C, XRCC2 Fanconi anemia
NBN Nijmegen breakage syndrome
Gene Autosomal Recessive Disorders
Clinical Indications for the Breast/Ovarian Cancer Panel
The Breast/Ovarian Cancer Panel is appropriate for individuals whose personal and/or family history are suggestive of a hereditary predisposition to breast and/or ovarian cancer. This includes:
• Women with ovarian, fallopian tube or primary peritoneal cancer
• Women diagnosed with breast cancer at an age ≤45
• Individuals with multiple related primary cancers
• Individuals with a type of cancer that is not common in the general population, such as male breast cancer
• Individuals with several relatives affected with related cancers, spanning multiple generations
Genetic Test Results and What They Mean
There are three possible outcomes of genetic testing: positive, negative and variant of unknown significance (VUS). All patients who undergo genetic testing should receive pre-test and post-test genetic counseling to understand the implications of testing. Genetic counseling services across the country can be found at: www.nsgc.org
Positive Result
A positive result indicates that a disease-causing mutation was identified in that individual, and the risk for cancer is increased. Knowledge of a positive result provides valuable information to the patient, physician and family members. Knowledge of a patient’s genotype can assist in making management and treatment decisions. Furthermore, testing of the patient’s family members can allow for accurate predictions of cancer risks.
Negative Result
A negative result means that a disease-causing mutation was not identified in the individual tested. A negative result can have different interpretations based on the following scenarios:
True Negative Result: An individual who tests negative for a known familial mutation is not a carrier of a known cancer-predisposing mutation that has been identified in another family member. The risk for cancer in this individual
is generally not expected to be greater than the general population. In some cases, this interpretation may be limited by the identification of a mutation in a gene with moderate cancer risk or a mutation in a gene that has been recently described (i.e. newer-risk genes previously described) with cancer risk and for which there are currently limited data to predict cancer risk. In these situations, not all of the cancer in the family may be attributed to the identified familial gene mutation. Therefore, clinical assessment of the complete family history of cancer and personal risk factors is important to determine appropriate management.
Uninformative Negative Result: If testing was performed on an individual
with a cancer diagnosis, this means that we do not have an explanation for why this individual developed cancer. Genetic counseling is recommended, as additional testing may be considered based on the individual’s medical and family history. If testing was performed on an individual without a personal history of cancer and based only on family history, the risk for cancer may remain increased as the exact cause of the cancer in the family remains unknown. Testing of an affected family member may help clarify this individual’s cancer risks.
Variant of Unknown Significance (VUS)
A variant of unknown significance (VUS) indicates that the pathogenicity of the variant cannot be clearly established. To further clarify the clinical significance of this variant, testing of family members may be helpful. If a relative with a related cancer is found to have the same variant, it may provide evidence that the variant may be disease-causing. The greater the number of affected family members who carry the VUS, the greater the likelihood that the VUS is pathogenic. With consistent linkage of the VUS with family members with related cancers, in addition to other evidence, the variant found may be reclassified as a family-specific mutation and predictive genetic testing can be offered to extended family members. Conversely, a VUS could be determined to be benign through this and other research. GeneDx will review a patient’s detailed family history to determine if family members are eligible for complementary targeted variant testing through our Variant Testing Program.
Genetic Counseling
Pre-test counseling is recommended for individuals who are either interested in understanding their risks and/or meet the clinical criteria for testing and are considering genetic testing. If a disease-causing mutation has already been identified in a family member, testing of the specific mutation is appropriate. If a disease-causing mutation has never been identified, an affected family member with the highest likelihood for a positive result (early onset disease, bilateral disease or multiple primaries) is ideally the best person for initial testing within a family. If an affected family member is not available for testing, testing of an unaffected family member can be performed, although a negative test result will not guarantee that the individual does not have an increased cancer risk. Once patients make the decision to undergo testing, post-test genetic counseling is recommended to understand the implications of the results. Genetic counseling services across the country can be found at: www.nsgc.org More information about risk assessment and genetic counseling for hereditary breast and ovarian cancer can be found at:
www.nchpeg.org/hboc
Management
BRCA1/2-positive PatientsThere are a variety of management strategies available to patients who test positive for a disease-causing mutation in BRCA1 or BRCA2. Various guidelines outline available options and recommendations for patients who have a positive BRCA1 or BRCA2 test result. These options include enhanced screening, risk reducing surgery, and medication to reduce risk. The guidelines below are examples of current recommendations as of 2014. Please visit www.nccn.org for the most up-to-date recommendations, including ages to begin surveillance and current recommended screening frequencies.
Increased Screening:
For women, increased screening includes the following:
• Increased breast awareness including self breast exam • Routine clinical breast exam
• Breast MRI and mammogram starting at an early age
• Consider transvaginal ultrasound of the ovaries and CA-125 blood tests • Consider full body skin exam
For men, increased screening includes the following:
• Increased breast awareness including self breast exam • Routine clinical breast exam
• Consider baseline mammogram with repeat imaging if gynecomastia or parenchymal/glandular breast density is noted on baseline study • Routine prostate cancer screening starting at an early age • Consider full body skin exam
• Consider pancreatic cancer screening
Risk Reducing Surgery:
• Consider prophylactic mastectomy; mastectomy reduces the chance of developing breast cancer by at least 90%.
• Salpingo-oopherectomy is recommended (as early as the 30s-40s) once a woman has completed childbearing; this reduces the risk of ovarian cancer by approximately 80% and, if performed prior to natural menopause without hormone replacement, reduces the risk of breast cancer by up to 50%.
Medication to Reduce Cancer Risk:
• Consider the use of breast cancer chemoprevention (such as tamoxifen and raloxifene),which may reduce the risk of breast cancer.
• Consider the use of oral contraceptives, which may reduce the risk of ovarian cancer by 45-50% in BRCA1 mutation carriers and by 60% in BRCA2 mutation carriers. Risks and benefits should be carefully
considered, given conflicting evidence on the impact of oral contraceptives on breast cancer risk.
Other Genes
Various screening and surgical options are available to individuals who test positive for other disease-causing mutations included on the Breast/Ovarian Cancer Panel as discussed in Table 5. The guidelines below are examples of current recommendations as of 2014. For comprehensive gene-specific guidelines, including ages to begin surveillance and current recommended screening frequencies, please visit: www.nccn.com
TP53 Increased breast awareness including self breast exam Routine clinical breast exam
Breast MRI and mammography starting at an early age Consider prophylactic mastectomy
Consider increased colonoscopy screening
Comprehensive physical exam including skin and neurologic exams Use caution regarding radiation therapy for cancer
PTEN Increased breast awareness including self breast exam Routine clinical breast exam starting at an early age Breast MRI and mammography starting at an early age
Consider endometrial biopsy and/or transvaginal ultrasound starting at an early age; encourage patient education and prompt response to symptoms Options of prophylactic mastectomy and hysterectomy can be discussed Annual physical exam
Routine colonoscopy starting at an early age Consider routine dermatologic exam
Baseline thyroid ultrasound at an early age and consider routinely thereafter Consider routine renal ultrasounds starting at an early age
CDH1 Increased breast awareness including self breast exam Routine clinical breast exam
Routine breast MRI and mammography Routine endoscopy starting at an early age Strong consideration of prophylactic gastrectomy Routine colonoscopy
STK11 Increased breast awareness including self breast exam Routine clinical breast exam
Routine breast MRI and mammography starting at an early age Frequent colonoscopy starting at an early age
Routine upper endoscopy starting at an early age
Routine pancreatic cancer screening (e.g. magnetic resonance
cholangiopancreatography (MRCP) or endoscopic ultrasound) starting at an early age Routine small bowel visualization starting at an early age
Routine pelvic exam with consideration of transvaginal ultrasound Annual physical exam
EPCAM, MLH1, MSH2, MSH6, PMS2
Frequent colonoscopy (every 1-2 years in many cases) starting at an early age Consider endometrial/ovarian cancer screening, which may include endometrial biopsy, transvaginal ultrasound and CA-125 blood tests
Consider prophylactic hysterectomy and salpingo-oophorectomy once a woman has completed childbearing
Consider routine esophagogastroduodenoscopy (EGD) with extended duodenoscopy starting at an early age
Consider routine urinalysis starting at an early age Annual physical exam
Consider the option of prophylactic colectomy, particularly if the patient requires surgery to address a colonic neoplasm not amenable to endoscopic resection
ATM, CHEK2, PALB2
Increased breast awareness including self breast exam Clinical breast exam
Annual breast MRI and mammogram Gene Management Guidelines
Resources for Patients
Bright Pink: www.brightpink.org
FORCE: www.facingourrisk.org
National Society of Genetic Counselors: www.nsgc.org
NCI: www.cancer.gov
Sharsheret: www.sharsheret.org
Susan G. Komen: www.komen.org
References
1. Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: Recommendations of the National Society of Genetic Counselors. J Genet Couns 16(3):241, 2007. 2. Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian cancer, fallopian tube,
and peritoneal carcinoma identified by massively parallel sequencing. PNAS 2011;108:18032, 2011. 3. Ford D, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast
cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998 Mar;62(3):676-89. 4. Lalloo F, Evans DG. Familial breast cancer. Clin Genet 82:105, 2012.
5. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Colorectal. Version 2.2014.
6. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2014.
7. Graeser MK, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009 Dec;27(35):5887-92.
8. Pennington KP, Walsh T, Lee M, et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer 119:332, 2013.
9. Levine DA et al. Fallopian Tube and Primary Peritoneal Carcinomas Associated With BRCA Mutations. J Clin Oncol. 2003 Nov 15;21(22):4222-7.
10. Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003 May;72(5):1117-30.
11. Chen S and Parmigiani G. Meta-anlaysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007 Apr;25(11):1329-33.
12. King MC, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003 Oct;302(5645):643-6.
13. Risch HA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario. J Natl Cncer Inst. 2006 Dec;98(23):1694-706.
14. Claus EB et al. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996 Jun 1;77(11):2318-24.
15. Brose MS et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002 Sep;94(18):1365-72.
16. Leongamornlert D et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012 May 8;106(10):1697-701.
17. Liede A et al. Cancer Risks for Male Carriers of Germline Mutations in BRCA1 or BRCA2: A Review of the Literature. J Clin Oncol. 2004 Feb 15;22(4):735-42.
18. Thompson D, Easton DF, and the Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002 Sep;94(18):1358-65.
19. Tai YC et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007 Dec;99(23):1811-4.
20. Biron-Shental T et al. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol. 2006 Dec;32(10):1097-100.
21. Ozcelik H et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet.1997 May;16(1):17-8.
22. The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999 Aug;91(15):1310-6.
23. Thompson D, Easton D, Breast Cancer Linkage Consortium. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001 Feb;68(2):410-9.
24. Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 392:402, 1998.
25. Gayther SA, Gorringe KL, Ramus SJ, et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res 58:4086, 1998.
26. Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. J Med Genet 47:436, 2010. 27. Kohlmann W et al. Lynch Syndrome. 2004 Feb 5 [Updated 2012 Sept 20]. In: Pagon RA, Adam MP,
Bird TD, et al., (Eds), GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993- 2014
28. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: Correction for ascertainment. J Med Genet. 42(6):491- 496, 2005
29. Vasen HF et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 110(4):1020-1027, 1996..
30. Dowty JG et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum Mutat. 34(3):490-497, 2013. 31. Bonadona V et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6
Genes in Lynch Syndrome. JAMA. 305(22):2304-2310, 2011.
32. Baglietto L et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 102(3):193-201, 2010.
33. Senter L et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 135(2):419-428, 2008.
34. Kempers MJ et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: A cohort study. Lancet Oncol. 12(1):49-55, 2011.
35. Brownstein MH, Wolf, M, Bikowski JB. Cowden’s disease: A cutaneuos marker of breast cancer. Cancer 41:2393, 1978.
36. Starnik TM, van der veen JP, Arwert F, et al. The Cowden syndrome: A clinical and genetic study in 21 patients. Clin Genet 29:222, 1986.
37. Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 18(2):400, 2012.
38. Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. ClinCancer Res 12(10):3209, 2006.
39. Giardiello FM et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. Dec;119(6):1447-53, 2000.
40. Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 46:689, 2009.
41. Mouchawar J, Korch C, Byers T, et al. Population-based estimate of the contribution of TP53 mutations to subgroups of early-onset breast cancer: Australian breast cancer family study. Cancer Res 70:4795, 2010.
42. Ruijs MWG, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: Mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet 47:421-428, 2010.
43. Chompret A et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 82(12):1932-7, 2000.
44. Olivier M et al. Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 63(20):6643-50, 2003.
45. Renwick A, Thompson D, Seal S, et al. ATM Mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38(8):873, 2006.
46. Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst 97:813, 2005.
47. Roberts NJ, Jio Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discovery 2(1):41, 2011.
48. Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31:55, 2002. 49. Narod SA. Testing for CHEK2 in the cancer genetics clinic: Ready for prime time? Clin Genet
2010;78(1):1-7.
50. Erkko H, Xia B, Nikkila J, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446(7133):316-9.
51. Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39(2):165-7.
52. Byrnes GB, Southey MC, Hopper JL. Are the so-called low penetrance breast cancer genes, ATM, BRIP1, PALB2, and CHEK2, high risk for women with strong family histories? Breast Cancer Res. 2008;10(3):208-14.
53. Heikkinen T, Karkkainen H, Aaltonen K, et al. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin Cancer Res. 2009;15(9):3214-22.
54. Antoniou et al. Breast-Cancer Risk in Families with Mutations in PALB2. N Engl J Med. 2014 Aug;371(6):497-506.
55. Ratajska M, et al. Cancer predisposing BARD1 mutations in breast–ovarian cancer families. Breast Cancer Res Treat. 2012 Jan;131(1):89-97.
56. Rafnar T, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011 Oct 2;43(11):1104-7.
57. Seal S, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006 Nov;38(11):1239-41.
58. Thompson ER, et al. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet. 2012 Sep;8(9):e1002894.
59. Pardini B, et al. NBN 657del5 heterozygous mutations and colorectal cancer risk in the Czech Republic. Mutat Res. 2009 Jun 18;666(1-2):64-7.
60. Kanka C, et al. Germline NBS1 mutations in families with aggregation of Breast and/or ovarian cancer from north-east Poland. Anticancer Res. 2007 Jul-Aug;27(4C):3015-8.
61. De Leeneer K, et al. Evaluation of RAD51C as cancer susceptibility gene in a large breast-ovarian cancer patient population referred for genetic testing. Breast Cancer Res Treat. 2012 May; 133(1):393-8.
62. Osher DJ, et al. Mutation analysis of RAD51D in non-BRCA1/2 ovarian and breast cancer families. Br J Cancer. 2012 Apr 10;106(8):1460-3.
63. Park DJ, et al. Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet. 2012 Apr 6;90(4):734-9.
64. Jiao L, et al. XRCC2 and XRCC3 gene polymorphism and risk of pancreatic cancer. Am J Gastroenterol. 2008 Feb;103(2):360-7.
207 Perry Parkway Gaithersburg, MD 20877
T 1 888 729 1206 • F 1 201 421 2010
How can I order this test?
You can order this test by taking the following steps:
1. Download the OncogeneDx test requisition form from the GeneDx website: www.genedx.com/forms
2. Complete all the forms with required information
3. Ship completed forms along with either two 4ml lavendar top tubes of blood OR saliva collected in mouthwash using our oral rinse collection kit and send to the following address:
Accessions GeneDx
207 Perry Parkway Gaithersburg, MD 20877
We provide shipping kits to physicians upon request. To place an order for shipping kits, please visit our website: www.genedx.com/supplies or email us at: wecare@genedx.com
About GeneDx
GeneDx is a highly respected genetic testing company, founded in 2000 by two scientists from the National Institutes of Health (NIH) to address the needs of patients and clinicians concerned with rare inherited disorders. Currently, GeneDx offers whole exome sequencing, oligonucleotide microarray-based testing for detecting chromosomal abnormalities, testing for inherited eye disorders and autism spectrum disorders and gene panels for testing various forms of inherited cardiac disorders, mitochondrial disorders, neurological disorders and inherited cancer disorders. At GeneDx, our technical services are matched by our scientific expertise and customer support. Our growing staff includes more than 80 geneticists and genetic counselors specialized in clinical genetics, molecular genetics, metabolic genetics and cytogenetics who are just a phone call or email away. We invite you to visit our website: www.genedx.com to learn more about us and the services we offer.