Copyright © 2002, American Society for Microbiology. All Rights Reserved.
Preadult Stage Parasites and Multiple Timed Exposure to Infective
Larvae Are Involved in Development of Limb Edema in
Brugia
malayi
-Infected Indian Leaf Monkeys (
Presbytis entellus
)†
P. K. Murthy,
1* M. A. Khan,
1H. B. Rajani,
2and V. M. L. Srivastava
1Divisions of Parasitology1and National Laboratory Animal Centre,2Central Drug Research
Institute, Lucknow 226001, India
Received 8 October 2001/Returned for modification 5 December 2001/Accepted 1 March 2002
The pathogenesis of filarial limb edema is not known. The role of parasitological variables and
parasite-mediated phenomena in the development of limb edema was investigated in thePresbytis entellus-Brugia malayi
model. Infection was initiated with subcutaneous inoculation of infective third-stage larvae (L3), and the
animals were reexposed to different doses of L3at the prepatent, patent, and diminishing microfilaremia (0 to
5% of peak microfilaremia count) stages of infection. A large L3inoculum size and repeated inoculation in the
ankle region during the prepatent, patent, and diminishing microfilaremia stages of infection were found to be necessary for reproducible induction of limb edema. The preadult stage of the parasite was found to be the most
potent inducer of limb edema, followed by L5and L4. The presence of the proinflammatory cytokines tumor
necrosis factor alpha, interleukin-1, and interleukin-6 in edema fluid in the leg receiving the parasite
challenge indicated that the limb edema development was due to parasite-mediated cytokine responses. The absence of bacterial infection or anti-streptolysin O titer in the edema fluid and blood indicated that bacterial infection is not necessary for the development of limb edema.
Lymphatic filarial manifestations caused byWuchereria
ban-croftiandBrugia malayiaffect about 120 million people
world-wide. The pathogenesis of the complex manifestations is not fully understood, because it is difficult to monitor the subjects from the time of exposure to infective third-stage larvae (L3) to
development of chronic filarial manifestations. However, it is thought to involve at least three components: parasitological, immunological, and bacteriological. The contribution of each of these components is currently the subject of intensive stud-ies. Epidemiological studies have shown positive correlations between lymphatic filarial infection and disease symptoms as development of symptoms follow a definite sequence, i.e., from infection to microfilaremia, then to amicrofilaremia, and finally to elephantiasis or hydrocele (2, 11, 12, 27).
Among the extrinsic factors that are thought to play a role in the development of filarial pathology, the most controversial are the secondary opportunistic bacterial and fungal infections. Exacerbation of acute and chronic episodes of lymphedema by opportunistic infections was reported to occur both in filarial patients (1, 12, 23, 24, 30) and in experimental animals (7). However, there are also reports that in many areas of ende-micity where local hygiene is not maintained, there is no con-current increase in pathology (30).
Although the basic information on immunological aspects of the filarial disease demonstrates differential immune respon-siveness among various clinically positive individuals, the trig-gering or inducing factor(s) that leads to the development of clinical disease is not known. Studies with animal models have
shown that the clinical and immunological outcomes of lym-phatic filarial infection depend upon the frequency and inten-sity of exposure to the parasites. In cats, exposure to a single inoculation ofBrugia pahangi induces lymphedema in about 15% of the exposed cats (6), whereas repeated small inocula-tions given at weekly intervals lead to the development of lymphedema in up to 40% of the animals (9). The Indian leaf monkey,Presbytis entellus, exposed to B. malayidevelops sys-temic and local filarial disease manifestations, such as fever, eosinophilia, and episodic limb edema (20, 32), which closely resemble those in human patients and depend largely on the mode of exposure to infective larvae (21). In this model we found that disease manifestations developed during either low or no microfilaremia and that the humoral and cellular im-mune responses in certain symptomatic animals are different from those shown by animals that never developed any mani-festations (8, 21). Also, some nonreactive parasite antigen mol-ecules were detected in the sera of symptomatic animals, sug-gesting their possible involvement in the pathogenesis of manifestations. Further investigations were made with this model to explore the factors that contribute to the develop-ment of manifestations, particularly limb edema. The param-eters considered were with a view to (i) develop an infection exposure protocol that increases the incidence of manifesta-tions, (ii) ascertain the possible contribution of nonparasite factors in the development of disease manifestations and (iii) identify the parasite life stage(s) that may be involved in the development of limb edema. In the present study, we report (i) the effect of L3exposure factors such as the size of inoculum
and the site and timing of inoculations on the incidence rate of manifestations, (ii) studies on edema fluid for investigation of bacterial infection and inflammatory cytokines, and (iii) the induction of limb edema in infected and normal monkeys by * Corresponding author. Mailing address: Division of Parasitology,
Central Drug Research Institute, P.O. Box 173, Lucknow 226001, India. Phone: 91-0522-212411-18, ext. 4429. Fax: 91-0522-223405. E-mail: psrkalpana@hotmail.com.
† CDRI communication no. 6254.
913
on August 17, 2020 by guest
http://cvi.asm.org/
administration of extracts derived from various life stages ofB.
malayi.
MATERIALS AND METHODS
Animals. Young adult male Indian leaf monkeys (P. entellus), commonly known in Hindi as langur, 3 to 4 kg in body weight were obtained from local suppliers. Immediately on receipt, the animals were kept in quarantine for 45 days, during which time they were subjected to routine health check procedures, including clinical biochemistry and hematology, and were thoroughly examined for tuberculosis (by the Mantoux test and chest X ray), intestinal helminthiasis (by examination of feces), and microfilariae (by night blood examination). Ani-mals found positive for intestinal helminths were treated with mebendazole (Zodex; Concept Pharmaceuticals, Bombay, India) at 20 mg/kg orally for 3 days, which was repeated after 3 weeks. None of the monkeys was positive forW. bancroftiorB. malayimicrofilariae. On completion of the quarantine and health check, the animals were transferred to the animal quarters of the experimental filariasis wing, where they remained under observation for not less than 4 weeks before the start of the study. Two days before the start of the study, the animals were again subjected to all of the tests described above, except those for tuber-culosis, for a final health check. A total of 20 disease-free monkeys that were negative in all of the tests were finally selected for the present study. Throughout the prestudy and study periods the animals were housed in temperature (24 to 28°C)- and photoperiod (12 h of dark and 12 h of light)-controlled quarters protected from mosquitoes and other vectors by wire netting. The animals were fed on a commercial pellet diet (Nav Maharashtra Chakan & Oil Mills, Pune, India) supplemented with calculated quantities of bread, Bengal gram, and seasonal fruits and vegetables. They had free access to safe drinking water.
Infection.Twenty male langurs were divided into four groups and inoculated with infective L3ofB. malayi. The L3were obtained from laboratory-bred female Aedes aegyptimosquitoes fed on microfilaremicMastomys couchaas described previously (22). Three animals each in groups I and II received a total of 250 L3
in the groin or ankle region in four divided doses within a span of 8 days. Animals in group III (n⫽4) received 500 L3in four divided doses spread over a period
of 45 days. In these animals the inoculations were given in the groin region. Group IV, consisting of eight animals, received inoculations in the ankle region. An initial inoculum of 500 L3in three or four divided doses (125 to 200 L3/dose)
at 13- to 15-day intervals was given to each animal (six animals received four doses of 125 L3, and two animals received doses of 125, 175, and 200 L3),
followed by a single inoculum of 125 L3during patency and one to three inocula
of 100 L3during the period of diminishing microfilaremia (we use the term
diminishing microfilaremia stage to indicate the period after patency during which microfilarial counts fell to and remained at 0 to 5% of the peak count). All of the animals received inoculations through the subcutaneous route. Animals in group V (n⫽2) received saline in the ankle region subcutaneously and served as controls.
Microfilaremia of infected animals was monitored between 9 and 10 p.m. by the membrane filtration technique (20) on days 60, 75, and 90 after the first L3
inoculation (p.f.i.) and thereafter at monthly intervals until the end of the study.
Clinical examination.All of the monkeys were examined daily for externally visible inflammation in the limbs. Rectal temperature was recorded on every alternate day. Quantitative assessment of swelling of limbs was made as de-scribed earlier (32). Briefly, peripheral (circumference) measurements were taken of the affected limb at three representative sites showing the lowest to maximal visible swelling between the knee and the ankle. The locations of these sites were determined in terms of distance from the knee joint towards the ankle, and measurements were then made at the same locations of the unaffected limb of the same animal. The swelling ratio was determined by dividing the sum of the three measurements for edematous limb by the sum of the three measurements for the unaffected limb of the same animal. The ratios were categorized into three range classes representing mild (ratio, 1.01 to 1.15), moderate (ratio, 1.16 to 1.30), and severe (ratio, more than 1.3) edematous swelling. Any inflammation in the toes was also considered.
Preparation of parasite whole-worm extracts.Microfilariae, L4, L5, and
pre-adult and pre-adult worms were recovered from the peritoneal cavities of experi-mentally infected jirds (Meriones unguiculatus). L3were isolated fromB. malayi
-infectedA. aegyptimosquitoes fed on microfilaria-positiveM. coucha9 or 10 days before. Soluble somatic extracts from all life stages of the parasite were prepared aseptically as described elsewhere (28), with some modifications. Briefly, the worms were washed thoroughly with sterile 0.01 M phosphate-buffered saline (pH 7.2) and homogenized in a Potter Elvehjem tissue grinder (A. Thomas Scientific, Philadelphia, Pa.) at 4°C. The homogenate was sonicated on ice at 20 kilocycles per s for 20 s, and 9 or 10 such strokes were applied to make the
extracts homogeneous. The protein content was measured by the method of Lowry et al. (18), and the antigen was stored in aliquots of 0.5 ml at⫺20°C until used.
Administration of extracts derived from various life stages of the parasite to monkeys.Animals of group IV harboring various stages of infection (between days 15 and 25 [prepatent], 50 and 60 [prepatent], 150 and 160 [patent], and 280 and 290 [period of diminishing microfilaremia] p.f.i.) were used in this study. In order to find out which stage of the parasite extract has the potential of inducing manifestation, extracts of various life stages of the parasite or saline were ad-ministered in the normal and filaria-infected monkeys through the subcutaneous route. The protein concentration of the extracts used was 1 mg in 0.5 ml. Each preparation was injected into both exposed and contralateral (unexposed) hind limbs at the ankle region. The inoculated areas were observed for development of manifestations if any, from 1 h after injection up to 8 h and thereafter at 24-h intervals for 7 days. Clinical assessment of the manifestations (i.e., edema and rectal temperature) was done until the disappearance of the manifestations as described above.
Collection of edema fluid.Edema fluid was collected from the edematous areas of the legs of the monkeys under aseptic conditions with the help of a heparinized syringe fitted with a 23-gauge needle. The fluid was stored at⫺20°C until use. The edema fluid from animals exposed to L3was collected on three
occasions: early stage (within 24 h of edema development), middle stage (the period when the volume of edema was maximum), and late stage (when the edematous reaction started receding). At each period the fluid was collected twice or thrice within 24 h. However, parasite extract-induced edema fluid was collected from animals when they showed well-developed pitting edema.
Collection of blood and sera.Sera were collected from symptomatic monkeys (during the period of manifestations), asymptomatic monkeys (which never showed any manifestations), and age-matched unexposed healthy monkeys 1 month after the first larval exposure and thereafter at monthly intervals until the termination of the experiment. The samples were stored at⫺20°C. Fresh hep-arinized blood from these monkeys was collected and immediately subjected to bacteriological culture.
Bacteriological examination in edema fluid and blood. Edema fluid and freshly collected blood were cultured for the presence of aerobic bacteria ( Strep-tococcusspp.,Staphylococcusspp., andCorynebacteriumspp. [gram positive] and enterobacteria,Escherichia coli,Salmonellaspp.,Shigellaspp.,Klebsiellaspp., Aerobacterspp.,Enterobacterspp.,Proteus spp., andPasteurellaspp. [gram neg-ative]) and anaerobic bacteria (Clostridiumgroup and anaerobicStreptococcus). For aerobic and anaerobic bacteria the techniques of Cruickshank et al. (4) and Wilson et al. (33) were generally followed. Briefly, blood or edema fluid in 0.1 ml was inoculated in duplicate nutrient broth tubes and blood agar and MacConkey lactose agar medium plates. The inoculum on the plates was distributed thinly by streaking it with a loop. The whole process was carried out under laminar flow aseptically. All of the tubes and plates were incubated aerobically at 37°C in the incubator and observed daily for 7 days.
For the culture of anaerobic bacteria the tubes and plates were kept in McIntosh Fildes anaerobic jars with a GasPak and incubated at 37°C in the incubator. The plates and tubes were examined after 48 h for any growth.
For identification of bacteria after completion of incubation, one loopful of inoculum was streaked on blood agar and MacConkey agar plates and incubated both aerobically and anaerobically for 48 h at 37°C. Colonies appeared on the solid media after incubation, and a single representative colony was picked up from the agar plate and confirmed on the basis of its cultural and staining characters with Gram stain. Bacteria were identified on the basis of the mor-phology, motility, hemolysis on blood agar plates, and biochemical and sugar fermentation reactions were done according to the techniques of Cowen and Steel (3) and Cruickshank et al. (4).
Measurement of ASO titer in edema fluid and serum.The anti-streptolysin O (ASO) titer in both edema fluid and serum was determined by using commer-cially available kits (Orthodiagnostics, Mumbai, India) according to the method described by the manufacturer.
Measurement of cytokine concentrations.Interleukin (IL-6) and tumor necro-sis factor alpha (TNF-␣) (PharMingen) and IL-1 (Biosource International, Camarillo, Calif.) in edema fluids were measured by sandwich enzyme-linked immunosorbent assay with paired cytokine-specific monoclonal antibodies ac-cording to the manufacturer’s instructions. The concentrations of the cytokines were calculated from optical densities of samples versus optical densities of standards with known concentrations. Means and standard deviations of cytokine concentrations in two samples of edema fluid collected at each period were calculated.
on August 17, 2020 by guest
http://cvi.asm.org/
RESULTS
Irrespective of the degree, site, and timing of L3exposure,
all of the animals became microfilaremic between days 75 and 90 p.f.i. Microfilaremia reached the maximum between days 90 and 180 p.f.i., followed by a sharp decline thereafter (data not shown). The level of microfilariae remained low after the peak microfilaremia except in those animals which received L3
dur-ing the period of diminishdur-ing microfilaremia; in that case, the levels showed a marginal and transient rise within 30 to 60 days following reexposure to L3(data not shown).
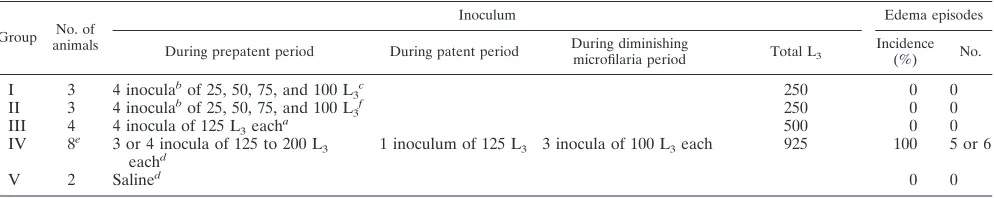
Clinical manifestations.The incidence of limb edema in the
monkeys exposed to L3 is shown in Table 1. The episodic
edema was the pitting and reversible type and was frequently associated with febrile attacks (102.5 to 104.6°C). The duration of edema was 3 to 15 days. None of the monkeys of groups I, II, and III, receiving 250 or 500 L3in the groin or ankle region,
developed edematous swelling in any part of the body. All eight animals of group IV, receiving inoculations in the ankle region, developed edema in the L3-inoculated limb. The
num-ber of episodes of edema was five or six. The edematous attacks were associated with low or no microfilaremia.
Bacteria and ASO titer in edema fluid and serum.Neither
edema fluid nor blood of any of the monkeys showed any growth of aerobic or anaerobic bacteria. The ASO titer was negative in the edema fluid and sera of all of the infected or uninfected (control) monkeys.
Effect of administration of somatic extracts.Table 2 shows
the development of edema in monkeys following inoculation of extracts derived from different life stages of the parasite.
Ju-venile adult worm extract, injected in the inoculated limbs of the animals which were first exposed to L350 to 60 days before,
induced the maximum edematous reaction. Extracts of L4and
L5 stages produced less intense edematous reactions in the
same category of animals. Microfilariae and adult worm ex-tracts did not induce any visible edematous reactions. The same monkeys harboring infections of other ages failed to develop limb edema when administered any of the parasite extracts. The intensity of edema was categorized as mild, mod-erate, or severe at ratios of 1.01 to 1.15, 1.16 to 1.3, and above 1.3, respectively. Control limbs (contralateral limbs) of ex-posed animals and limbs of uninfected animals (which did not receive any L3 inoculation) administered saline did not show
any visible edematous reaction when injected with the parasite extracts.
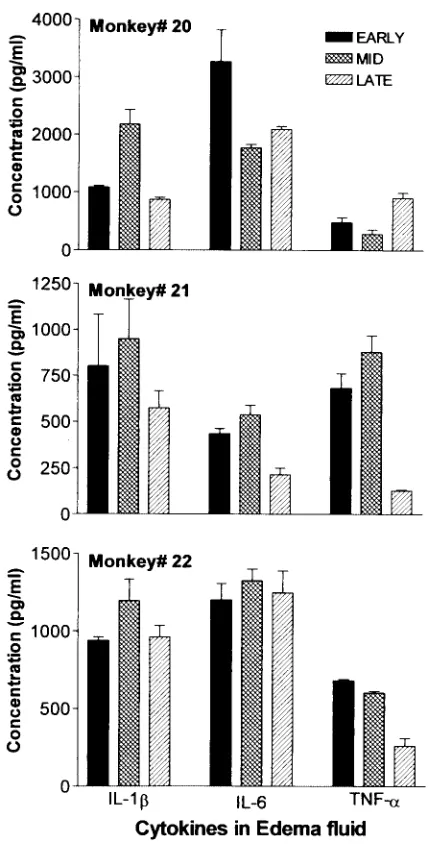
Cytokine levels in edema fluid.Figure 1 shows the
concen-trations of the proinflammatory cytokines IL-1, IL-6, and TNF-␣in edema fluids of limbs of monkeys (no. 20, 21, and 22) of group IV which developed spontaneous edema following exposure to B. malayi L3, measured at three different time
points, i.e., within 24 h of first appearance (early period), at maximum swelling (middle period), and when the edema started receding (late period).
In all three monkeys the patterns for the IL-1 concentra-tion were comparable. In one of the three monkeys (no. 22), the concentration of IL-6 was same at all the time points. Monkey 20 showed elevated levels of IL-6 during early devel-opment of edema, but at the middle and late periods the levels declined. In the edema fluid of the third monkey (no. 21), the IL-6 concentration was highest during the middle period of edema development, but it dropped to very low levels by the late period. In two of the three monkeys (no. 21 and 22) the concentration of TNF-␣in edema fluid at the early and middle time points was comparatively higher than that at the late time point. In contrast, in monkey 20 this cytokine increased during the late period of the edema development compared to the other two periods.
In all three monkeys (no. 38, 39, and 53) receiving juvenile whole-worm extract through the subcutaneous route, of the three cytokines determined in the edema fluid during the late period of edema, the IL-6 concentration was highest, followed by IL-1. The TNF-␣ concentration was the lowest (Fig. 2). These cytokines could not be determined in L4or L5
extract-TABLE 1. Incidence and number of episodes of limb edema in Indian leaf monkeys (P. entellus) infected withB. malayi
Group animalsNo. of
Inoculum Edema episodes
During prepatent period During patent period During diminishingmicrofilaria period Total L3 Incidence(%) No.
I 3 4 inoculabof 25, 50, 75, and 100 L
3c 250 0 0
II 3 4 inoculabof 25, 50, 75, and 100 L
3f 250 0 0
III 4 4 inocula of 125 L3eacha 500 0 0
IV 8e 3 or 4 inocula of 125 to 200 L 3
eachd 1 inoculum of 125 L3 3 inocula of 100 L3each 925 100 5 or 6
V 2 Salined 0 0
aInoculations were given between days 1 and 45 p.f.i. in the groin region. bInoculations were given within 8 days.
cInoculations were given in the groin region.
dInoculations were given at 13- to 15-day intervals in the ankle region. eSix animals received 125 L
3/dose⫻4 and two animals received 125, 175 and 200 L3/dose. f Inoculations were given in the ankle region.
TABLE 2. Effects of challenge with extracts of differentB. malayi
life forms on the development of edematous swelling in normal and L3-induced infection in Indian leaf monkeys (P. entellus)
Group (n)
Reactionawith the following somatic extract:
Microfilariae L3 L4 L5 Juvenile adultworm Adultworm
Naive (2) ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
Infected (6)b ⫺ ⫺ ⫹ ⫹⫹ ⫹⫹⫹ ⫺
aGrading of edematous reaction (ratio of circumference between control and
experimental limbs):⫺, no swelling (1.0);⫹, mild swelling (1.01 to 1.15);⫹⫹, moderate swelling (1.16 to 1.3);⫹⫹⫹, severe swelling (more than 1.3).
bAnimals harboring 50- to 60-day-old infections.
on August 17, 2020 by guest
http://cvi.asm.org/
induced edema due to insufficient edema fluid from the very low intensity of edema developed by the animals.
DISCUSSION
It is well understood that multiple acute attacks of ad-enolymphangitis lead to chronic irreversible deformity. How-ever, it is not clear what causes more episodes in some indi-viduals than in others. Is it the consequence of parasite burden, immunological reactions, bacterial involvement, or a combina-tion of these or other factors? In our previous study we re-ported that 33 to 60% of Indian leaf monkeys exposed to single or multiple doses of B. malayi L3 developed acute disease
manifestations such as episodic limb edema, systemic symp-toms of fever, and malaise. Earlier we also reported that 1 of the 12 symptomatic monkeys had developed limb edema that persisted for more than 10 months, with some signs of
chro-nicity of the manifestations at late stage (8). In the present investigation we have tried to determine the parasitological variables responsible for the development of disease manifes-tations in this monkey model.
Five major factors appeared to be associated with the de-velopment of edematous swelling in the present model. First, repeated exposure ofP. entellustoB. malayiL3following the
first larval exposure induced edematous swelling in the limbs of all (100%) of the animals. This occurred when animals were repeatedly exposed to different doses of L3 at the prepatent,
patent, and diminishing microfilaremia stages of infection fol-lowing the first larval inoculation. Using logistic regression analysis of the present and earlier data, we found that the incidence of edema development in P. entellus could be in-creased if L3was injected between days 30 and 60 p.f.i. Limb
edema was also induced by administration of somatic extracts of specific parasite life stages into the limbs of monkeys har-boring 50- to 60-day-old infections. This finding suggested that repeated exposure during these periods perhaps was necessary for the development of edema in this model, as administration of the parasite extracts during other periods, such as between days 15 and 25, 150 and 160, or 280 and 290 p.f.i., failed to elicit any edematous reaction. Klei et al. (16, 17) have shown that jirds with B. pahangi infection developed a large number of lymph thrombi in the lymphatics between 60 and 90 days posti-noculation in response to embolization of soluble somatic ex-tract-coated cyanogen bromide activated Sepharose. This co-incides with the time at which the female worms start releasing microfilariae. This evidence indicated that parasite-specific factors or products, reproductive products, or larvae or their metabolites might be responsible for the induction of edema-tous reactions. Several investigators have suggested that fre-quent and repeated exposure to infective mosquito bites pos-itively correlates with infection prevalence, intensity of infection, and disease symptoms (11, 12, 14, 25). Our present findings, viewed against the background of reports in the lit-erature, indicate that repeated and continuous exposure to parasites at the time when existing parasites are at molting FIG. 1. Cytokines in edema fluid of symptomaticB. malayi-infected
Indian leaf monkeys (P. entellus). Error bars indicate standard devia-tions.
FIG. 2. Proinflammatory cytokines in edema fluid of limbs of In-dian leaf monkeys (P. entellus) infected withB. malayiand challenged with whole worm extract (values are means and standard deviations for three monkeys [monkeys 38, 39, and 53]).
on August 17, 2020 by guest
http://cvi.asm.org/
stages or when female worms start releasing microfilariae might be necessary for the induction of edematous reaction.
Second, the most interesting finding of the present study is that the somatic extract of the preadult stage of the worm, when inoculated into the parasitized limb, evoked the most intense edematous swelling, whereas the extracts of L4or L5
stages were much less effective. Further, the nonparasitized limb did not show any edematous swelling following adminis-tration of any of the worm extracts. These findings thus clearly indicate a decisive role of preadult stage parasite components in the development of limb edema. Recently, the lipopolysac-charides (LPS) of the endosymbiotic Wolbachia bacteria present in all the stages of the parasite have been considered to be potential stimulators of the inflammatory reaction (29). It is then likely that induction of intense edema in our study pre-dominantly by the extracts of preadult stage is due to LPS of
Wolbachiain the extracts. However, why the extracts of other
stages injected in quantitatively identical amounts should fail to elicit identical edematous responses is not clear and remains to be investigated. Perhaps the density of the bacterial popu-lation in other stages is below the threshold required for ini-tiating and sustaining the edematous reaction, but no informa-tion is presently available on this aspect. Nevertheless, the edematous reaction observed in the present model resembles those in patients after diethylcarbamazine therapy due to re-lease of a large amount of parasite antigen and/or LPS-like molecule (13). With rodent models, Klei et al. (15) showed that prior sensitization of jirds withB. pahangifacilitated the de-velopment of an inflammatory reaction in tissues, but the iden-tity of the parasite stage involved was not known.
Third, in the present study it was observed that a large inoculum size was required to induce an inflammatory (edem-atous) reaction, as a smaller inoculum (250 L3) failed to induce
such a reaction. In areas of endemicity a human subject ac-quires, on average, approximately 4,000 to 6,000 infective lar-vae through around 1,500 mosquito bites per year (10). It is thought that such repeated exposures to large numbers of larvae increase the chances for the development of adenolym-phangitis. The site of L3 exposure is an equally important
determinant for development of an inflammatory reaction, since the monkeys inoculated with L3in the ankle region
de-veloped edematous swelling while those inoculated in the groin region did not. Three features related to the site of disease development in malayan filariasis are that (i) the lower extrem-ities of the human body are the most preferred biting sites for
B. malayi-carrying mosquitoes, (ii) leg edema is common, and
(iii) B. malayi has a special preference for residing in the popliteal lymph nodes. In the present study too it was found that when the forearm instead of the hind limb was used for inoculation of L3, subsequent challenge of the same forearm
with parasite extracts failed to induce an edematous reaction in the arm (data not shown), indicating that the axial lymph nodes are not the preferred abodes for the parasite. These findings suggest that possibly all of these combinations of elements of the exposure profile may not occur uniformly in all subjects in areas of endemicity and hence only a certain percentage of the population, which has such a schedule or combination of ex-posures, shows manifestations of the disease.
Fourth, in the present study the absence of ASO titer or bacterial infection in serum or edematous fluid of monkeys
indicated that bacterial infection was not involved in the de-velopment of acute episodic attacks of limb edema. This find-ing agrees with the report of Taylor and Turner (30), who considered the role of bacterial infection in enhancing lym-phatic pathology to be controversial.
Finally, the presence of significant amounts of local inflam-matory cytokines in the edema fluid of our monkeys indicates that cytokines play an important role in the development of limb edema. Rao et al. (26) found that infection of immuno-deficient mice with Brugiaspecies results in development of lymphedema and is associated with production of proinflam-matory cytokines IL-1, IL-6, TNF-␣, and granulocyte-macro-phage colony-stimulating factor in lymph fluid of parasitized dilated lymphatics. They suggested that the regulatory activity of a network of these localized cytokines might cause the lymphatic lesions. In the present study we could demonstrate the cytokines directly in the edema fluid. Also, peripheral blood mononuclear cells from our infected monkeys (group IV), when stimulated withB. malayiantigen, produced signif-icant amounts of IL-1, IL-6, and TNF-␣, of which the level of IL-6 was the highest (data not shown). In vitro and in vivo studies with filaria-infected animals and humans have shown that filarial parasites could stimulate production of IL-1, IL-6, IL-12, TNF-␣, and granulocyte-macrophage colony-stimulat-ing factor, and they correlated with the development of acute and chronic disease manifestations (5, 13, 19, 29, 31; P. F. Turner, K. A. Rockett, H. Francis, K. Awadzi, E. A. Ottesen, and A. Clark, Abstr. Meet. Aust. N. Zealand Soc. Parasitol., 1992; M. Yazdanbakhsh, L. Duyun, L. Aarden, and F. Partono, Letter, J. Infect. Dis.166:453–454, 1992). Regarding the iden-tity of the parasite products or factors stimulating the cytokine production, not much is known at present, but they may in-clude, as mentioned above, the LPS of the endosymbiotic
Wol-bachiaspp. (29).
In conclusion, the present findings indicate that following first larval exposure, the mode of subsequent larval exposure, such as the size, site, and timing of exposure, plays a decisive role in the development of manifestations in the Indian leaf monkey model. The preadult stage appeared to be predomi-nantly involved in evoking an acute edematous reaction in the infected monkeys, and the edematous reaction developed only in the limb that was challenged with parasite extract. The presence of inflammatory cytokines in systemic and edematous fluids suggests that the development of edema is evidently mediated by cytokines produced during the interaction be-tween the host and the parasite or its products. The present study also shows that bacterial infection is probably not nec-essary for the development of acute episodic attacks of limb edema in the monkey model.
ACKNOWLEDGMENTS
We thank C. M. Gupta for necessary facilities, P. S. R. Murthy for critical review, S. K. Mandal for statistical analysis of the data, and V. K. Bose and R. C. Rai for technical assistance.
REFERENCES
1.Addis, D. G., M. L. Eberhard, and P. J. Lammie.1994. ‘Filarial’ adenolym-phangitis without filarial infection. Lancet343:597.
2.Bundy, D. A., B. T. Grenfell, R. Spark, J. W. Kazura, and M. P. Alpers.1991. Age specific patterns of changes in the dynamics ofWuchereria bancrofti infections in Papua New Guinea. Am. J. Trop. Med. Hyg.44:518–527.
on August 17, 2020 by guest
http://cvi.asm.org/
3.Cowan, S. T., and K. J. Steel.1965. Manual for the identification of medical bacteria. Cambridge University Press, London, United Kingdom. 4.Cruickshank, R., J. P. Duguid, B. P. Marmion, and R. H. A. Swain.1975.
Medical microbiology, 12th ed., vol. II. The practice of medical microbiology, p. 96–189. Churchill Livingstone, London, United Kingdom.
5.Das, B. K., P. K. Sahoo, and B. Ravindran.1996. A role of tumor necrosis factor-alpha in acute lymphatic filariasis. Parasite Immunol.18:421–424. 6.Denham, D. A., T. Ponnudurai, G. S. Nelson, and R. Rogers.1972. Studies
withBrugia pahangi. II. The effect of repeated infection on parasite levels in cats. Int. J. Parasitol.2:401–407.
7.Ewert, A., J. C. Reitmeyer, and D. Folse.1980. Chronic infection of cats with Brugia malayi and Streptococcus. Southeast Asian J. Trop. Med. Public Health11:31–39.
8.Ghosh, R. P., P. K. Murthy, K. Tyagi, P. S. R. Murthy, and R. K. Chatterjee.
1999. Longitudinal cellular immune responses in asymptomatic and symp-tomaticBrugia malayi-infected leaf monkeyPresbytis entellus. J. Parasitol.
85:861–866.
9.Grenfell, B. T., E. Michael, and D. A. Denham.1992. The cat-Brugia pahangi system as a model for the dynamics of human lymphatic filariasis. Parasitol. Today7:318–323.
10.Gubler, D. J., and N. C. Bhattacharya.1974. A quantitative approach to the study of bancroftian filariasis. Am. J. Trop. Med. Hyg.23:1027–1036. 11.Gyapong, J. O.1998. The relationship between infection and disease in
Wuchereria bancroftiinfection in Ghana. Trans. R. Soc. Trop. Med. Hyg.
92:390–392.
12.Gyapong, J. O., M. Gyapong, and S. Adjei.1996. Epidemiology of acute adenolymphangitis (ADL) due to lymphatic filariasis in northern Ghana. Am. J. Trop. Med. Hyg.54:591–594.
13.Harbrink, M., A. J. Terhell, G. K. Abadi, Y. Mitsui, and M. Yazdanbakhsh.
1999. Inflammatory cytokines following diethylcarbamazine (DEC) treat-ment of different clinical groups of lymphatic filariasis. Trans. R. Soc. Trop. Med. Hyg.93:665–672.
14.Kazura, J. W., R. Spark, K. Forsyth, G. Brown, P. Heywood, P. Peters, and M. Alpers.1984. Parasitological and clinical features of bancroftian filariasis in a community in East Sepik province, Papua New Guinea. Am. J. Trop. Med. Hyg.33:119–123.
15.Klei, T. R., F. M. Enright, D. P. Blanchard, and S. A. Uhl.1982. Effects of presensitization on the development of lymphatic lesions inBrugia phangi -infected jirds. Am. J. Trop. Med. Hyg.31:280–291.
16.Klei, T. R., F. M. Enright, K. McDonough, and S. U. Coleman.1988.Brugia pahangi: granulomatous lesion development in jirds following single and multiple infections. Exp. Parasitol.66:132–139.
17.Klei, T. R., C. S. McVay, V. A. Dennis, S. U. Coleman, F. M. Enright, and H. W. Casy.1990.Brugia pahangi:effects of infection duration and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp. Parasitol.71:393– 405.
18.Lowry, O. H., N. R. Rosebrough, A. L. Farr, and R. J. Randall.1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem.193:265–275.
19.Mahanty, S., M. Ravichandran, U. Raman, K. Jayraman, V. Kumaraswami, and T. B. Nutman.1997. Regulation of parasite-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect. Immun.65:
1742–1747.
20.Murthy, P. K., K. Tyagi, and R. K. Chatterjee.1990. Indian langur (Presbytis entellus) as an experimental host forBrugia malayi infection. Curr. Sci.
59:1236–1239.
21.Murthy, P. K., K. Tyagi, R. P. Ghosh, P. S. R. Murthy, and R. K. Chatterjee.
1999. Longitudinal humoral immune responses of Indian leaf monkey ( Pres-bytis entellus) toBrugia malayiinfection. Parasitology119:53–66. 22.Murthy, P. K., K. Tyagi, T. K. Roy Chowdhury, and A. B. Sen.1983.
Sus-ceptibility ofMastomys natalensis(GRA strain) to subperiodic strain of Brugia malayi. Indian J. Med. Res.77:623–630.
23.Olsewski, W. L., S. Jamal, G. Manokaran, S. P. Pani, V. Kumaraswami, U. Kubicka, B. Lukomsaka, A. Dworezynski, E. Swoboda, and F. Meisel-Miko-lajcyk.1997. Bacteriologic studies of skin, tissue fluid, lymph and lymph nodes in patients with filarial lymphedema. Am. J. Trop. Med. Hyg.57:7–15. 24.Pani, S. P., J. Yuvaraj, P. Vanamail, V. Dhanda, E. Michael, B. T. Grenfell, and D. A. P. Bundy.1995. Episodic adenolymphangitis and lymphoedema in patients with bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg.89:72–74. 25.Partono, F.1987. The spectrum of disease in lymphatic filariasis. CIBA
Found. Symp.127:15–31.
26.Rao, U. R., A. C. Vickery, B. H. Kwa, and J. K. Nayar.1996. Regulatory cytokines in the lymphatic pathology of athymic mice infected withBrugia malayi. Int. J. Parasitol.26:561–565.
27.Srividya, A., S. P. Pani, P. K. Rajagopalan, D. A. P. Bundy, and B. T. Grenfell.1991. The dynamics of infection and disease in Bancroftian filari-asis. Trans. R. Soc. Trop. Med. Hyg.85:255–259.
28.Tandon, A., P. K. Murthy, R. P. Saxena, A. B. Sen, and K. C. Saxena.1988. Dot-ELISA for diagnosis of lymphatic filariasis. Indian J. Med. Res.87:429– 433.
29.Taylor, M. J., H. F. Cross, and K. Bilo.2000. Inflammatory responses induced by the filarial nematodeBrugia malayiare mediated by lipopolysac-charide-like activity from endosymbioticWolbachiabacteria. J. Exp. Med.
191:1429–1435.
30.Taylor, M. J., and P. F. Turner.1997. Control of lymphatic filariasis. Para-sitol. Today13:85–87.
31.Turner, P. F., K. A. Rockett, E. A. Ottesen, H. Francis, K. Awadzi, and A. Clark.1994. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J. Infect. Dis.169:1071–1075.
32.Tyagi, K., P. K. Murthy, and R. K. Chatterjee.1996. Response of Indian leaf monkey (Presbytis entellus) to subperiodic strain ofBrugia malayi. Curr. Sci.
70:164–167.
33.Wilson, G. S., A. Miles, and M. T. Parker.1983. Principles of bacteriology, virology and immunology, 7th ed., vol. 2. Edward Arnold, London, United Kingdom.